Abstract
♦ Objective: Our aim in the present study was to identify outcomes in children with special needs or social disadvantage, or both, receiving chronic peritoneal dialysis (CPD) treatment in a pediatric dialysis unit.
♦ Methods: Among 110 children started on CPD in our unit during the period between November 1995 and November 2008, we identified 13 patients (8 girls, 5 boys) with major physical, mental, or psychosocial problems. Age at CPD initiation in the group with disability ranged from 4.0 years to 16.5 years (median: 7.5 years). Under lying diseases were vesicoureteral reflux (4 patients), neuropathic bladder and vesicoureteral reflux (3 patients), chronic pyelonephritis (3 patients), amyloidosis (2 patients), and Alport syndrome (1 patient). Challenges encountered were adverse family or social circumstances (4 patients), cerebral palsy (3 patients), Down syndrome (1 patient), rectovesical fistula in conjunction with ectopic anus and previous multiple abdominal surgery (1 patient), blindness and deafness (1 patient), ventriculoperitoneal shunt (1 patient), colostomy and malnutrition (1 patient), and mental retardation and blindness (1 patient). All catheters were implanted percutaneously.
♦ Results: Median duration of dialysis was 18 months (range: 6 - 124 months). The frequency of peritonitis was not different between children with and without disability (p > 0.05). In children with disability compared with children without disability, the frequencies of catheter-related infections (1 episode/79.3 patient-months vs 1 episode/32.4 patient-months) and of catheter-related non-infectious complications (1 episode/238 patient-months vs 1 episode/115.7 patient-months) were lower (p < 0.05). Chronic peritoneal dialysis was terminated in 5 children (for renal transplantation in 3, switch to hemodialysis in 1, death in 1).
♦ Conclusions: Our results suggest that, with appropriate family support and an experienced multidisciplinary team, CPD can be effectively performed in children with special needs or social disadvantage, or both.
Keywords: Chronic peritoneal dialysis, contraindications, end-stage renal disease, disability, children
Children with end-stage renal disease (ESRD) and their parents are faced with the question of dialysis modality choice. Peritoneal dialysis (PD) and hemodialysis (HD) differ technically and in the type of effort required from patients. The registry of the North American Pediatric Renal Transplant Cooperative Study has reported data about treatment options for patients initiating renal replacement therapy (RRT) in pediatric centers: One quarter of the children studied underwent preemptive renal transplantation, one half were started on PD, and one quarter were started on HD. Thus, chronic peritoneal dialysis (CPD) is the most commonly used mode of RRT in children (1,2).
When preemptive transplantation is not an option, technical, social, and compliance issues, together with family preference, generally dictate the choice between HD and CPD. Compared with HD, CPD allows for the least disruption of home life and school and work attendance. It is particularly useful in very small patients with lack of vascular access, contraindications for anticoagulation, cardiovascular instability, and lack of proximity to pediatric HD centers. Chronic PD is associated with better control of blood pressure, better correction of acidemia and fluid balance, better growth and rehabilitation, and more liberalized food and water intake (3-5).
But despite those advantages, the application of CPD is sometimes very difficult or even impossible. Inappropriate social conditions, mental retardation, previous abdominal surgery, ventriculoperitoneal shunt, and ostomies, which all make CPD difficult, are some of the adverse factors (6). Published experiences of physically or socially challenged children on CPD are lacking. Our aim in the present study was to analyze the outcome of CPD treatment in children with special needs or social disadvantages (or both) identified at a pediatric center during a 13-year experience with PD.
METHODS
The study was conducted at the Department of Pediatric Nephrology, Izmir Tepecik Training and Research Hospital, Turkey. Children with ESRD who were on CPD from November 1995 to November 2008 were included in the study. Children were excluded if the PD catheter was used for acute renal failure. Medical records for the study patients were retrospectively reviewed using a standardized data collection form. Physically or psychosocially challenged children with ESRD receiving CPD were studied in depth.
