Abstract
♦ Background: Nephrotic syndrome (NS) in children has been associated with a systemic circulating permeability factor. Therefore, once peritoneal dialysis (PD) has been started, peritoneal protein losses should be higher in the nephrotic than in the non-nephrotic population.
♦ Objective: We compared peritoneal protein losses in children with and without NS on PD.
♦ Methods: Our retrospective 4-year study analyzed Hispanic patients with NS under PD. Data at dialysis entry and 6 months later were compared. Nutritional support was given according to recommended dietary allowances and recommendations from the Kidney Disease Outcomes Quality Initiative. Clinical and biochemical data were obtained, and 24-hour dialysate and urine samples were collected to measure protein losses. Dialysis dose (Kt/V), daily protein intake (DPI), normalized protein equivalent of nitrogen appearance (nPNA), peritoneal equilibration test (PET), and peritonitis rate were determined. All measurements took place at least 4 weeks after resolution of a peritonitis episode. All patients received automated PD using a HomeChoice PD System cycler (Baxter Healthcare Corporation, Deerfield, IL, USA), with an exchange volume of 1100 mL/m2 and a dextrose concentration of 1.5% - 2.5%. A control group of non-NS children on PD matched by age and sex were also studied. Data are reported as mean ± standard deviation. Differences between groups were calculated using the Mann-Whitney U-test, and p < 0.05 was considered significant.
♦ Results: Each study group consisted of 10 patients [NS patients: 4 boys, mean age of 7.3 ± 4.1 years; control patients: 6 boys, mean age of 7.2 ± 4.7 years (p = nonsignificant)]. In the group with NS, 8 patients were diagnosed by biopsy as having focal segmental glomerulosclerosis, and 2 as having minimal-change disease. At study entry, patients with NS had hourly urinary protein losses of 398 ± 313 mg/m2 and daily peritoneal protein losses of 3.4 ± 1.9 g/m2, compared with 29.9 ± 31 mg/m2 and 1.5 ± 1.1 g/m2 respectively in the control group (p < 0.05). The same statistical difference was found 6 months later. We observed no statistical differences in PET results, daily exchange volume, and mean dextrose concentration of dialysate. Similarly, no significant between-group differences were observed for Kt/V, DPI, nPNA, and biochemical parameters.
♦ Conclusions: Hispanic children with NS on PD show higher peritoneal protein losses than do their control counterparts. Such differences could be secondary to increased peritoneal permeability caused by a systemic permeability factor.
Keywords: Peritoneal protein loss, plasma permeability factor, nephrotic syndrome, focal segmental glomerulosclerosis
Idiopathic nephrotic syndrome (NS) is a glomerular disease that typically affects children between 2 and 8 years of age. The estimated annual incidence is 2 - 7 per 100,000 population under the age of 18, and the estimated prevalence is 12 - 16 per 100,000 (1). Minimal-change disease (MCD) remains the most common cause of NS, especially in children less than 8 years of age (2). Some studies have suggested a changing trend in the histopathology of NS, with an increasing incidence of focal segmental glomerulosclerosis (FSGS) in adults as well as in children—reaching up to 55% in adolescents (3). As a consequence, it might be expected that more children on dialysis will be carriers of FSGS in the coming years.
The causes of NS are unclear. The hypothesis of circulating factors involved in the genesis of abnormal glomerular permeability to plasma proteins has been accepted as a plausible theory since the 1970s. Originally proposed by Shalhoub in 1974 (4), these factors have been presumed to be linked to T-cell dysfunction. Meyrier (5) proposed that MCD represents a systemic abnormality in lymphocyte function. There is some evidence that a circulating permeability factor is present in patients with some forms of NS, mainly FSGS, which may render the glomerular basement membrane more permeable to plasma proteins (6-8). The observation that up to 35% of patients diagnosed with FSGS and receiving a renal graft develop recurrent NS soon after transplantation, and evidence that plasmapheresis helps to lower or even to stop proteinuria and a recurrence of NS, have been considered to be strong evidence supporting the systemic theory (9,10).
Sharma et al. (8) identified a focal segmental permeability protein factor associated with a low molecular weight fraction from plasma and showed that the protein’s in vitro activity correlates with severity of disease and risk of recurrence. McCarthy et al. recently proposed that cardiotrophin-like cytokine factor 1 (CLCF1) could be an active factor in FSGS. Soluble urokinase receptor has been also identified in the plasma of patients with recurrent FSGS (11).
