Abstract
♦ Background: A multicenter, multi-country randomized controlled trial (the balANZ study) recently reported that peritonitis rates significantly improved with the use of neutral-pH peritoneal dialysis (PD) solutions low in glucose degradation products (“biocompatible”) compared with standard solutions. The present paper reports a secondary outcome analysis of the balANZ trial with respect to peritonitis microbiology, treatment, and outcomes.
♦ Methods: Adult incident PD patients with residual renal function were randomized to receive either biocompatible or conventional (control) PD solutions for 2 years.
♦ Results: The safety population analysis for peritonitis included 91 patients in each group. The unadjusted geometric mean peritonitis rates in those groups were 0.30 [95% confidence interval (CI): 0.22 to 0.41] episodes per patient-year for the biocompatible group and 0.49 (95% CI: 0.39 to 0.62) episodes per patient-year for the control group [incidence rate ratio (IRR): 0.61; 95% CI: 0.41 to 0.90; p = 0.01]. When specific causative organisms were examined, the rates of culture-negative, gram-positive, gram-negative, and polymicrobial peritonitis episodes were not significantly different between the biocompatible and control groups, although the biocompatible group did experience a significantly lower rate of non-pseudomonal gram-negative peritonitis (IRR: 0.41; 95% CI: 0.18 to 0.92; p = 0.03). Initial empiric antibiotic regimens were comparable between the groups. Biocompatible fluid use did not significantly reduce the risk of peritonitis-associated hospitalization (adjusted odds ratio: 0.80; 95% CI: 0.48 to 1.34), but did result in a shorter median duration of peritonitis-associated hospitalization (6 days vs 11 days, p = 0.05). Peritonitis severity was more likely to be rated as mild in the biocompatible group (37% vs 10%, p = 0.001). Overall peritonitis-associated technique failures and peritonitis-related deaths were comparable in the two groups.
♦ Conclusions: Biocompatible PD fluid use was associated with a broad reduction in gram-positive, gram-negative, and culture-negative peritonitis that reached statistical significance for non-pseudomonal gram-negative organisms. Peritonitis hospitalization duration was shorter, and peritonitis severity was more commonly rated as mild in patients receiving biocompatible PD fluids, although other peritonitis outcomes were comparable between the groups.
Keywords: Biocompatibility, glucose degradation products, peritonitis, outcomes, randomized controlled trial, technique survival, end-stage renal disease
Peritonitis is a serious complication of peritoneal dialysis (PD), resulting in considerable morbidity and mortality, depending on the underlying organism (1-10). Overall rates of peritonitis have declined since the early 1990s (11-13) because of advances in connectology and Staphylococcus decolonization protocols (12,14-16).
Recent evidence suggests that the type of dialysis fluid used for PD may also have an impact on peritonitis rates through local effects on the peritoneal membrane and host defenses against infection. Conventional PD fluids are considered “unphysiologic,” based on their acidic pH (5.0 - 5.8), high lactate concentration (30 - 40 mmol/L), high osmolality (320 - 520 mOsm/kg), high glucose concentration (31 - 236 mmol/L), and contamination by glucose degradation products (GDPs) generated during the heat sterilization process (17). Such solutions reduce the viability and growth of peritoneal mesothelial cells and fibroblasts in vitro, alter the turnover of structural collagen, and modify the homeostatic balance of cytokines and growth factors (18-20). The viability and function of peritoneal phagocytic cells such as leukocytes and macrophages are also impaired by standard PD fluids (17,18,21-23). Most of these adverse effects of dialysate on the peritoneal membrane appear to be accounted for by acidic pH and high GDP concentrations, because in vivo studies showed that they were largely abrogated by the use of neutral-buffered low-GDP (“biocompatible”) fluids (17,19,24-26).
The recently published balANZ randomized controlled trial (27) found that, in incident PD patients, clinically important and statistically significant reductions in peritonitis were associated with administration of a neutral-pH, lactate-buffered, low-GDP fluid (Balance: Fresenius Medical Care, Bad Homburg, Germany) compared with a conventional lactate-buffered PD solution (Stay·Safe: Fresenius Medical Care). Specifically, patients receiving biocompatible fluid had a lower probability of experiencing peritonitis (30% vs 50%, p = 0.006), a significantly longer time to a first peritonitis episode (p = 0.01), and a significantly lower overall peritonitis rate (0.30 episodes vs 0.49 episodes per patient-year, p = 0.01), which persisted after adjustment for age, sex, body mass index, diabetes, cardiovascular disease, baseline renal function, and peritoneal transport status.
To further evaluate the impact of biocompatible fluid on peritonitis microbiology and outcomes, the present study aimed to determine whether the beneficial effects on peritonitis of neutral-pH, low-GDP (“biocompatible”) PD fluid compared with conventional dialysate in the balANZ trial were specific for particular micro-organisms or led to improved peritonitis outcomes (peritonitis-associated hospitalization, peritonitis severity, peritonitis relapse, peritonitis-related technique failure, and peritonitis-related death).
METHODS
The study design and methodology have previously been described (28), as have the results of the main primary and secondary analyses specified in the statistical analysis plan (27). The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12606000044527), and the study protocol was approved by ethics committees at all participating centers. All patients provided written informed consent before trial participation.
Incident adult PD patients who at enrollment had both a measured residual glomerular filtration rate of 5 mL/min/1.73 m2 or more, and a measured urine volume of 400 mL/day or more, were included in the study. Pregnant or breastfeeding patients, patients expected to die within 12 months, patients participating in trials targeting residual renal function in PD, and patients with a significant cancer history in the preceding 5 years, with acute infection at enrollment, with contraindications to PD, with any physical or mental disorder that appreciably hampered study protocol compliance, or with a known or suspected allergy to the trial product or related products were excluded.
