Abstract
♦ Purpose: Decline in physical function is commonly observed in patients with kidney failure on dialysis. Whether lung congestion, a predictable consequence of cardiomyopathy and fluid overload, may contribute to the low physical functioning of these patients has not been investigated.
♦ Methods: In 51 peritoneal dialysis (PD) patients, we investigated the cross-sectional association between the physical functioning scale of the Kidney Disease Quality of Life Short Form (KDQOL-SF: Rand Corporation, Santa Monica, CA, USA) and an ultrasonographic measure of lung water recently validated in dialysis patients. The relationship between physical functioning and lung water was also analyzed taking into account the severity of dyspnea measured using the New York Heart Association (NYHA) classification currently used to grade the severity of heart failure.
♦ Results: Evidence of moderate-to-severe lung congestion was evident in 20 patients, and this alteration was asymptomatic (that is, NHYHA class I) in 11 patients (55%). On univariate analysis, physical functioning was inversely associated with lung water (r = -0.48, p < 0.001), age (r = -0.44, p = 0.001), previous cardiovascular events (r = -0.46, p = 0.001), and fibrinogen (r = -0.34, p = 0.02). Physical functioning was directly associated with blood pressure, the strongest association being with diastolic blood pressure (r = 0.38, p = 0.006). The NYHA class correlated inversely with physical functioning (r = -0.51, p < 0.001). In multiple regression analysis, only lung water and fibrinogen remained independent correlates of physical functioning. The NYHA class failed to maintain its independent association.
♦ Conclusions: This cross-sectional study supports the hypothesis that symptomatic and asymptomatic lung congestion is a relevant factor in the poor physical functioning of patients on PD.
Keywords: KDQOL-SF, physical functioning, lung comets, NYHA class
Decline in physical function is commonly observed in patients with chronic kidney disease on dialysis (CKD-5D). Anemia, mineral and bone disorders, inflammation-muscular wasting complex, cardiomyopathy, neuropathy, and depression (conditions that all frequently occur in CKD-5D patients) may be involved in the pathogenesis of weakness and impaired physical health in this population (1-3).
Fluid overload is highly prevalent in CKD-5D (4-6), and pulmonary congestion is the most concerning consequence of volume excess (7). However, whether lung congestion may contribute to the low physical functioning of CKD-5D patients—an obvious possibility in a population with an exceedingly high cardiovascular burden—has not been studied.
With that background in mind, we investigated the relationship between pulmonary congestion and physical functioning in peritoneal dialysis (PD) patients, a category of patients at risk for volume excess when diuresis is minimal or exhausted (6). Because cardiomyopathy per se may lead to fluid accumulation in the lung, the relationship between physical functioning and lung water was also analyzed, taking into account the severity of dyspnea measured using the New York Heart Association (NYHA) classification currently used to grade the severity of heart failure.
METHODS
The study protocol conformed to the Declaration of Helsinki and was approved by the local ethics committee. All patients provided informed consent.
STUDY POPULATION
Patients treated in two renal units (Reggio Calabria and Acireale, Catania, Italy) were enrolled if they had been on PD for more than 6 months. Other criteria for enrolment included freedom from peritonitis for at least 3 months, no intercurrent acute illness, and no neurologic or orthopedic disease potentially impairing physical activity.
The 51 patients who met the criteria represented about 90% of the entire PD population from the two units. Of those 51 patients, 40 were on continuous ambulatory PD, and 11 were on automated PD. Dialysis prescriptions aimed at obtaining a weekly total Kt/V of at least 1.8. In 23 patients, 1 daily bag contained an icodextrin solution (7.5 g/dL). The median duration of PD treatment in the group was 27 months (interquartile range: 9 - 47 months). The median residual diuresis by 24-hour urine collection was 400 mL (interquartile range: 0 - 925 mL). The cause of chronic renal disease was glomerulonephritis in 13 patients, nephroangiosclerosis in 11, unknown in 10, tubulointerstitial nephritis in 8, and polycystic kidney in 3. Six patients had diabetic nephropathy, but diabetes as a comorbidity was present in 8 additional patients. Two patients had mild chronic obstructive pulmonary disease.