Before starting RRT, all children and their families are introduced to the clinicians and nurses that constitute our dialysis team. They are educated about renal replacement therapy, including dialysis modality choices. After home visits from our nurses, a variety of factors, including patient or family choice, patient size, medical comorbidities, and family support, are taken into account before the selection of CPD as a dialysis modality. Patients and their legal guardians are actively involved in making the choice of treatment.
Negative conditions that make CPD treatment difficult are grouped into these categories (6):
Relative contraindications: A living situation inadequate for home dialysis, lack of an appropriate caregiver, impending or recent major abdominal surgery, imminent living-related donor transplantation
Absolute contraindications: Omphalocele, gastroschisis, bladder extrophy, diaphragmatic hernia, obliterated peritoneal cavity, peritoneal membrane failure
Despite the presence of adverse conditions, CPD was chosen instead of HD for the treatment of the challenged children with ESRD because of difficulties of vascular access (in small children), long distance from a pediatric HD center, family preference, or a combination of those reasons. Written informed consent for the choice of RRT modality was obtained from all parents of the children. In our unit, no patient with special needs or social disadvantage was denied treatment over the study period. Although the study group of children with disability did not contain any young infants, the same policies on RRT choice were applied to all pediatric age groups.
All CPD catheters were placed percutaneously under local prilocaine anesthesia in a ward treatment room by an experienced pediatric nephrologist as described in detail in a previous paper (7). We used swan-neck, double-cuffed Tenckhoff catheters with coiled tips. A 1-week training course for continuous ambulatory PD (CAPD) or automated PD (APD) was started 1 week after implantation. Our policy is to train, whenever possible, 2 family members. Except when dialysis was urgently needed, CPD treatment was started 2 weeks after catheter placement in all patients. In patients requiring immediate dialysis, CPD was initiated just after catheter insertion using low dialysate volumes (500 mL/m2), with heparin (1000 U/L) administered every 1 - 2 hours during the first 2 - 3 days. During that period, patients stayed in a supine position with minimal ambulation. The amount of dialysate was then increased gradually to 1100 - 1400 mL/m2 within 2 weeks. The remaining children were seen at 1 week, at which time their incisions were checked and their dressings changed.
The parents performed the PD (either CAPD or APD) at home. Subsequently, all children were assessed at least once monthly in the nephrology clinic, when a complete blood count, blood chemistry (including estimation of residual renal function), and microbiologic and biochemical examinations of the dialysate effluent were done. At that time, the dialysis nurse checked parental skills in PD by asking them to do a bag change (for CAPD patients) or to complete the cycler set-up, showing how they connect to and disconnect from the cycler (for APD patients), under our supervision during the clinic visit. In addition, the dialysis nurses made home visits at least twice annually.
We studied the demographic characteristics of the children (age, sex, primary renal diagnosis) and the incidence of catheter-related complications. All catheters were evaluated for mechanical and infectious complications. For children who transferred to another center while still on dialysis therapy, the day of transfer was counted as the last day of follow-up, with a minimum follow-up of 3 months.
A diagnosis of peritonitis was made if effluent showed a white blood cell count greater than 100/mm3, with at least 50% polymorphonuclear neutrophils, with or without the presence of abdominal pain and cloudiness of the effluent. We defined exit-site infection as pericatheter erythema or drainage or both; a positive culture result was not mandatory. Criteria for diagnosis of tunnel infection were erythema, edema, and tenderness over the subcutaneous portion of the catheter. When an infection was suspected, samples from the exit site or the effluent were obtained for culture before initiation of treatment. All samples were processed in our hospital. Empiric antibiotic therapy with cefazolin and ceftazidime was the initial therapy in patients with peritonitis. For exit-site infection, the initial therapy was local application of mupirocin ointment. We defined mechanical complications as noninfectious complications related to the catheter: drainage problems, catheter migration, catheter kink (8). The complication rate was calculated as the average number of patient-months between episodes.