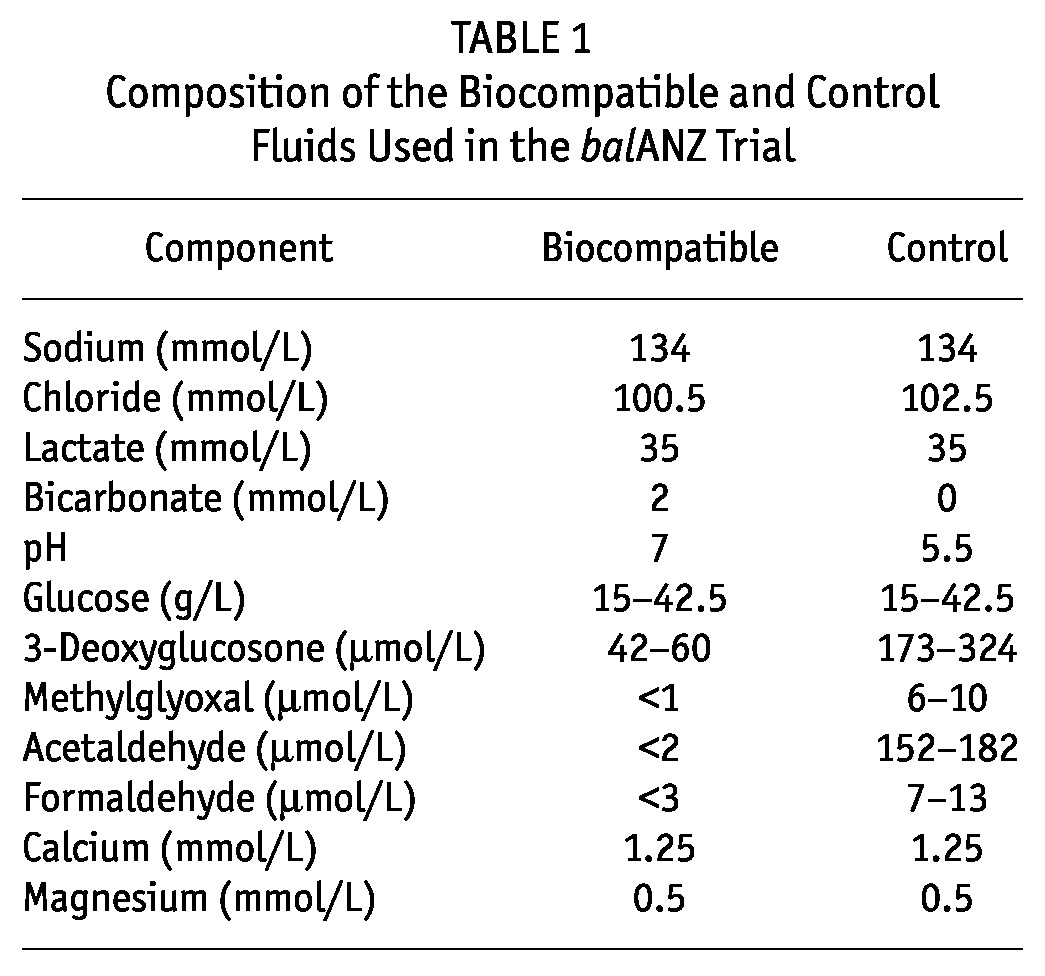
Using central computer-generated randomization over a Web-based link, participants were randomly allocated to receive either Balance (neutral-pH, lactate-buffered, low-GDP solution) or Stay·Safe (conventional lactate-buffered PD solution), stratified by center and diabetic nephropathy. The PD connectology was identical for both systems, and patients were treated according to local unit practices. Table 1 shows the chemical composition of the biocompatible and control fluids used in the balANZ trial.
TABLE 1.
Composition of the Biocompatible and Control Fluids Used in the balANZ Trial
Peritonitis was defined as a dialysate white cell count greater than 100/μL, with more than 50% polymorphonuclear leukocytes in a patient with a compatible clinical picture (abdominal pain, fever, or cloudy dialysate) (29). The organisms present in each episode of peritonitis during the study, excluding relapses, were classified as gram-positive (coagulase-negative staphylococci, S. aureus, Streptococcus, or other), gram-negative (Pseudomonas or non-pseudomonal), fungal, culture-negative, and polymicrobial (2 or more organisms isolated on effluent culture). When a combination of classifications existed for a peritonitis episode, the episode was included in both classifications for the purposes of the Poisson analysis.
Peritonitis-associated hospitalization was defined as hospitalization primarily for treatment of peritonitis. Peritonitis severity was rated subjectively as mild, moderate, or severe on serious adverse event forms by local clinicians at the time of each peritonitis episode. Peritonitis relapse was defined according to the International Society for Peritoneal Dialysis guidelines as peritonitis with the same organism or a sterile episode occurring within 4 weeks of an earlier episode (29-31). In keeping with International Society for Peritoneal Dialysis recommendations (30,32), a peritonitis episode complicated by relapse was counted as a single episode. Peritonitis-related death was recorded if the patient’s death was directly attributable to peritonitis in the clinical opinion of the treating nephrologist. Each patient was followed for up to 24 months, and the results were analyzed on an intention-to-treat basis. Study participants and clinicians were not blinded to PD fluid treatment.