MEASUREMENTS OF PHYSICAL FUNCTION
We used the physical functioning scale of the Kidney Disease Quality of Life Short Form (KDQOL-SF: Rand Corporation, Santa Monica, CA, USA), which has specifically been validated on CKD patients in its Italian translation (8). This 10-question scale captures abilities to deal with the physical requirements of life, such as attending to personal needs and walking. For the analysis, we used the raw scores, which can range from 30 (no limitation at all in the activities) to 10 (greatly limited in the activities). The questionnaire was self administered by most patients. In a few cases, patients were helped by nurses.
In a group of 19 patients, physical activity was also evaluated using a pedometer. Patients were asked to wear the Omron Walking Style II step counter (Omron Healthcare Europe, Hoofddorp, Netherlands) for 3 days. The mean daily value of the reading across the 3 days was used in the analysis.
LUNG WATER EVALUATION
Lung water was estimated by ultrasonography of the chest (9). We described the technique in detail and tested its validity in hemodialysis patients (7). The rational of the method is that, in the presence of extravascular lung water, the ultrasound beam finds subpleural interlobular septa thickened by edema. The reflection of the beam generates comet-tail reverberation artifacts, called “B lines” or “ultrasound lung comets.” The sum of lung comets observed in well-identified chest areas produces a score reflecting the extent of lung water accumulation (0 being no detectable lung comets).
A standard 3.0-MHz echocardiography probe was used. As previously described (7), ultrasonography of the anterior and lateral chest was performed on the right and left hemithorax from the second to the fourth (and, on the right side, to the fifth) intercostal space, and from the parasternal to the axillary line. One of three severity classes of lung congestion was assigned as described elsewhere (7): Patients with a lung comets score of 15 - 30 were considered to have moderate lung congestion, and those with a score exceeding 30 were considered to have severe congestion.
One operator was responsible for lung comets determination at each center. In a previous paper, the interoperator reproducibility of a lung comets determination was found to be excellent after formal training (7). The sonographer at the second nephrology unit participating in the present study was originally trained at CNR-IBIM, and his performance was scored as good by the trainer.
Lung water and physical functioning were assessed on the same day.
NYHA SCORE
Patients were allocated to one of four dyspnea classes according to their NYHA classification. In CKD-5D patients, NYHA class is a strong predictor of mortality, with reasonable agreement between assessors (10).
LABORATORY METHODS
Plasma fibrinogen, serum albumin, and C-reactive protein were determined by routine autoanalyzer methods.
STATISTICAL ANALYSIS
Data are presented as mean ± standard deviation, median and interquartile range, or frequencies. Comparisons between groups were made using t-test, Mann-Whitney test, or chi-square test, as appropriate. Non-normally distributed variables were log-transformed before being compared.
The association between lung comets and physical functioning assessed using the KDQOL-SF was analyzed using single and multiple ordinal regression analyses, adjusting for factors associated (p ≤ 0.05) with physical activity and lung comets on univariate analysis.
All calculations were performed using a standard statistical package (SPSS for Windows: SPSS, Chicago, IL, USA).
RESULTS
The median physical functioning score on the KDQOL-SF was 20 (interquartile range: 15 - 25), and most of the patients (n = 41, 80%) scored lower than a normal Italian population of similar age. Table 1 shows the main baseline characteristics of the patients grouped by physical functioning score (≤20 vs >20). The table also lists the correlation coefficients of the relations between the variables.
TABLE 1.