Steroid-resistant patients with NS show poor prognosis; most progress to end-stage renal failure (ESRF) and dialysis over time. If steroid-resistant NS were to be associated with the systemic permeability factor demonstrated by Sharma et al., then peritoneal protein losses should be greater in NS patients on peritoneal dialysis (PD) than in a non-NS dialyzed population. The implications of increased peritoneal protein losses include further exacerbation of malnutrition, increased risk of infection, growth retardation, developmental delay, and increased mortality in pediatric PD patients with NS (12). To date, only one study has showed that peritoneal protein losses are higher in children with NS on PD, and clinical observations suggest that these patients need careful nutrition and dialytic supervision (13). The objective of the present study was to evaluate peritoneal protein losses in Hispanic children with steroid-resistant NS on PD therapy, comparing them with dialyzed patients not having NS.
METHODS
Our retrospective 4-year analysis (2003 - 2007) evaluated chronic PD patients with ESRF secondary to NS. All patients were attending the Luis Calvo Mackenna Children’s Hospital, University of Chile, Santiago, Chile.
Data from the 2nd and 6th month on PD were analyzed. Age, sex, weight, height, and time on PD were recorded. Biochemical data included serum creatinine (Jaffe reaction), electrolytes, blood urea nitrogen (enzyme assay), calcium, phosphate, parathyroid hormone [PTH (Advantage Nichols Intact PTH Assay: Quest Diagnostics Nichols Institute, San Juan Capistrano, CA, USA)], serum albumin, and protein (turbidimetric assay). At the beginning and end of follow-up, 24-hour protein losses and nitrogen were measured in dialysate and urine. Thimerosal was added to the urine and dialysate samples to avoid urea generation secondary to bacterial activity. All samples obtained under conditions of noncompliance were discarded.