STATISTICAL ANALYSIS
Results are expressed as frequencies and percentages, means ± standard deviations, or medians with range, depending on data distribution. Group comparisons were performed using the chi-square test, unpaired t-test, or Mann-Whitney test as appropriate. Organism-specific peritonitis rates were compared using Poisson regression, with no adjustment for multiple episodes or multiple organisms per subject. Kaplan-Meier survival analyses and multivariate Cox proportional hazards model analyses were used for time-to-event analyses. The Poisson and Cox analyses were adjusted for center, diabetic nephropathy status, baseline glomerular filtration rate, and PD modality (automated PD or continuous ambulatory PD). Data were analyzed by Statistical Revelations Pty Ltd. (http://www.statisticalrevelations.com.au/). Values of p < 0.05 were considered statistically significant.
RESULTS
PATIENT CHARACTERISTICS
The study randomized 185 patients to receive either biocompatible (n = 92) or control fluid (n = 93). Of those patients, 91 in each group (182 overall) were included in the safety analysis in the present study. As previously reported (27), the two groups were well matched for all baseline characteristics, including age, sex, cause of end-stage renal failure, presence of cardiovascular disease, body mass index, initial dialysis modality, prescribed medications, blood pressure, prescribed dialysate volumes and glucose exposure, residual renal function and urine volume, peritoneal ultrafiltration, peritoneal permeability, and laboratory parameters (serum albumin, calcium, hemoglobin). There were also no significant differences between the biocompatible and control groups with respect to the proportions of patients receiving peritonitis prophylaxis measures during the study, including exit-site mupirocin prophylaxis (32% vs 40%, p = 0.35), nasal screening for S. aureus (69% vs 62%, p = 0.35), prophylactic antibiotic administration at catheter insertion (93% vs 90%, p = 0.59), and prophylactic antifungal therapy at the time of peritonitis antibiotic therapy (55% vs 52%, p = 0.77). No patients in the study used exit-site gentamicin cream. Of the 16 units participating in the study, 14 routinely prescribed antibiotics at the time of PD catheter insertion; 6 routinely prescribed antibiotics at the time of procedures (for example, colonoscopy); 10 routinely screened PD patients for nasal S. aureus; 10 routinely performed topical mupirocin eradication of nasal staphylococcal carriage; 4 routinely prescribed exit-site mupirocin; and 7 routinely prescribed antifungal prophylaxis concomitantly with antibiotic therapy.