Differences between the children with and without disability were analyzed using the chi-square test for categorical variables and the Mann-Whitney U-test for continuous variables. Values of p less than 0.05 were accepted as significant. The SPSS statistical software program (version 13.0: SPSS, Chicago, IL, USA) was used for the analysis.
RESULTS
Between November 1995 and November 2008, 110 children diagnosed with ESRD at the department of Pediatric Nephrology, Izmir Tepecik Training and Research Hospital [mean age: 9.8 ± 4.0 years (range: 1.1 months - 19 years); 59 girls, 51 boys], were dialyzed with CPD. Of those 110 children, 13 with special needs or social disadvantage, or both, were identified (Table 1).
TABLE 1.
Demographic and Clinical Features of 13 Children with Special Needs or Social Disadvantages on Chronic Peritoneal Dialysis
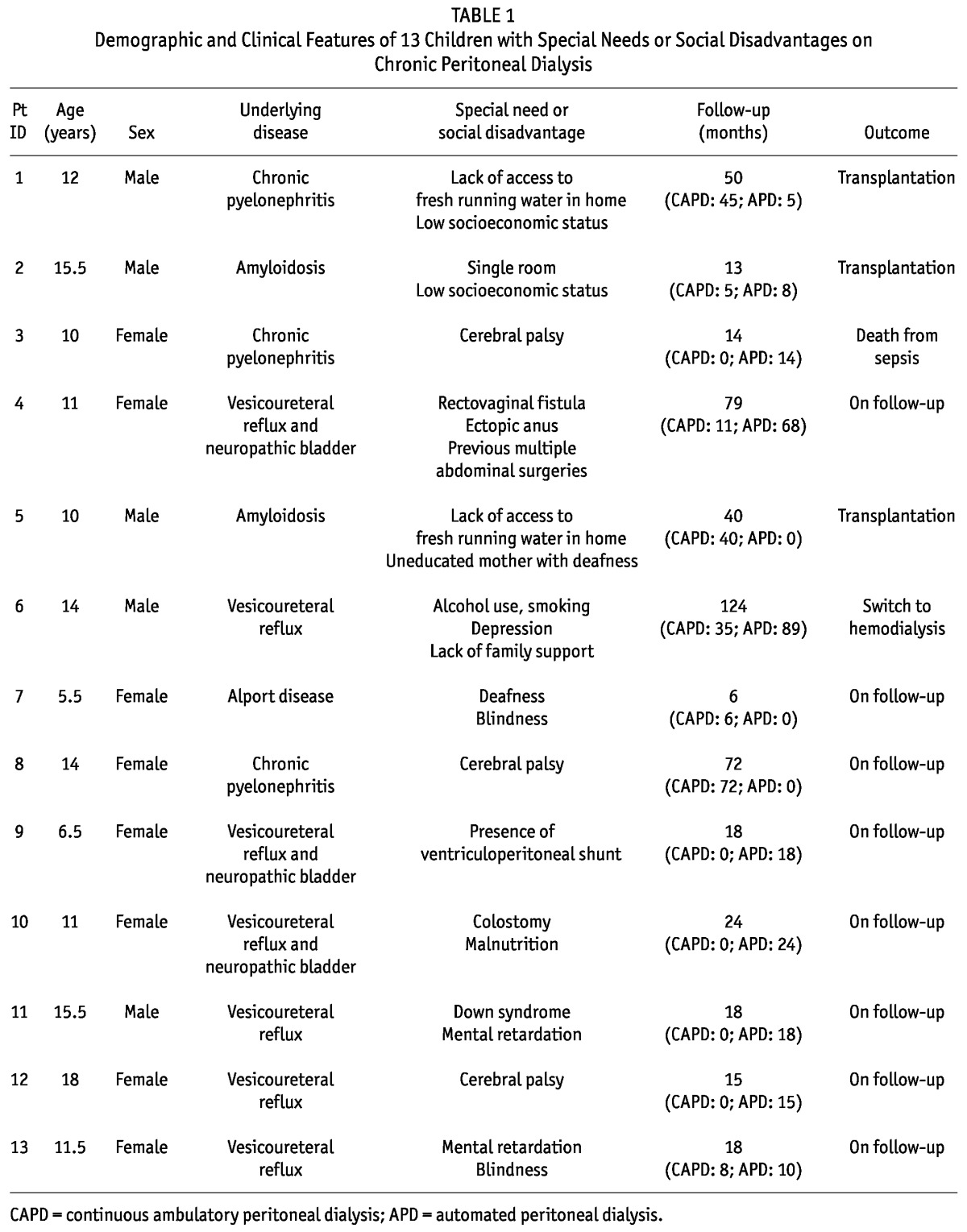
In children with disability, CPD was preferred instead of HD because of distance from the pediatric HD centers (patients 1, 2, 3, 5, 8, 9, 10), difficulties of vascular access (patients 4, 6, 7, 9, 10), and family or patient preference (all patients). Median duration of CPD was 18 months (range: 6 - 124 months). During a total of 476 dialysis months, 31 episodes of peritonitis (1 episode/15.3 patient-months), and 6 catheter infections (5 at the exit-site and 1 of the tunnel; 1 episode/79.3 patient-months) were observed. Of those 37 episodes of infection, 23 (62.1%) were culture-negative. Another 7 involved Pseudomonas species (18.9%); 5 (13.5%), Staphylococcus aureus; 1 (2.7%), Enterobacter species; and 