Dialysate volume, dialysate dextrose concentration, and daily urine output were recorded. All serum, urine, and dialysate samples were processed on Hitachi 917 and 717 analyzers (Boehringer Mannheim, Mannheim, Germany) in our laboratory. Patients with fever, infection, gastrointestinal absorption disturbances, active steroid treatment, endocrine diseases, and compliance or behavioral disturbances were excluded.
Daily protein intake, residual and peritoneal dialysis dose (Kt/V), and normalized protein equivalent of nitrogen appearance [nPNA, Borah equation (14)] were measured every 3 months. A peritoneal equilibration test (PET) was performed every 6 months, as usual. If a peritonitis episode occurred, peritoneal protein loss, Kt/V, and PET evaluations took place at least 4 weeks after antibiotic therapy was completed. The peritonitis rate was recorded.
Patients on PD for ESRF secondary to non-NS causes were matched by age and sex to constitute a control group (Table 1). All patients received erythropoietin alfa and calcitriol as recommended in the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines (15,16). No patient was on growth hormone therapy.
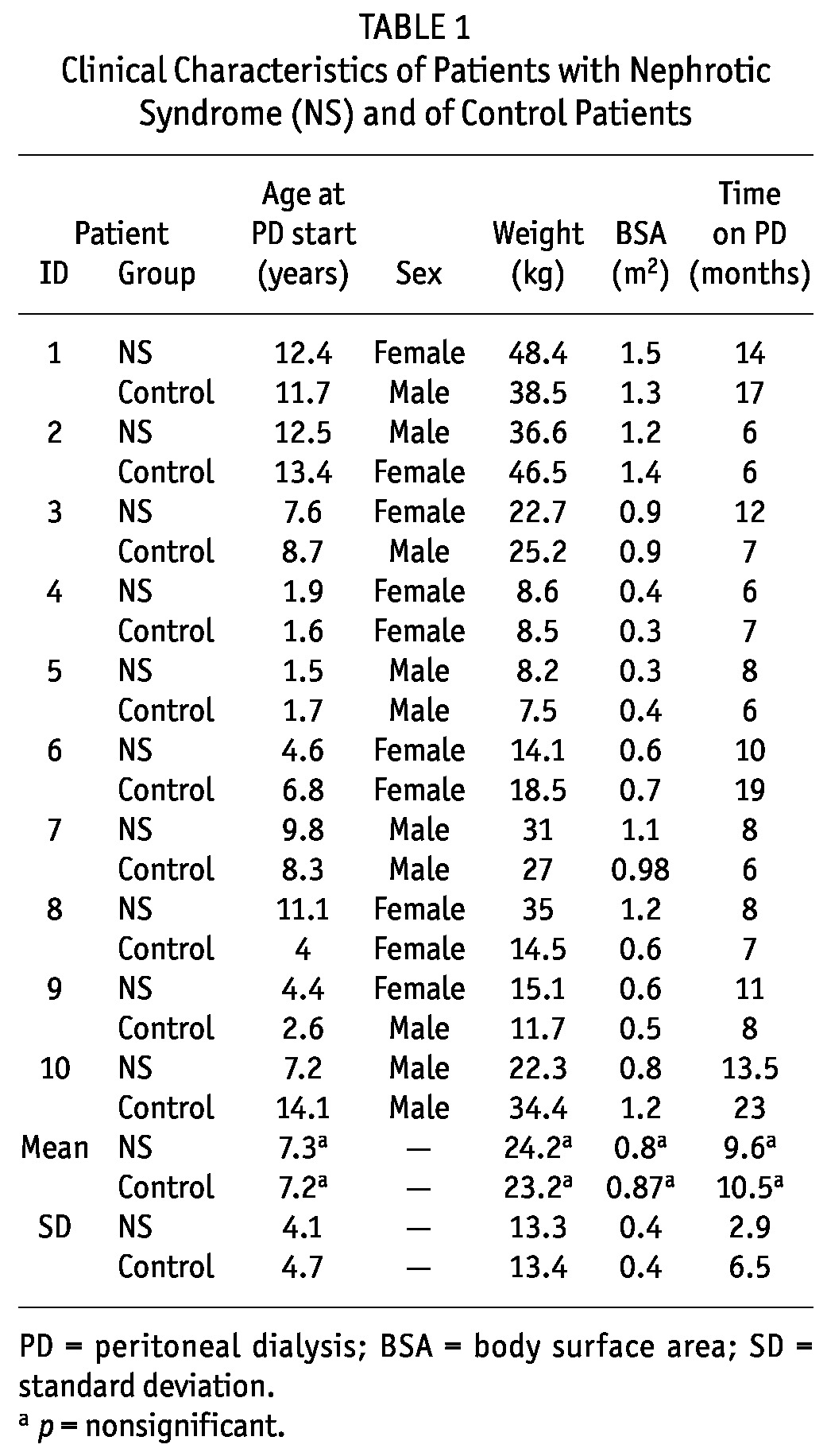
TABLE 1.
Clinical Characteristics of Patients with Nephrotic Syndrome (NS) and of Control Patients
Nutritional support was given in all patients to reach 100% of the recommended dietary allowance for energy and the K/DOQI recommendations for protein (17,18). During follow-up, monthly monitoring of nutrition was performed by a renal dietitian to assure adequate protein and energy intake. All patients underwent automated PD using a HomeChoice PD System cycler (Baxter Healthcare Corporation, Deerfield, IL, USA), with an exchange volume of 1100 mL/m2 and a dextrose concentration of 1.5% - 2.5% to reach a minimum Kt/V of 2.1 (19).
The study was approved by the Clinical Ethical Committee of the Luis Calvo Mackenna Children’s Hospital. Data are reported as mean ± standard deviation. Differences between the groups were determined using the Mann-Whitney U-test on SOCR (the Statistics Online Computational Resource, University of California, Los Angeles, CA, USA), and p < 0.05 was considered significant.