PERITONITIS MICROBIOLOGY
Based on the 38 episodes of peritonitis that occurred in 27 patients in the biocompatible group and the 67 episodes that occurred in 45 controls, the unadjusted geometric mean peritonitis rates in the biocompatible and control groups were 0.30 [95% confidence interval (CI): 0.22 - 0.41] and 0.49 (95% CI: 0.39 to 0.62) episodes per patient-year respectively, with an incidence rate ratio (IRR) of 0.61 (95% CI: 0.41 to 0.90; p = 0.01) in favor of the biocompatible group.
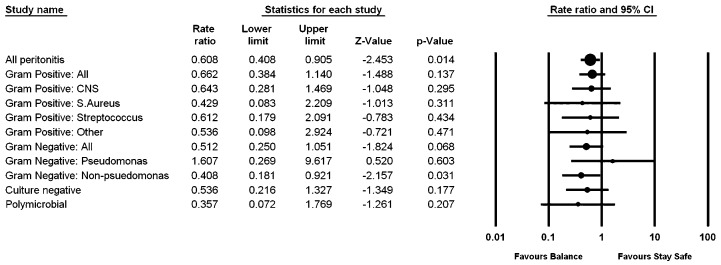
Table 2 shows the micro-organisms recovered during peritonitis episodes. Figure 1 shows a forest plot representing the IRR for the analysis of each group of organisms and all organisms isolated from peritonitis episodes. Compared with control patients, patients in the biocompatible group experienced generally lower rates of culture-negative, gram-positive, gram-negative, and polymicrobial peritonitis episodes, although the reduction was statistically significant only for non-pseudomonal gram-negative peritonitis (IRR: 0.41; 95% CI: 0.18 to 0.92; p = 0.03).
TABLE 2.
Micro-organisms Recovered from Dialysatesa during Peritonitis Episodes in balANZ Trial Participants
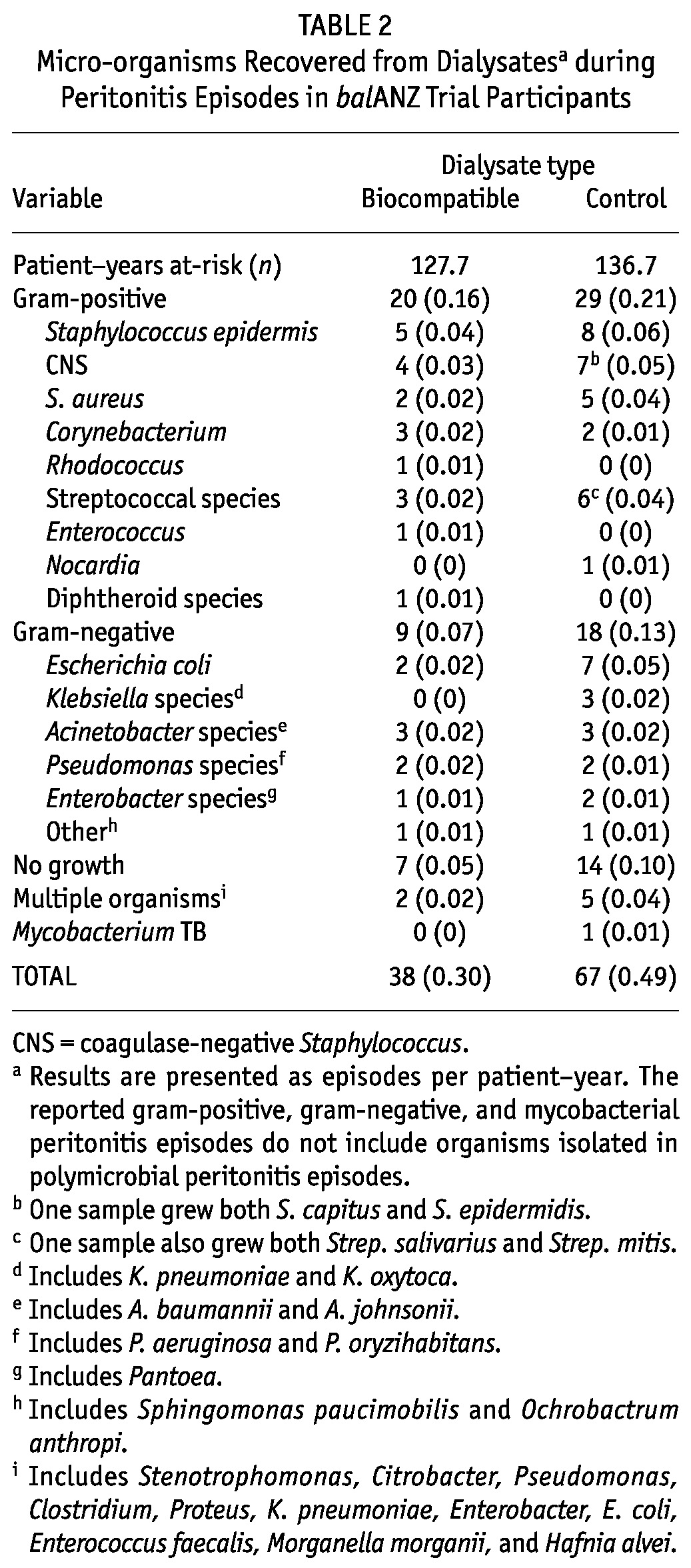
Figure 1.
— Forest plot of organisms responsible for peritonitis episodes in the biocompatible and control groups. Results are presented as incidence rate ratios with 95% confidence intervals (CIs). When a peritonitis episode represented a combination of classifications, the episode was included in both classifications for the Poisson analysis.
INITIAL PERITONITIS TREATMENT
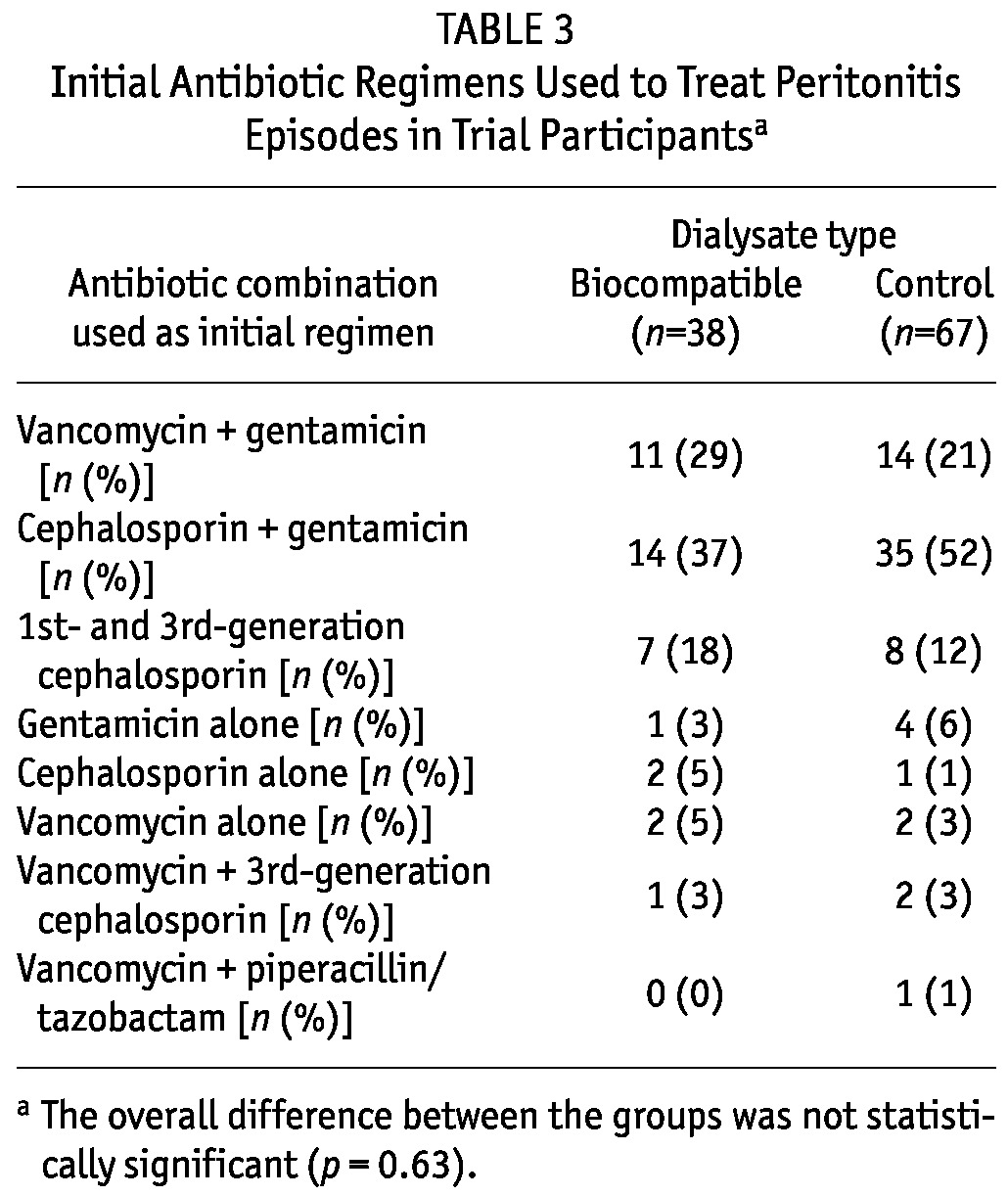
Table 3 shows the initial antibiotic regimens used to treat peritonitis episodes in the intervention and control groups. The three most common initial empiric antibiotic regimens used in each group were (in descending order) cephalosporin (primarily cephazolin) and gentamicin, vancomycin and gentamicin, and first- and third-generation cephalosporins. There were no significant differences in the initial empiric antibiotic selections between the groups (p = 0.63).