1 (2.7%), Staphylococcus epidermidis. No peritonitis episodes were observed in patients 7, 9, 10, and 12. Two patients had 1 peritonitis episode each (patients 3 and 11), two patients had 2 peritonitis episodes each (patients 5 and 8), two patients had 3 peritonitis episodes each (patients 1 and 2), one patient had 4 peritonitis episodes (patient 13), one patient had 7 peritonitis episodes (patient 4), and one patient had 8 peritonitis episodes (patient 6). Two catheter-related noninfectious complications were observed: a drainage problem (patient 2) and an incisional hernia (patient 4). The rate of noninfectious complications was 1 episode in 238 patient-months. In the 13 patients with disability, 14 PD catheters were inserted (patient 4 required catheter replacement because of resistant peritonitis).
In the 97 children without disability, the median duration of PD was 17 months, and the total dialysis time was 3010 months. A total of 179 episodes of peritonitis, 92 catheter infections (82 exit-site infections, 10 tunnel infections), and 26 catheter-related noninfectious complications (12 dislocations, 7 drainage problems, 7 kinks) were observed. These 97 patients received a total of 101 PD catheters.
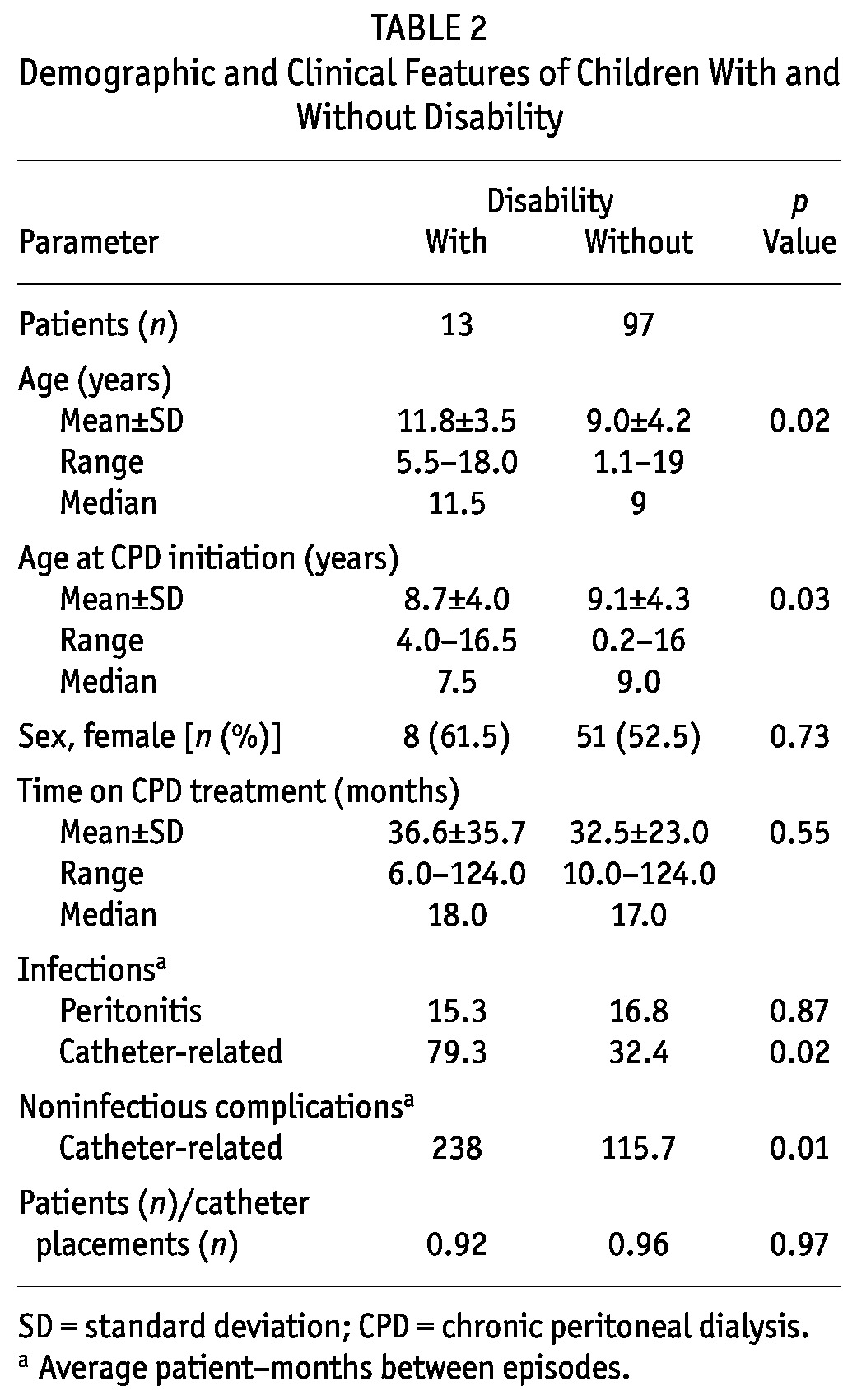
Table 2 compares the children with and without disability. The frequency of peritonitis was not statistically different between the groups (p > 0.05). Surprisingly, the frequencies of catheter-related infection and noninfectious catheter complications were significantly lower in children with disability than in those without disability (p < 0.05).
TABLE 2.
Demographic and Clinical Features of Children With and Without Disability
DISCUSSION
Few studies in the literature have looked at CPD treatment in children with special needs (9,10). In the present study, we report our single-center experience of children with disability receiving CPD treatment. Although the number of patients was limited, this study is the first from a developing country to compare children with and without disability on CPD. The time on CPD treatment, the rate of peritonitis, and the number of PD catheters placed in children with disability were similar to those in children without disability. Surprisingly, the rates of catheter-related infections and noninfectious catheter complications were lower in children with disability than in those without disability. These results show that CPD might be an applicable RRT option in ESRD children with special needs in our country.
Mental retardation can be regarded as a contraindication or relative contraindication for CPD treatment because of the higher risk of peritonitis caused by the patient’s difficulty with maintaining personal hygiene and compliance. In pediatric patients undergoing PD, the need for the exchanges to be performed by another person is highly important and could explain the compromised quality of life of the parents. Mental retardation in the PD patient further increase dependence, and hence, parents are often constrained by family commitments. The primary caregiver for a patient who is not self-sufficient in a CPD program is therefore exposed to burnout syndrome (11-13).