RESULTS
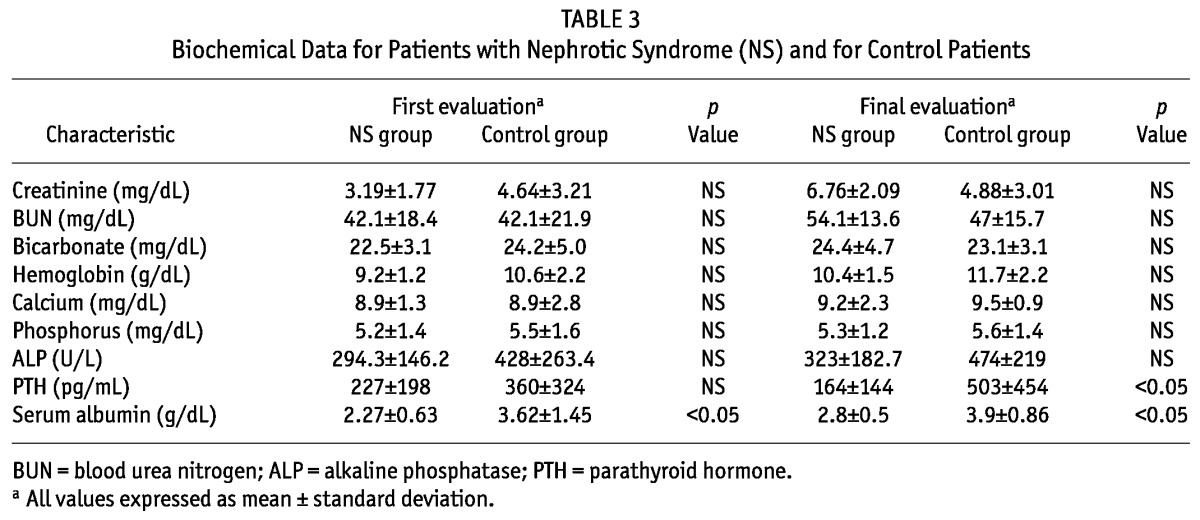
Each study group consisted of 10 patients [NS patients: 4 boys, mean age of 7.3 ± 4.1 years; control patients: 6 boys, mean age of 7.2 ± 4.7 years (p = nonsignificant)]. The causes of NS were diagnosed by renal biopsy. In the NS group, 8 patients had FSGS, and 2, MCD. In the control group, 6 patients had renal dysplasia, 2 had obstructive uropathy, and 2 had renal disease of unknown cause. The groups did not differ in weight, body surface area, months on PD at the time of data extraction, or dialysis volume prescription (Tables 1 and 2). Height-to-age standard deviation score was -1.5 ± 0.6 in the study group and -1.9 ± 0.8 in the control group. Daily protein intake (grams per kilogram) was similar in both groups at the beginning and the end of the study. Mean nPNA was 1.502 for the NS group and 0.902 for the control group (p < 0.05, Table 2). Tables 2 and 3 detail dialytic and biochemical data for the groups, showing values for creatinine, blood urea nitrogen, bicarbonate, hemoglobin, calcium, phosphorus, alkaline phosphatase, serum protein, albumin, and PTH for both groups. Only PTH and serum albumin showed a significant difference between groups. At the end of the protocol, PTH was 164 ± 144 pg/mL in the NS group and 503 ± 454 pg/mL in the control group (p < 0.05).
TABLE 2.
Dialysis Characteristics of Patients with Nephrotic Syndrome (NS) and of Control Patients
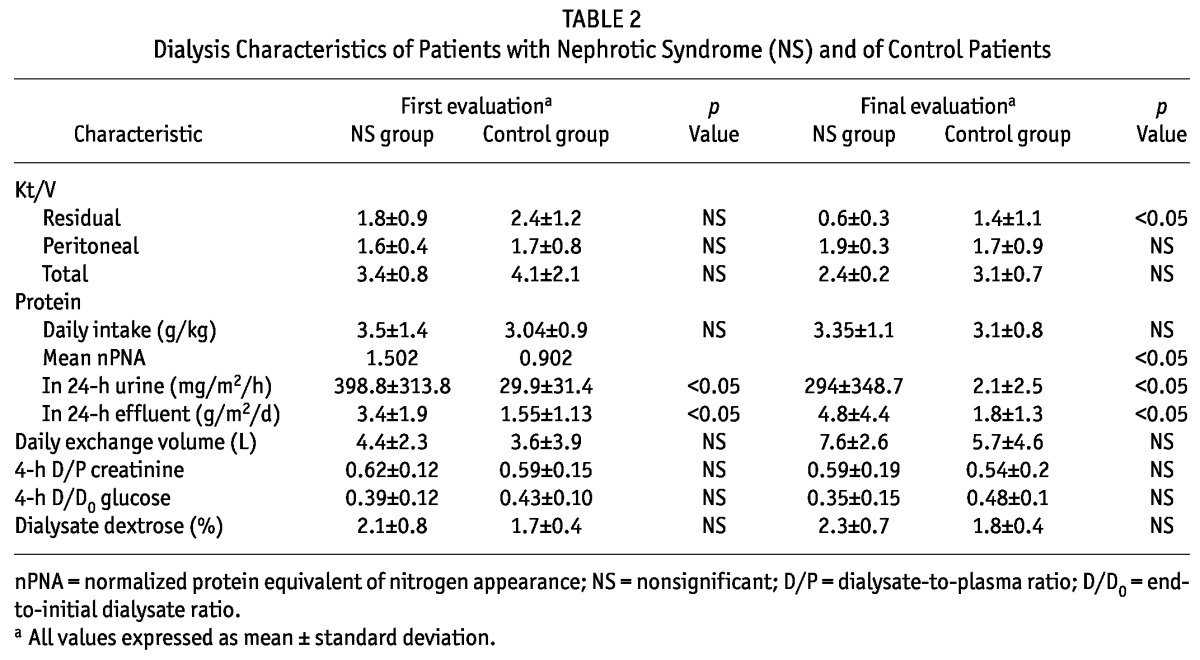
TABLE 3.