TABLE 3.
Initial Antibiotic Regimens Used to Treat Peritonitis Episodes in Trial Participantsa
PERITONITIS-ASSOCIATED HOSPITALIZATION
Excluding relapses, hospitalization was required for 23 peritonitis episodes (61%) in the Balance group and for 41 episodes (61%) in the control group (p = 0.89). Using negative binomial regression with adjustment for age, sex, body mass index, diabetes mellitus, cardiovascular disease, PD modality, baseline glomerular filtration rate, and peritoneal transport status, biocompatible PD fluid was not associated with a significant reduction in the rate of peritonitis-associated hospitalization (IRR: 0.80; 95% CI: 0.48 to 1.34). The only independent predictor of hospitalization was male sex (p = 0.03).
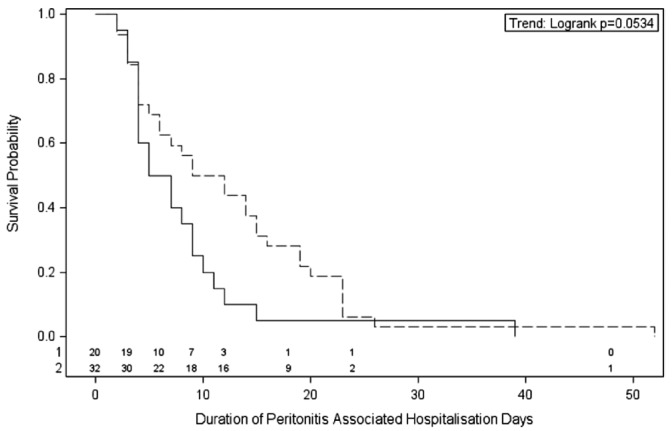
Exposure to biocompatible PD fluid was associated with a shorter duration of hospitalization for peritonitis that just failed to reach statistical significance (p = 0.05, Figure 2). The median durations of peritonitis-associated hospitalization were 6 days (95% CI: 4 to 9 days) in the Balance group and 11 days (95% CI: 5 to 15 days) in the control group (p = 0.05). When peritonitis-associated hospitalization was examined according to causative organism, exposure to biocompatible fluid was associated with a significant reduction in time to hospital discharge for culture-negative peritonitis (p = 0.04) and a trend to a reduction in time to discharge for gram-negative peritonitis (p = 0.07). No differences were observed between the biocompatible and control groups for time to discharge for gram-positive (p = 0.36) or polymicrobial peritonitis (p = 0.16). On multivariate Cox proportional hazards model analysis, no significant interaction was seen between treatment assignment (biocompatible vs control) and causative organism (p = 0.20). Duration of peritonitis-associated hospitalization varied significantly between centers (p = 0.002) and was significantly longer in patients with diabetic nephropathy (p = 0.005). Hospitalization duration was not significantly associated with baseline glomerular filtration rate, PD modality, or causative organism.
Figure 2.
— Kaplan-Meier analysis for duration (days) of peritonitis-associated hospitalization in the balANZ trial participants during the 2-year study period. The difference between the biocompatible (solid line) and control (dashed line) groups was of borderline statistical significance (p = 0.05). Numbers at risk are indicated above the abscissa.
PERITONITIS SEVERITY
Peritonitis severity was rated by attending clinicians in the biocompatible group as mild in 14 episodes (37%), moderate in 17 episodes (45%), and severe in 7 episodes (18%). In contrast, peritonitis episodes were significantly more likely to be rated moderate rather than mild in the control group [mild, 7 episodes (10%); moderate, 52 episodes (78%); severe, 8 episodes (12%); p = 0.001].
RELAPSED, RECURRENT, AND REPEAT PERITONITIS
In the biocompatible group, 5 peritonitis episodes (13%) were complicated by 7 relapses, which compares with 2 peritonitis episodes (3%) complicated by 2 relapses in the control group (p = 0.11). In the biocompatible group, 5 relapses (71%) necessitated hospitalization; in the control group, 1 relapse (50%) necessitated hospitalization.
Two episodes of recurrent peritonitis occurred in both the biocompatible and the control group (IRR: 1.07; 95% CI: 0.15 to 7.6; p = 0.95).
Repeat peritonitis occurred on 4 occasions in the biocompatible group and on 5 occasions in the control group (IRR: 0.86; 95% CI: 0.23 to 3.19; p = 0.82).