For parents who are unable to devote themselves to treating their mentally retarded child and providing everyday care, the decision to initiate PD treatment can be difficult. In this situation, the decision can be made with the help of an ethics committee. Our hospital has no committee to deal with this issue; however, we have not encountered such a case to date. On the other hand, mentally retarded children are often not able to remain quiet or immobile for the 3 - 4 hours required to complete a HD treatment (11,14), and it is also clear that these patients can develop severe peritonitis that might require conversion to temporary HD. Mentally retarded PD patients can be prepared for accepting HD treatment by a graduated approach using play therapists and psychologists. In the present study, 5 - 13 children with disabilities had mental retardation. Fortunately, their parents devoted themselves to serving their child and providing everyday care. The frequency of peritonitis was not significantly higher in the mentally retarded children than in non-retarded children. We believe that the personal characteristics of the parents are important for the success of CPD treatment. Additionally, it has been our observation that increased well-being in the patient is reflected in a gradual improvement in parental emotional status.
Adhesions resulting from previous abdominal surgery can cause catheter migration, kinking, and obstruction in patients starting CPD. These complications can lead to malfunction of the catheter (15). But even if the catheter functions well, these patients usually have a smaller effective peritoneal surface area because of adhesions and loculations, potentially resulting in either or both of underdialysis and ultrafiltration failure (11). Incisional hernia is a frequent complication of abdominal surgery. Currently, laparoscopy is the only practical way to reliably investigate the suitability of the peritoneal cavity for dialysis in patients with a history of abdominal surgery (16). In our series, only 1 child (patient 4) had a history of 2 previous abdominal surgeries (for rectovaginal fistula and ectopic anus). We were not able to undertake laparoscopy before PD catheter placement in that patient. During follow-up on CPD, an incisional hernia developed and was repaired. This patient also required catheter replacement because of resistant peritonitis. As expected, the patient had negative experiences associated with the earlier abdominal surgeries. Yet, despite all of the adverse events, she has been on CPD treatment for more than 6 years.
The presence of a colostomy in infants with ESRD receiving PD is associated with an inherent risk for contamination and the development of a PD catheter-associated infection. The swan-neck presternal catheter could be a better option in a patient with an ostomy (11,17,18). Malnutrition is another situation that argues against the choice of CPD treatment (11). In the present study, 1 of the children with disability (patient 10) had a colostomy because of Hirschsprung disease. Additionally, she was undernourished. She therefore had 2 relative contraindications, but the choice for CPD had to be made because of her distance from pediatric HD centers, difficulties of vascular access, and patient and family preference. A swan-neck presternal catheter was not selected because of our lack of experience with that technique. We instead applied the method that we know best. The patient has been on follow-up for 2 years.
Some authors consider that the presence of a ventriculoperitoneal shunt is a relative contraindication to PD because of the potential risks of shunt-induced peritoneal damage and of infection ascending through the shunt (11,19). Patients with a ventriculoperitoneal shunt on CPD present specific diagnostic challenges because of overlapping symptoms secondary to PD or to complications of the ventriculoperitoneal shunt (3). More recent reports suggest that PD under close monitoring is not contraindicated in children with ESRD regardless of the presence of ventriculoperitoneal shunt (3,10). In our series, patient 9, with a ventriculoperitoneal shunt, has been maintained on CPD treatment for 18 months. We share the view of Ram Prabahar et al. (3) that physicians, nurses, patients, and parents need to be forewarned about possible complications, especially in developing countries.
If the patient has a helper who is committed to doing the dialysis, then blindness is not an absolute contraindication to PD in adulthood (11). In childhood, the presence of dedicated parents makes the application of CPD easier. Among our children with challenges, the patient with blindness and Alport disease (patient 7) has been on CPD treatment for the shortest time (6 months). Our experience with that patient is therefore limited. The other patient with blindness (patient 13) also has mental retardation; however, she has remained on CPD therapy for 18 months.