Biochemical Data for Patients with Nephrotic Syndrome (NS) and for Control Patients
With regard to protein losses, patients with NS showed an hourly urinary loss of 398.8 ± 313.8 mg/m2 and a daily dialysate loss of 3.4 ± 1.9 g/m2 at the start of the study; in the control group, the parallel values were 29.9 ± 31.4 mg/m2 and 1.55 ± 1.13 g/m2 (p < 0.05). Both groups showed the same statistical differences 6 months later (Table 2, Figures 1 and 2).
Figure 1.
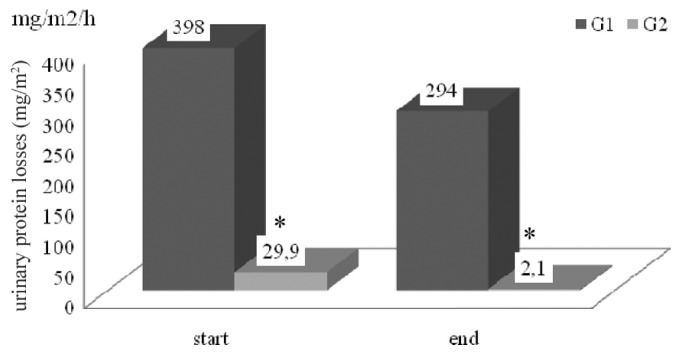
— Hourly urinary protein losses (milligrams per square meter). G1 = children with nephrotic syndrome; G2 = children with renal disease from other causes. * p < 0.05.
Figure 2.
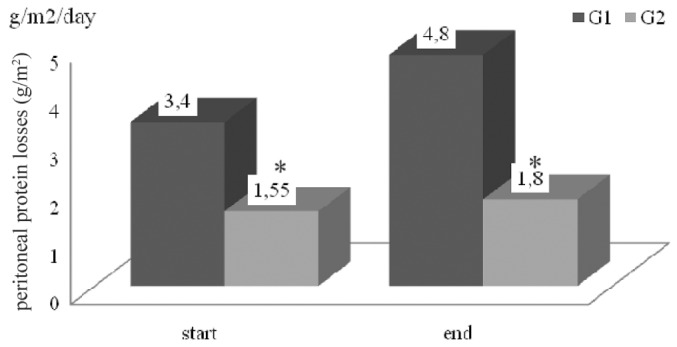
— Daily peritoneal protein losses (grams per square meter). G1 = children with nephrotic syndrome; G2 = children with renal disease from other causes. * p < 0.05.
At the start of the protocol, total and residual Kt/V were 3.4 ± 0.8 and 1.8 ± 0.9 in the NS group, and 4.1 ± 2.1 and 2.4 ± 1.2 in the control group. Values at the end of the study were 2.4 ± 0.2 and 0.6 ± 0.3 in the NS group, and 3.1 ± 0.7 and 1.4 ± 1.1 in the control group (p = nonsignificant).