Main Demographic, Clinical, and Biochemical Dataa for the Study Patients
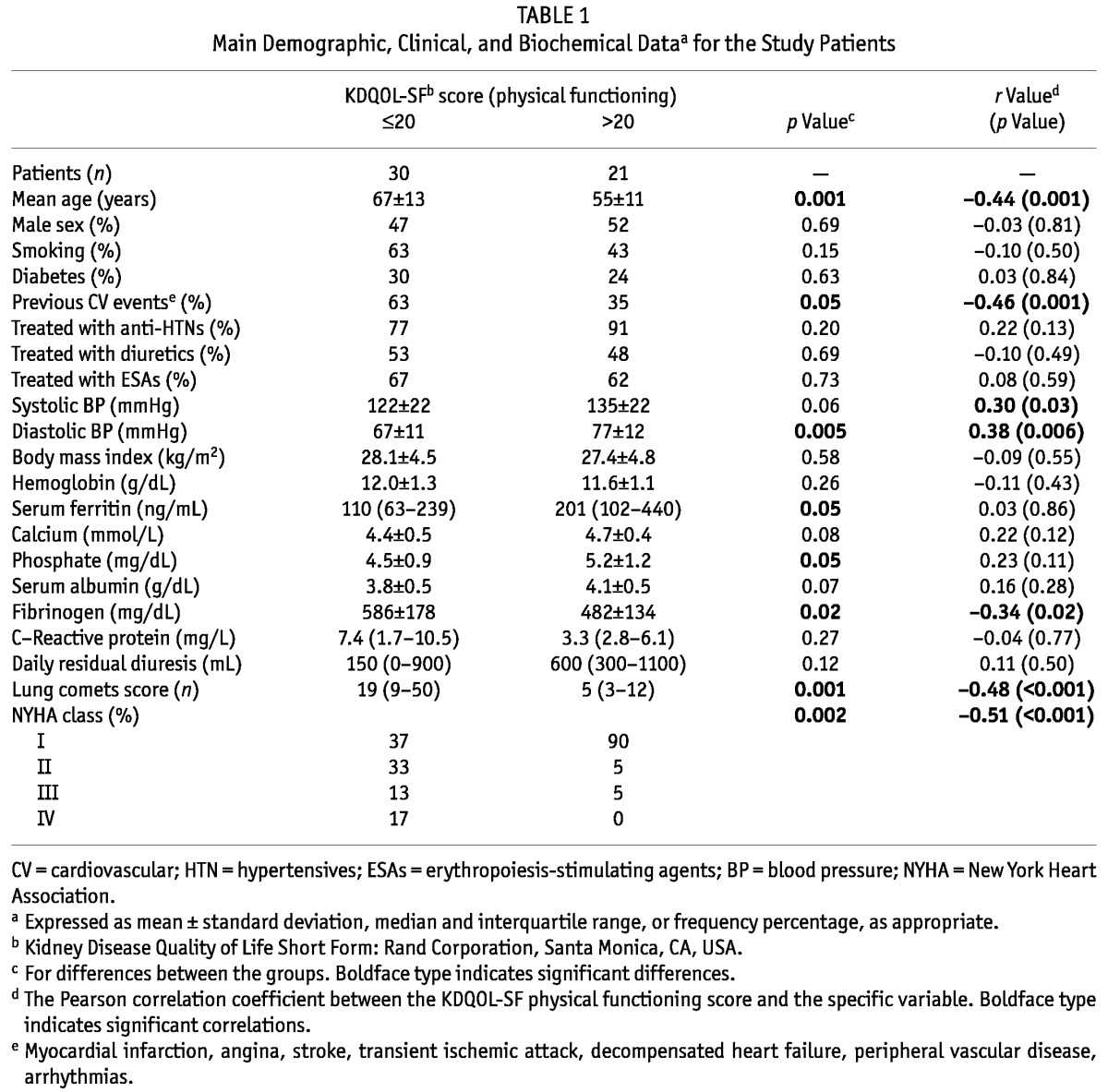
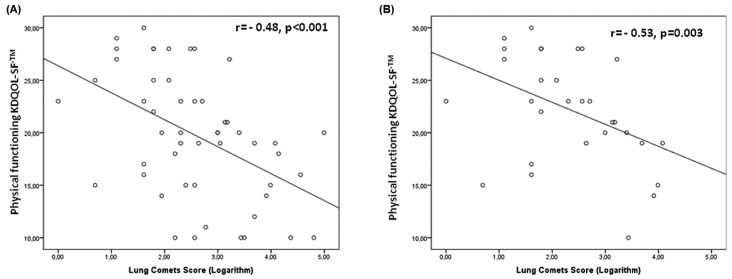
Patients in the lower-score category were older and more frequently had a history of cardiovascular events, lower blood pressure (BP), higher serum fibrinogen, and lower serum ferritin and phosphate. The lung comets score was markedly higher in patients with lower physical activity (Table 1), and lung comets were strongly and inversely (r = -0.48, p < 0.001) related with physical functioning [Figure 1(A)]. Physical functioning was also inversely associated with age (r = -0.44, p = 0.001) and fibrinogen (r = -0.34, p = 0.02), and directly associated with BP (systolic BP: r = 0.30, p = 0.03; diastolic BP: r = 0.38, p = 0.006). Notably, poor physical functioning accompanied background cardiovascular events (r = -0.46, p = 0.001).