TECHNIQUE SURVIVAL AND PATIENT SURVIVAL
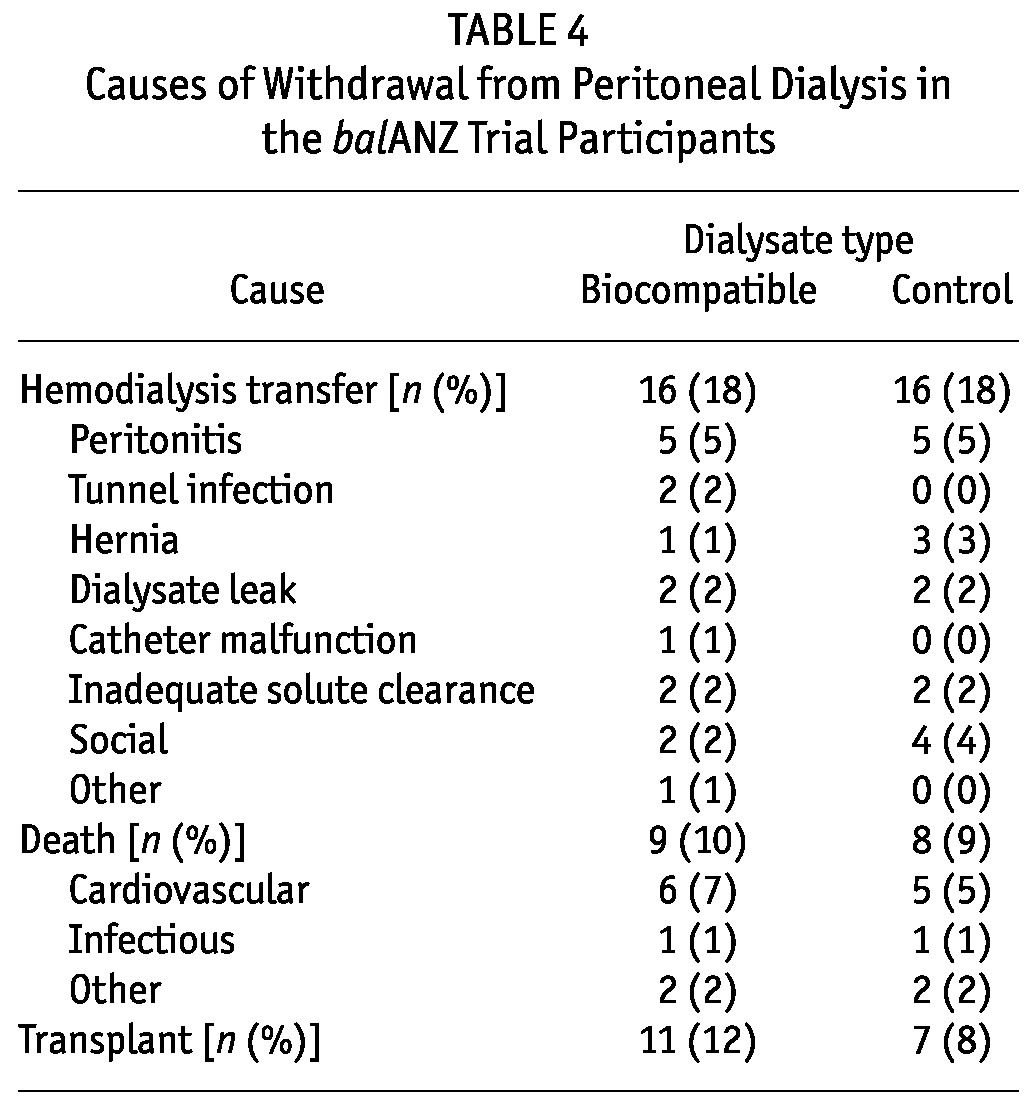
Table 4 shows the causes of withdrawal from PD among the balANZ trial participants. In both the biocompatible group and the control group, 5 patients had their catheters removed and were converted to hemodialysis as a result of peritonitis (p = 1.0). One death related to Pseudomonas peritonitis occurred in a patient who was using biocompatible fluid.
TABLE 4.
Causes of Withdrawal from Peritoneal Dialysis in the balANZ Trial Participants
DISCUSSION
This large multicenter, multi-country randomized controlled trial demonstrates that, compared with prescription of conventional dialysate, prescription of neutral-pH, lactate-buffered, PD solution low in glucose degradation products (Balance) in PD patients was associated with a marked reduction in overall peritonitis rates. When specific causative organisms were examined, the rates of culture-negative, gram-positive, gram-negative, and polymicrobial peritonitis were not significantly different between the biocompatible and control groups, although the biocompatible group did experience a significantly lower rate of non-pseudomonal gram-negative peritonitis. The initial antibiotic regimens used to treat peritonitis episodes in the intervention and control groups were similar. Compared with conventional PD fluid use, biocompatible PD fluid use was also associated with a shorter duration of peritonitis-associated hospitalization (p = 0.05) and a higher likelihood of peritonitis severity being rated as mild by attending clinicians. However, peritonitis-related technique failure and peritonitis-related death were comparable between the groups.
The foregoing results are in keeping with an earlier 3-year nonrandomized study of incident PD patients in which 50 participants using bicarbonate-buffered biocompatible fluid (BicaVera: Fresenius Medical Care) experienced a peritonitis rate of 0.34 episodes per patient-year compared with 0.60 episodes per patient-year in 50 patients using conventional PD fluid (Stay·Safe), despite the fact that patients in the biocompatible group had a higher score on the Charlson comorbidity index and a higher frequency of diabetes mellitus. Using negative binomial regression to adjust for age, sex, nasal staphylococcal carriage, cause of end-stage renal failure, weekly Kt/V, and a 3-point subjective nursing assessment of the patients’ PD skills and ability, biocompatible fluid use was associated with a significant reduction in the risk of peritonitis (adjusted odds ratio: 0.64; 95% CI: 0.43 to 0.98) (33). Similar findings were observed when the results were expressed as patient-exchanges (0.30 vs 0.50 peritonitis episodes per 1000 patient-exchanges; adjusted odds ratio: 0.65; 95% CI: 0.43 to 0.98). Of peritonitis episodes in both groups, the greater proportion were caused by gram-positive organisms. Moreover, compared with PD patients receiving conventional dialysate, those receiving biocompatible PD fluid experienced shorter peritonitis durations (based on peritoneal white cell counts).