With the aim of examining the effects of peritoneal permeability and dialysate dextrose concentration on peritoneal protein losses, PET and mean dextrose concentration were compared. At dialysis start and at 6 months, the 4-hour dialysate-to-plasma (D/P) creatinine was, respectively, 0.62 and 0.59 (low average) for the NS group and 0.59 and 0.54 (low average) for the control group; the end-to-initial dialysate (D/D0) glucose was, respectively, 0.39 and 0.35 (low average) for the NS group, and 0.43 and 0.48 (low average/low) for the control group (p = nonsignificant, Table 2). Nonsignificant differences in the mean dialysate dextrose concentration and in the daily exchange volumes were found between the groups (Table 2). The number of peritonitis episodes during the observation period, divided by the total months, then divided by 12, showed a peritonitis index of 1.12 ± 0.34 in the NS group compared with 1.34 ± 0.46 in the control group (p = nonsignificant).
DISCUSSION
In children, NS is the most common chronic glomerular disease, being the primary cause in 95% of patients (20). The pathogenesis is unclear, but there is evidence of a dysregulation involving cell-mediated immunity. Some authors have proposed that a systemic circulating factor could be involved in the pathogenesis of NS, resulting in increased glomerular permeability. A favorable response to immunosuppressive medications and a lack of inflammatory changes in the renal parenchyma suggest an extrarenal factor as the causative agent for proteinuria (21). Focal segmental glomerulosclerosis could be considered a typical disorder in which the circulating permeability factor ought to be operative. After kidney transplantation, as many as 40% of patients with FSGS develop recurrence of their original disease (22). Some authors have shown that pre-transplantation levels of the permeability factor are predictive of FSGS recurrence in the graft, although this finding has not been consistent (9). Several vascular permeability factors have been implicated in nephrotic proteinuria, including vascular growth factors, heparanase, and hemopexin. Vascular endothelial growth factor (VEGF) is a potent permeability factor produced in vivo by normal glomerular podocytes, and receptors for VEGF are located on glomerular endothelial and mesangial cells. However, serum and urinary levels of VEGF in nephrotic patients are not different in remission or relapse, and VEGF does not increase glomerular permeability during in vivo experiments (22). In nephrotic patients with MCD, lymphocyte-derived vascular permeability factors have also been identified. The production of those factors is apparently stimulated by the cytokines interleukin 2, 12, and 15, and inhibited by cyclosporine and steroids (23). However, the specificity of these factors is poor, and their ability to reproduce increased glomerular permeability in vivo is not convincing. Heparanase is postulated to increase the permeability of glomerular capillary wall by degrading heparan sulphate glycosaminoglycans. The degradation of these anionic glycans has long been hypothesized to be a cause of increased glomerular permeability to proteins (21), but the exact role of heparanase as a permeability factor is still unclear, and no conclusions can be drawn at present. Currently, the best-characterized permeability factor in steroid-sensitive NS patients is hemopexin, a 100-kD heme-binding acute-phase reactant. Hemopexin has been shown to reduce the density of anionic sites in the lamina rara interna of glomeruli, and serum levels of hemopexin increase during relapse in steroid-sensitive NS (23).
Sharma, working with various colleagues (7-10), identified a 30-kD to 50-kD glycoprotein permeability factor in FSGS nephrotic patients. This permeability factor is weakly anionic, heat-labile, and sensitive to protease (8). In rats, it is capable of inducing proteinuria, which can, in vitro, be inhibited by cyclosporine and cyclooxygenase inhibitors. Proteinuria recurs after initial renal transplantation in approximately 30% of patients whose underlying diagnosis is FSGS. It has been shown that the pre-transplant activity levels of this permeability factor could be predictive of recurrence of FSGS in the renal allograft. In a recent study, McCarthy et al. proposed that CLCF1 could be an active factor in the recurrence of FSGS (11,24). It has been observed that this cytokine mimics the effect of FSGS plasma on glomerular albumin permeability and that it lowers nephrin expression in the glomeruli and cultured podocytes. Authors have also reported that a monoclonal antibody to CLCF1 blocks the glomerular albumin-loss effect of active FSGS sera, and it has been reported that CLCF1 levels in the circulation of patients with recurrent FSGS may be up to 100 times that seen in normal subjects (24).
Based on the hypothesized systemic effect of a circulating factor affecting protein permeability in non-glomerular membranes, Kopanati et al. performed a retrospective study to evaluate the protein permeability of the peritoneal membrane in patients with NS on PD. They found that peritoneal protein losses in steroid-resistant NS were more than twice those in patients without NS. The permeability ratio of the peritoneal membrane to macromolecules was higher in NS than in non-NS patients, a finding consistent with the systemic effect of a “circulating factor” (13).