Figure 1.
— Relationship of lung comets score with physical functioning in (A) all patients, and (B) patients scored as New York Heart Association class I.
The NYHA classification was class I in 30 patients (58.8%); class II in 11 (21.6%); and classes III and IV in 5 (9.8%). The NYHA class was strongly and inversely associated with physical functioning (r = -0.51, p < 0.001, Table 1).
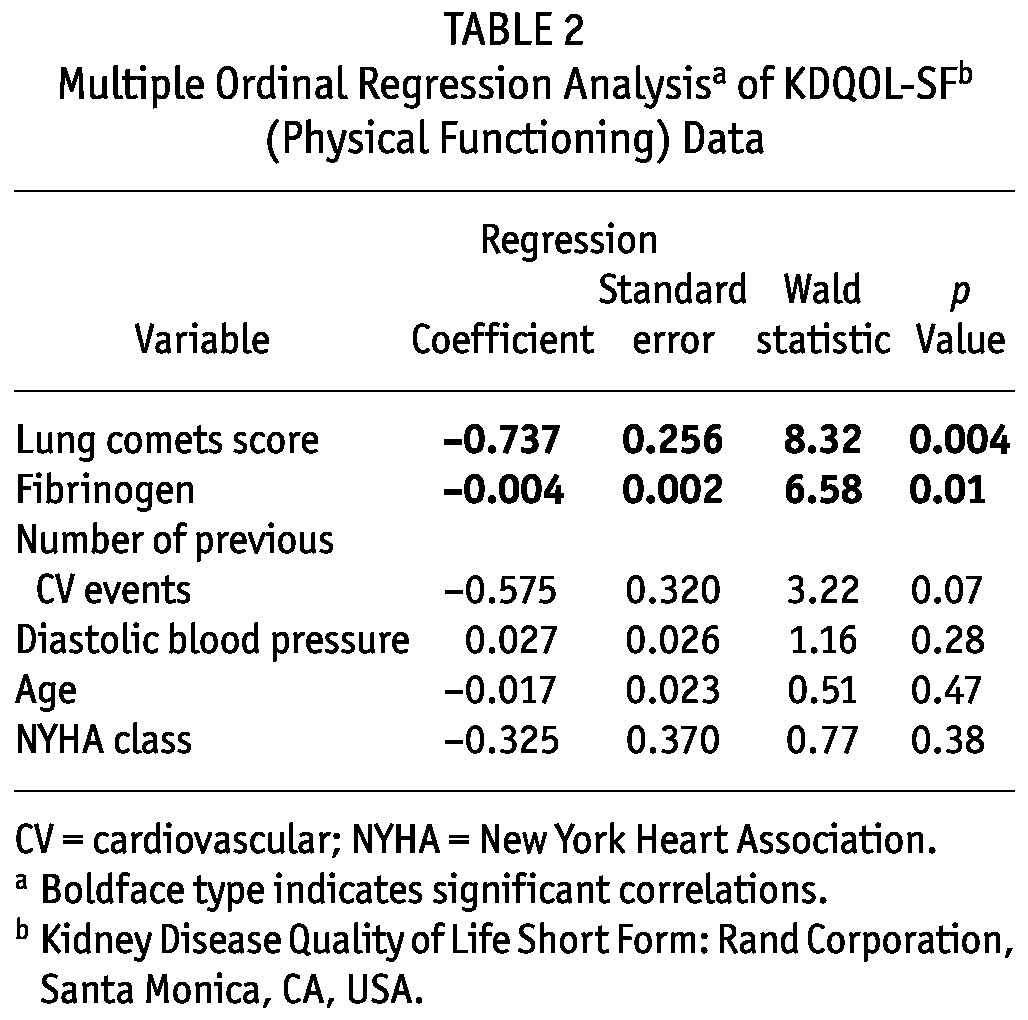
In the multiple regression model, only lung comets and fibrinogen remained as independent correlates of physical functioning (Table 2); NYHA class was no longer a significant correlate in that model (Table 2). In patients without dyspnea (n = 30, NYHA class I), moderate-to-severe lung congestion was observed in 11 (37%), and lung comets score was strongly associated with physical functioning [r = -0.53, p = 0.003, Figure 1(B)].