Furkert and colleagues (34) similarly observed that, in 53 patients receiving biocompatible fluid between 2000 and 2005, peritonitis rates were appreciably lower than rates in 67 patients receiving conventional dialysates between 1990 and 1999 (0.24 episodes vs 0.60 episodes per year respectively, p = 0.002), despite the fact that patients in the biocompatible group were older and more likely to be diabetic. However, this retrospective analysis relied on historical controls and may have been affected by co-intervention bias, because overall peritonitis rates have generally improved in most PD centers since the early 1990s.
In contrast to those nonrandomized studies, a number of small randomized controlled trials have failed to observe a difference in peritonitis rates between patients receiving biocompatible and conventional dialysis fluids (35-43). In a 6-month extension substudy of one of those trials (35), which involved 57 of the original 106 participants, peritonitis rates were significantly lower in the biocompatible group than in the control group (0.24 episodes vs 0.63 episodes per patient-year respectively, p < 0.05). However, longer-term follow-up of an augmented number of trial participants in another randomized controlled trial reported by Srivastava et al. (44) did not demonstrate a significant difference in peritonitis rates between the biocompatible and control groups (0.52 episodes vs 0.45 episodes per patient-year respectively, p = 0.82). In that trial, 86% of patients in the intervention arm used Physioneal (Baxter Healthcare Corporation, Deerfield, IL, USA), which contains appreciably higher levels of GDPs than Balance does (28,43,45,46) and therefore may have been less effective at preserving peritoneal host defenses against infection. Other potential explanations for the apparent disparity in results between the balANZ trial and earlier randomized controlled trials may be insufficient statistical power, short-term follow-up, low event rates, high drop-out rates, enrollment of prevalent PD patients, and single-center designs in the earlier studies. Alternatively, the possibility of a type 1 statistical error (that is, chance finding of a significant result) in the balANZ trial cannot be excluded.
Nevertheless, the marked beneficial effect of biocompatible fluid on peritonitis occurrence and resolution in the present investigation is biologically plausible insofar as experimental evidence has shown that, compared with the effects of conventional acidic-pH, high-GDP dialysates, the effects of neutral-pH, low-GDP “biocompatible” fluids on the peritoneal membrane lead to improved local immune defenses. Numerous in vitro (17,18,18-23) and animal experimental studies (47,48) have demonstrated that conventional PD fluids impair the viability and function of peritoneal mesothelial cells, leukocytes, and macrophages, and that those adverse effects are largely abrogated by exposure to biocompatible fluids. Several clinical studies have further observed that compared with the use of conventional dialysates, the use of biocompatible fluids is associated with improved levels of peritoneal biomarkers such as cancer antigen 125 (36,38), suggesting possibly enhanced preservation of the peritoneal mesothelium. Moreover, a randomized controlled trial (38) reported significantly lower levels of C-reactive protein at 52 weeks of treatment in 25 incident PD patients using biocompatible solution than in 25 patients using conventional PD fluid, suggesting that biocompatible fluids may also exert favorable effects on systemic inflammatory responses. Any ensuing improvement in local peritoneal host defenses may have accounted for the observations in the present study of a generalized reduction in peritonitis rates across gram-positive and gram-negative organisms (although the reduction was statistically significant only for non-pseudomonal gram-negative organisms).
The balANZ trial has also previously reported that patients using biocompatible fluids (compared with conventional dialysate) experienced a significantly delayed onset of anuria (27). Because the development of anuria is associated with increased mortality, technique failure, and peritonitis episodes (49,50), it is possible that a renoprotective benefit of biocompatible fluids may also have enhanced general resistance to infection, such as peritonitis. In keeping with that possibility, the balANZ trial previously demonstrated a lower risk of non-PD-related infections in the biocompatible group compared with the control group (27).
Although the initial empiric antibiotic regimens used and the microbiology of peritonitis episodes were comparable between the two groups, another novel finding of the balANZ study is that patients in the biocompatible group who experienced peritonitis spent a shorter period of time in hospital (p = 0.05). That finding may have been related to the initially less severe peritonitis episodes in the biocompatible group, because compared with peritonitis episodes in control patients, such episodes were significantly more likely to be rated as mild in severity by attending clinicians. No interaction was observed between biocompatible fluid use and causative organism with respect to the duration of peritonitis-associated hospitalization. The effects of center-specific practices (“center effect”) on peritonitis outcomes were balanced between the intervention and control groups by stratification of randomization according to center and were additionally adjusted for in the multivariate Cox proportional hazards model analyses.
The present study had a number of limitations, the principal one being that the trial was not formally powered for peritonitis outcomes, which were secondary analyses of the balANZ trial. The possibility of type 2 statistical errors for some of the outcomes (particularly peritonitis-related technique failure and death) is high. The open-label design potentially introduced observer bias, although that potential was countered by the use of clearly defined objective peritonitis outcome measures. Nevertheless, the subjective rating of peritonitis severity by clinicians may have been influenced by their knowledge of which type of PD fluid the patient was receiving. Co-intervention bias also cannot be excluded. A number of important results were not recorded in the balANZ study, including catheter insertion technique, catheter type, PD fluid sampling method, and laboratory investigations (for example, peritonitis white cell counts and antimicrobial susceptibilities of isolated micro-organisms).