Homelessness, poor home conditions, poor hygiene, lack of a place to store supplies, lack of a telephone, severe depression, drug abuse, and inadequate social support are all adverse factors that argue against CPD treatment. Some of those factors are challenges in both PD and HD (11). According to the limited data available from developing countries, poor socioeconomic conditions do not appear to play a role in peritonitis rates and CPD failure (20). In the present study, the socioeconomic conditions of 4 patients (patients 1, 2, 5, 6) were poor. But CPD treatment was the real option for dialysis in those patients because of their distance from pediatric HD centers and the difficulties of vascular access. Our pediatric dialysis team educated the families rigorously. Home visits were more frequent. Their knowledge and skills in CPD application were rigorously checked by dialysis nurses. The social services unit of our hospital gave financial and logistics support to the families to ensure more convenient indoor conditions (modifications to the dialysis room, electrical hook-up, provision of running water, and donation of the materials required for dialysis, among others). The hospital psychologists intermittently provided psychological support to the families and the patients. We also implemented patient-specific initiatives. The mother of patient 5 was deaf and had a poor education level. Training in CPD was given to the patient’s sister (25 years old and married), who was willing to help and who lived near the parental home. Patient 6 was using alcohol and was smoking. He also had depression. On follow-up, psychiatric aid was provided in an attempt to solve those problems. The attempt was not entirely successful; his family was enthusiastic initially, but they did not give appropriate support. In course of time, the dialysis team overcame the problems, partially through close attention. The support of the family then gradually increased. At the end of dialysis therapy, CPD was a bridge to transplantation in 3 of these 4 psychosocially challenged children.
Despite the absolute and relative contraindications to the use of CPD in some children (6), our study supports the decision to offer that modality to children with special needs or social disadvantage when parents who can provide dedicated and meticulous care are identified. Indeed, in our group, successful PD treatment has been achieved for these patients with the help of their dedicated families.
The successful application of CPD also requires a dedicated dialysis team. The experience of the team is another important feature (7). Our pediatric dialysis team has worked together since 1995. We believe that our comprehensive CPD training and monitoring programs for parents and our social support programs are key factors in the success of CPD. Our pediatric dialysis team acts based on those principles.
CONCLUSIONS
Our single-center experience supports the idea that CPD can be a very effective therapy in children with disability. The incidence of complications is within acceptable limits. In countries with limited resources, the effectiveness of CPD treatment is more important for this group of patients. The success of CPD treatment in children with special needs or social disadvantage (or both) depends on an experienced and dedicated pediatric dialysis team, proper education and training of the CPD patients and their families, and appropriate family support.
DISCLOSURES
No grant support and no additional people provided direct help for the preparation of this study. The authors have no conflicts of interest to declare.
REFERENCES
- 1. Neu AM, Ho PL, McDonald RA, Warady BA. Chronic dialysis in children and adolescents. The 2001 NAPRTCS annual report. Pediatr Nephrol 2002; 17:656–63 [DOI] [PubMed] [Google Scholar]