TABLE 2.
Multiple Ordinal Regression Analysisa of KDQOL-SFb (Physical Functioning) Data
In the 19 patients whose actual physical activity was determined by a count of daily steps, the correlation between lung comets and this objective measurement of physical functioning was strong and inverse (r = -0.60, p = 0.007, Figure 2). The agreement between physical activity by KDQOL-SF and by step counter was excellent, as shown by regression analysis (r = 0.61, p = 0.006, Figure 3).
Figure 2.
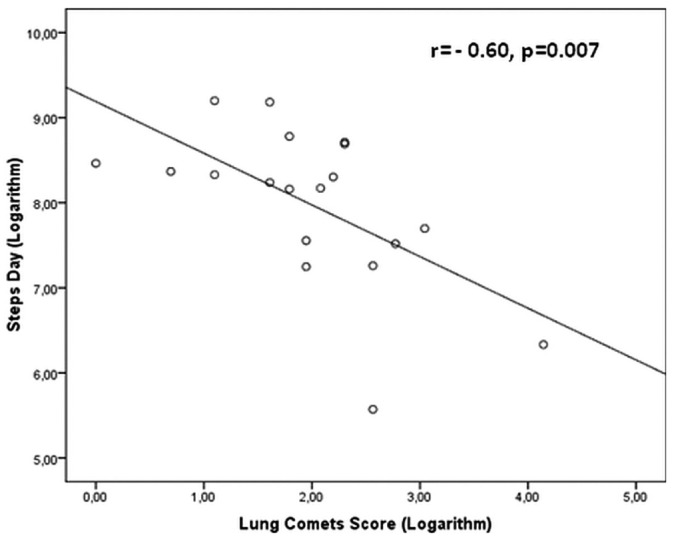
— Relationship between lung comets score and daily walking.
Figure 3.
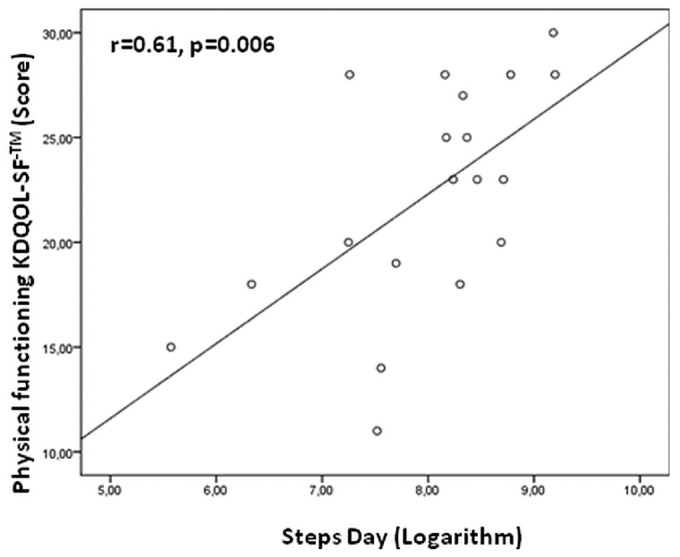
— Relationship between Kidney Disease Quality of Life Short Form [physical functioning scale (KDQOL-SF: Rand Corporation, Santa Monica, CA, USA)] and daily walking.
DISCUSSION
Our observations support the hypothesis that lung congestion is a relevant factor in the poor physical functioning of CKD-5D patients. The strong association between lung water and physical functioning was observed in both symptomatic and asymptomatic (that is, NHYA class I) patients, the latter representing the greater proportion (59%) of the population studied.