In our study, NS and non-NS PD patients showed some significant differences. So as to avoid the impact of time on the peritoneal membrane, data were obtained from the first 6 months on dialysis, regardless of how long patients or controls had been on PD treatment. The main differences between the children observed by Kopanati and colleagues and those observed by our group were age (14.7 ± 1 years vs 7.3 - 4.1 years respectively) and ethnicity; however, the results were similar in both studies. As expected, plasma albumin was lower and cholesterol levels were higher (data not shown) in patients with NS, and urinary and peritoneal protein losses were higher in NS patients than in control patients, with the difference between the groups being significant. Peritoneal protein losses were standardized as 24-hour losses per square meter of body surface area to adjust for the different ages, heights, and weights of the patients. A daily mean final value of 4.8 ± 4.4 g/m2 in NS patients compared with 1.8 ± 1.3 g/m2 in non-NS patients was found, which is similar to the results reported by Kopanati et al., who found that daily peritoneal protein losses in steroid-resistant NS patients were more than twice those in non-NS patients: 9.6 ± 3.25 g/m2 and 4.1 ± 0.367 g/m2 respectively.
Some variables that could potentially affect peritoneal protein losses in PD patients were also evaluated: daily exchange volume, peritoneal and residual Kt/V, glucose concentration in dialysate, and PET parameters. The peritoneal Kt/V was not significantly different between the groups (Table 2). The residual Kt/V at protocol start was also similar in both groups; however, at the end of the study, it was lower in patients with NS, possibly as a consequence of the higher ultrafiltration requirement in that group. Residual Kt/V decreased 60% in NS and 40% in non-NS children during follow-up (p = nonsignificant). Loss of residual renal function before study entrance could not be observed, because patient data were recorded from the 2nd month after PD start. Another plausible explanation could be a more rapid decline in residual renal function in children with glomerulopathies (the NS group) than in those with congenital renal hypoplasia and dysplasia (the non-NS group), in which residual renal function decreases more slowly.
As shown in Table 2, PD parameters did not differ between the groups with respect to the number of cycles or hours, or the type of dialysis. Small differences in the dialysis prescription were noted between the groups in terms of dialysate exchange volume and dialysate dextrose concentration because, to minimize edema, patients with NS often have higher ultrafiltration requirements; however, neither difference reached statistical significance. The exchange volumes were 990 ± 440 mL for patients with NS and 880 ± 440 mL for patients without NS, values calculated using a dialysis dose of 1100 mL/m2 as recommended. The dextrose concentration was also similar in both groups. Kopanati et al. founded higher exchange volumes in their patients, 1231 ± 80 mL and 1168 ± 44 mL respectively for the NS and non-NS groups, which accords with the greater age and body surface area in their patients.
In the present study, the 4-hour D/P creatinine and D/D0 glucose were also similar for both groups. With the exception of the low D/D0 glucose category in the control group at the end of the study, all patients fell into the low-average category for D/P creatinine and D/D0 glucose during the protocol.
A very interesting finding was the significant difference in mean nPNA between the groups, with a significantly higher value being seen in children with NS. That difference may be a result of the difference in urinary and peritoneal protein losses, suggesting that nPNA may not accurately reflect the nutrition status of NS patients on PD.
CONCLUSIONS
Hispanic children with NS on automated PD had higher peritoneal protein losses than did their control counterparts. These peritoneal protein losses could be secondary to increased peritoneal permeability caused by a systemic permeability factor. More studies involving a greater number of patients are needed in the future to prove or disprove this hypothesis.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
Acknowledgments
The present study was supported by Fondecyt Grant 11090045.