- 2. Watson AR, Gartland C. on behalf of the European Paediatric Peritoneal Dialysis Working Group. Guidelines by an ad hoc European committee for elective chronic peritoneal dialysis in pediatric patients. Perit Dial Int 2001; 21:240–4 [PubMed] [Google Scholar]
- 3. Ram Prabahar M, Sivakumar M, Chandrasekaran V, Indhumathi E, Soundararajan P. Peritoneal dialysis in a patient with neurogenic bladder and chronic kidney disease with ventriculoperitoneal shunt. Blood Purif 2008; 26:274–8 [DOI] [PubMed] [Google Scholar]
- 4. Lingens N, Dobos E, Witte K, Busch C, Lemmer B, Klaus G, et al. Twenty-four-hour ambulatory blood pressure profiles in pediatric patients after renal transplantation. Pediatr Nephrol 1997; 11:23–6 [DOI] [PubMed] [Google Scholar]
- 5. Kaiser BA, Polinsky MS, Stover J, Morgenstern BZ, Baluarte HJ. Growth of children following the initiation of dialysis: a comparison of three dialysis modalities. Pediatr Nephrol 1994; 8:733–8 [DOI] [PubMed] [Google Scholar]
- 6. Verrina E. Peritoneal dialysis. In: Avner ED, Harman WE, Niaudet P, Yoshikawa N, eds. Pediatric Nephrology. 6th ed. Berlin, Germany: Springer-Verlag; 2009: 1785–816 [Google Scholar]
- 7. Aksu N, Yavascan O, Anil M, Kara OD, Erdogan H, Bal A. A ten-year single-centre experience in children on chronic peritoneal dialysis—significance of percutaneous placement of peritoneal dialysis catheters. Nephrol Dial Transplant 2007; 22:2045–51 [DOI] [PubMed] [Google Scholar]
- 8. Rahim KA, Seidel K, McDonald RA. Risk factors for catheter-related complications in pediatric peritoneal dialysis. Pediatr Nephrol 2004; 19:1021–8 [DOI] [PubMed] [Google Scholar]
- 9. Yavascan O, Kara OD, Anil M, Bal A, Pehlivan O, Aksu N. Chronic peritoneal dialysis treatment in a pediatric patient with Down syndrome. Perit Dial Int 2008; 28:558–9 [PubMed] [Google Scholar]
- 10. Grünberg J, Verocay MC, Rébori A, Pouso J. Comparison of chronic peritoneal dialysis outcomes in children with and without spina bifida. Pediatr Nephrol 2007; 22:573–7 [DOI] [PubMed] [Google Scholar]
- 11. Shetty A, Oreopoulos DG. Peritoneal dialysis: its indications and contraindications. Dial Transplant 2000; 29:71–7 [Google Scholar]
- 12. Hausmann MJ, Landau D. A Down syndrome patient treated by peritoneal dialysis. Nephron 2002; 92:484–6 [DOI] [PubMed] [Google Scholar]
- 13. Kotzabassaki S, Parissopoulos S. Burnout in renal care professionals. EDTNA ERCA J 2003; 29:209–13 [DOI] [PubMed] [Google Scholar]
- 14. Kujdych N, Lowe DA, Sparks J, Dottes A, Crook ED. Dignity or denial? Decisions regarding initiation of dialysis and medical therapy in the institutionalized severely mentally retarded. Am J Med Sci 2000; 320:374–8 [DOI] [PubMed] [Google Scholar]
- 15. Chen SY, Chen TW, Lin SH, Chen CJ, Yu JC, Lin CH. Does previous abdominal surgery increase postoperative complication rates in continuous ambulatory peritoneal dialysis? Perit Dial Int 2007; 27:557–9 [PubMed] [Google Scholar]
- 16. Crabtree JH. Previous abdominal surgery is not necessarily a contraindication for peritoneal dialysis. Nat Clin Pract Nephrol 2008; 4:16–17 [DOI] [PubMed] [Google Scholar]
- 17. Warchol S, Roszkowska-Blaim M, Latoszynska J, Jarmolinski T, Zachwieja J. Experience using presternal catheter for peritoneal dialysis in Poland: a multicenter pediatric survey. Perit Dial Int 2003; 23:242–8 [PubMed] [Google Scholar]
- 18. Chadha V, Jones LL, Ramirez ZD, Warady BA. Chest wall peritoneal dialysis catheter placement in infants with a colostomy. Adv Perit Dial 2000; 16:318–20 [PubMed] [Google Scholar]
- 19. Müller T, Arbeiter K, Aufricht C. Renal function in meningomyelocele: risk factors, chronic renal failure, renal replacement therapy and transplantation. Curr Opin Urol 2002; 12:479–84 [DOI] [PubMed] [Google Scholar]
- 20. Katz IJ, Sofianou L, Hopley M. An African community-based chronic ambulatory peritoneal dialysis programme. Nephrol Dial Transplant 2001; 16:2395–400 [DOI] [PubMed] [Google Scholar]