In our survey, physical functioning was estimated by the well-validated KDQOL-SF physical functioning scale (8), and lung water was evaluated by ultrasonography (applied for the first time in a PD population). Chest ultrasonography has previously been applied in patients with congestive heart failure and allows for the direct quantification of water accumulating in the lung interstitium (9). The technique was recently validated by our group in hemodialysis patients (7).
The main finding of our study is the strong link between physical functioning and pulmonary congestion. Remarkably, almost one quarter (23%) of the variance in physical functioning was explained by the simultaneous variability in lung water, a relationship which, on multivariate analysis, proved to be independent of dyspnea (as assessed by NYHA class), anemia, and other factors commonly related to poor physical functioning in patients with kidney failure. That observation generates the hypothesis that interventions aimed at reducing asymptomatic lung congestion may translate into improved physical functioning in PD patients. The issue is of relevance because, in line with previous studies (11), we found that physical activity was impaired in about 80% of patients compared with a normal population of similar age. As expected, other factors were associated with physical functioning. For instance, decline in physical functioning is a feature of normal aging. The process is accentuated by comorbidities that often accompany aging, and as expected, such an association was confirmed in our PD cohort.
In the present study low blood pressure was one of the strongest correlates of reduced physical functioning, a phenomenon likely reflecting impaired myocardial performance and pump failure. On the other hand, physical functioning assessed by the KDQOL-SF was independent of hemoglobin, a parameter considered a fundamental determinant of physical health. We believe that, in the context of the present survey, the lack of an association between hemoglobin and physical functioning reflects the fact that anemia was adequately treated in most patients (in 84%, hemoglobin was ≥10.5 g/dL).
As to markers of protein-energy wasting and inflammation, we found no association for KDQOL-SF score with albumin and C-reactive protein, but we did observe a strong and independent association of physical functioning with serum fibrinogen, an established biomarker of inflammation and high risk for death in patients with end-stage renal disease, including those on continuous ambulatory PD (12-14). Although this association cannot be interpreted as causal, it is intriguing, in that it accords with the notion that fibrinogen synthesis is also increased in response to volume expansion in dialysis patients (14-16).
Our study has limitations. First, the sample size was relatively small. Furthermore, the cross-sectional design prevents the possibility of making a causal interpretation of the findings. The analysis was based on a single measurement and does not account for prescription changes. Another limitation is the use of a self-reported scale of physical activity. However, the perception by patients of their physical functioning is one of the most important aspects of quality of life, and in a subset of patients, we found that physical functioning measured using the KDQOL-SF showed excellent agreement with actual physical activity measured using a step counter. Finally, another aspect that might limit the applicability of our data to the greater PD population is the method used for the lung water estimate. It is well known that echocardiographic techniques are mainly qualitative and depend on the sonographer’s skills. However, the technique of lung ultrasonography has previously been studied in patients with heart disease and found to require a short learning curve and to be reliable even in the hands of beginners using a low-technology apparatus (17). Moreover, we previously validated chest ultrasonography in CKD patients on hemodialysis, showing that, in that population also, inter-operator reproducibility is excellent after a short training period (7).
Reduced physical functioning in patients with kidney failure is often considered an inexorable consequence of uremia, and efforts toward improving this alteration (which has a high impact on quality of life) are often laxly pursued by renal physicians. Hidden pulmonary congestion secondary to volume overload or left ventricular disorders (or both) is a potential cause of reduced physical functioning and a modifiable risk factor in this population. The lack of reliable noninvasive measures of lung water is probably an important factor explaining clinical inertia in tackling pulmonary congestion in dialysis patients. Our observations may provide a solid basis for furthering research aimed at targeting asymptomatic lung congestion in PD patients.
CONCLUSIONS
Our study supports the hypothesis that lung congestion is a major factor implicated in the poor physical functioning of patients with end-stage renal disease on PD even before the appearance of clinically detectable dyspnea. Because lung congestion is a modifiable factor, intervention studies are warranted to definitively test this hypothesis and to lay appropriate groundwork before chest ultrasonography can be recommended as a tool for routine assessment of PD patients.
DISCLOSURES
The authors have no financial conflicts of interest to disclose.