REFERENCES
- 1. Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet 2003; 362:629–39 [DOI] [PubMed] [Google Scholar]
- 2. The primary nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int 1978; 13:159–65 [DOI] [PubMed] [Google Scholar]
- 3. Bonilla-Felix M, Parra C, Dajani T, Ferris M, Swinford RD, Portman RJ, et al. Changing patterns in the histology of idiopathic nephrotic syndrome in children. Kidney Int 1999; 55:1885–90 [DOI] [PubMed] [Google Scholar]
- 4. Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet 1974; 2:556–60 [DOI] [PubMed] [Google Scholar]
- 5. Meyrier A. Mechanisms of disease: focal segmental glomerulosclerosis. Nat Clin Pract Nephrol 2005; 1:44–54 [DOI] [PubMed] [Google Scholar]
- 6. Trachtman H, Greenbaum LA, McCarthy ET, Sharma M, Gauthier BG, Frank R, et al. Glomerular permeability activity: prevalence and prognostic value in pediatric patients with idiopathic nephrotic syndrome. Am J Kidney Dis 2004; 44:604–10 [PubMed] [Google Scholar]
- 7. Savin VJ, McCarthy ET, Sharma M. Permeability factors in focal segmental glomerulosclerosis. Semin Nephrol 2003; 23:147–60 [DOI] [PubMed] [Google Scholar]
- 8. Sharma M, Sharma R, McCarthy ET, Savin VJ. “The FSGS factor:” enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma. J Am Soc Nephrol 1999; 10:552–61 [DOI] [PubMed] [Google Scholar]
- 9. Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med 1996; 334:878–83 [DOI] [PubMed] [Google Scholar]
- 10. Artero ML, Sharma R, Savin VJ, Vincenti F. Plasmapheresis reduces proteinuria and serum capacity to injure glomeruli in patients with recurrent focal glomerulosclerosis. Am J Kidney Dis 1994; 23:574–81 [DOI] [PubMed] [Google Scholar]
- 11. McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2010; 5:2115–21 [DOI] [PubMed] [Google Scholar]
- 12. Schärer K, Essigmann HC, Schaefer F. Body growth of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 1999; 13:828–34 [DOI] [PubMed] [Google Scholar]
- 13. Kopanati S, Baum M, Quan A. Peritoneal protein losses in children with steroid-resistant nephrotic syndrome on continuous-cycler peritoneal dialysis. Pediatr Nephrol 2006; 21:1013–19 [DOI] [PubMed] [Google Scholar]
- 14. Cano F, Azocar M, Cavada G, Delucchi A, Marin V, Rodriguez E. Kt/V and nPNA in pediatric peritoneal dialysis: a clinical or a mathematical association? Pediatr Nephrol 2006; 21:114–18 [DOI] [PubMed] [Google Scholar]
- 15. National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 2006; 47(Suppl 3):S11–146 [Erratum in Am J Kidney Dis 2006; 48:518] [DOI] [PubMed] [Google Scholar]
- 16. Eknoyan G, Levin A, Levin NW. on behalf of the National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(Suppl 3):S1–201 [PubMed] [Google Scholar]
- 17. United States, The National Academies, Institute of Medicine, Food and Nutrition Board (FNB). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: FNB; 2005. [Available online at: http://books.nap.edu/openbook.php?record_id=10490&page=21; accessed 20 December 2011] [Google Scholar]
- 18. National Kidney Foundation. KDOQI clinical practice guidelines: nutrition in chronic renal failure. Am J Kidney Dis 2000; 35(Suppl 2):S1–140 [DOI] [PubMed] [Google Scholar]
- 19. NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis 1997; 30(Suppl 2):S69–133 [DOI] [PubMed] [Google Scholar]
- 20. Behrman RE, Kliegman RM, Jenson HB, eds. Nephrotic syndrome. In: Behrman RE, Kliegman RM, Jenson HB, eds. Nelson Textbook of Pediatrics. 17th ed. Philadelphia, PA: Saunders; 2004: 1753–8 [Google Scholar]
- 21. Bagga A, Mantan M. Nephrotic syndrome in children. Indian J Med Res 2005; 122:13–28 [PubMed] [Google Scholar]
- 22. Glassock RJ. Circulating permeability factors in the nephrotic syndrome: a fresh look at an old problem. J Am Soc Nephrol 2003; 14:541–3 [DOI] [PubMed] [Google Scholar]
- 23. Brenchley PE. Vascular permeability factors in steroid sensitive nephrotic syndrome and focal segmental glomerulosclerosis. Nephrol Dial Transplant 2003; 18(Suppl 6):vi21–5 [DOI] [PubMed] [Google Scholar]
- 24. Savin VJ, Sharma M, McCarthy ET, Sharma R, Reddy S, Dong JW, et al. Cardiotrophin-like cytokine-1: candidate for the focal glomerulosclerosis permeability factor (Abstract). J Am Soc Nephrol 2008; 19:59A 18045850 [Google Scholar]