Acknowledgments
The authors are grateful to Diego Delfino, who was responsible for the clinical management of PD patients, together with Vincenzo Panuccio and Giuseppe Enia; to Giuseppina Ventura, Domenica Pustorino, and Antonella Biondo, nurses in our PD program; to Maurizio Postorino, manager of the research database; and to Maria Concetta Postorino who helped with data extraction.
REFERENCES
- 1. Feroze U, Noori N, Kovesdy CP, Molnar MZ, Martin DJ, Reina-Patton A, et al. Quality-of-life and mortality in hemodialysis patients: roles of race and nutritional status. Clin J Am Soc Nephrol 2011; 6:1100–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anand S, Chertow GM, Johansen KL, Grimes B, Kurella Tamura M, et al. Association of self-reported physical activity with laboratory markers of nutrition and inflammation: the Comprehensive Dialysis Study. J Ren Nutr 2011; 21:429–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anand S, Kaysen GA, Chertow GM, Johansen KL, Grimes B, Dalrymple LS, et al. Vitamin D deficiency, self-reported physical activity and health-related quality of life: the Comprehensive Dialysis Study. Nephrol Dial Transplant 2011; 26:3683–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 2009; 119:671–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 2009; 24:1574–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Enia G, Mallamaci F, Benedetto FA, Panuccio V, Parlongo S, Cutrupi S, et al. Long-term CAPD patients are volume expanded and display more severe left ventricular hypertrophy than haemodialysis patients. Nephrol Dial Transplant 2001; 16:1459–64 [DOI] [PubMed] [Google Scholar]
- 7. Mallamaci F, Benedetto FA, Tripepi R, Rastelli S, Castellino P, Tripepi G, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC Cardiovasc Imaging 2010; 3:586–94 [DOI] [PubMed] [Google Scholar]
- 8. Klersy C, Callegari A, Giorgi I, Sepe V, Efficace E, Politi P. Italian translation, cultural adaptation and validation of KDQOL-SF, version 1.3, in patients with severe renal failure. J Nephrol 2007; 20:43–51 [PubMed] [Google Scholar]
- 9. Gargani L. Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound 2011; 9:6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Postorino M, Marino C, Tripepi G, Zoccali C. Prognostic value of the New York Heart Association classification in end-stage renal disease. Nephrol Dial Transplant 2007; 22:1377–82 [DOI] [PubMed] [Google Scholar]
- 11. Procaccini DA, Angelini P, Aucella F, Avanzi C, Brusasco S, Carta G, et al. Health-related quality of life in patients with chronic kidney disease [Italian]. G Ital Nefrol 2008; 25:694–701 [PubMed] [Google Scholar]
- 12. Zoccali C, Mallamaci F, Tripepi G, Cutrupi S, Parlongo S, Malatino LS, et al. Fibrinogen, mortality and incident cardiovascular complications in end-stage renal failure. J Intern Med 2003; 254:132–9 [DOI] [PubMed] [Google Scholar]
- 13. Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Cutrupi S, Parlongo S, et al. Fibrinogen, inflammation and concentric left ventricular hypertrophy in chronic renal failure. Eur J Clin Invest 2033; 33:561–6 [DOI] [PubMed] [Google Scholar]
- 14. Enia G, Panuccio V, Mallamaci F, Tripepi G, Mandalari A, Zoccali C. Fibrinogen and calcium × phosphate product are predictors of cardiovascular events in the CAPD population. J Nephrol 2005; 18:764–9 [PubMed] [Google Scholar]
- 15. Giordano M, De Feo P, Lucidi P, dePascale E, Giordano G, Infantone L, et al. Increased albumin and fibrinogen synthesis in hemodialysis patients with normal nutritional status. J Am Soc Nephrol 2001; 12:349–54 [DOI] [PubMed] [Google Scholar]
- 16. Goodship TH. Fibrinogen in hemodialysis: the worst of both worlds? Kidney Int 2003; 63:379–80 [DOI] [PubMed] [Google Scholar]
- 17. Bedetti G, Gargani L, Corbisiero A, Frassi F, Poggianti E, Mottola G. Evaluation of ultrasound lung comets by hand-held echocardiography. Cardiovasc Ultrasound 2006; 4:34 [DOI] [PMC free article] [PubMed] [Google Scholar]