CONCLUSIONS
Administration of a neutral-pH, lactate-buffered fluid low in GDPs (Balance) to incident PD patients was associated with a statistically significant and clinically important reduction in overall peritonitis rates. Examination of organism-specific peritonitis demonstrated a significant reduction in non-pseudomonal gram-negative peritonitis rates in the biocompatible group. Although the initially prescribed empiric antibiotic regimens were similar, peritonitis-related hospitalization duration was shorter in patients receiving biocompatible PD fluids than in those receiving conventional solutions (p = 0.05), and peritonitis episodes were more commonly rated as mild in severity. Overall peritonitis-related technique failure and peritonitis-related death were comparable between the groups. Further trials examining the effects of biocompatible fluids on peritonitis are warranted.
DISCLOSURES
DWJ is a consultant for Baxter Healthcare Pty Ltd. and has previously received research funds from that company. He has also received speaker’s honoraria and research grants from Fresenius Medical Care. He has previously been a consultant to Gambro Pty Ltd. He is an International Society for Peritoneal Dialysis Councillor and a current recipient of a Queensland Government Health Research Fellowship. FGB is a consultant for Baxter Healthcare and Fresenius Medical Care. MC is an employee of Fresenius Medical Care. NB has previously received research funds from Roche, travel grants from Roche, Amgen, and Janssen-Cilag, and speaking honoraria from Roche. MGS has participated in company-sponsored research for both Baxter Healthcare and Fresenius Medical Care. In the past, but not currently, he has also been a member of the Clinical Advisory Boards of Baxter Healthcare and Fresenius Medical Care, and has attended sponsored meetings and received honoraria. All other authors have no financial conflicts of interest to declare.
The balANZ trial was funded by Fresenius Medical Care.
Acknowledgments
Collaborators (BalANZ Investigators): Australian centers: G. Rangan, L. Liew - Blacktown Hospital, Sydney, NSW; U. Steinwandel - Fremantle Hospital, Fremantle, WA; L. Garvey - John Hunter Hospital, Newcastle, NSW; M. Gilbert - Liverpool Hospital, Sydney, NSW; I. Abraham, J. Nandkumar - Monash Medical Centre, Melbourne, VIC; A. Coburn, V. Bali - Princess Alexandra Hospital, Brisbane, QLD; S. McDonald, S. Frasca, M. Hockley, C. Russ - The Queen Elizabeth Hospital, Adelaide, SA; K. Bannister, M. Hockley, K. Pirone - Royal Adelaide Hospital, SA; L. Williams - Royal Brisbane Hospital, Brisbane, QLD; K. Warr, G. Smith - Perth, WA; S. Pellicano - Sir Charles Gairdner Hospital, Perth, WA; E. O’Flaherty - St. Vincent’s Hospital, Melbourne, VIC. New Zealand Centers: L. Reed, L. Anderson - Dunedin Hospital, Dunedin; B. Jagannathan, P. Nicholls - Middlemore Hospital, Auckland. Singapore Centers: C.K. Tam - Singapore General Hospital; R. Lee - Tang Tock Seng Hospital.
The invaluable assistance provided by Caro Badcock from Statistical Revelations Pty Ltd. with regard to all statistical analyses in this study is gratefully acknowledged.
The invaluable assistance of Dr. Feidhlim Woods (former Fresenius employee and current Fresenius consultant) and Ms. Vanessa Wilson (current Fresenius employee) in providing advice on study design, and coordination and critical review of the manuscript is gratefully acknowledged.
REFERENCES
- 1. Barraclough K, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Corynebacterium peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 82 cases. Nephrol Dial Transplant 2009; 24:3834–9 [DOI] [PubMed] [Google Scholar]
- 2. Edey M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Enterococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 116 cases. Nephrol Dial Transplant 2010; 25:1272–8 [DOI] [PubMed] [Google Scholar]
- 3. Fahim M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Coagulase-negative staphylococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 936 cases. Nephrol Dial Transplant 2010; 25:3386–92 [DOI] [PubMed] [Google Scholar]
- 4. Govindarajulu S, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Staphylococcus aureus peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 503 cases. Perit Dial Int 2009; 30:311–19 [DOI] [PubMed] [Google Scholar]
- 5. Jarvis EM, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Predictors, treatment, and outcomes of non-Pseudomonas gram-negative peritonitis. Kidney Int 2010; 78:408–14 [DOI] [PubMed] [Google Scholar]
- 6. O’Shea S, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Streptococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 287 cases. BMC Nephrol 2009; 10:19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miles R, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Predictors and outcomes of fungal peritonitis in peritoneal dialysis patients. Kidney Int 2009; 76:622–8 [DOI] [PubMed] [Google Scholar]
- 8. Siva B, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Pseudomonas peritonitis in Australia: predictors, treatment, and outcomes in 191 cases. Clin J Am Soc Nephrol 2009; 4:957–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fahim M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Culture-negative peritonitis in peritoneal dialysis patients in Australia: predictors, treatment, and outcomes in 435 cases. Am J Kidney Dis 2010; 55:690–7 [DOI] [PubMed] [Google Scholar]
- 10. Ghali JR, Bannister KM, Brown FG, Rosman JB, Wiggins KJ, Johnson DW, et al. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit Dial Int 2011; 31:651–62 [DOI] [PubMed] [Google Scholar]
- 11. Johnson DW, Chang S, Excell L, Livingston B, Bannister K, McDonald SP. Peritoneal dialysis. In: McDonald SP, Excell L, eds. ANZDATA Registry Report 2006. Adelaide, Australia: Australian and New Zealand Dialysis and Transplant Registry; 2007: 87–103 [Google Scholar]
- 12. Zelenitsky S, Barns L, Findlay I, Alfa M, Ariano R, Fine A, et al. Analysis of microbiological trends in peritoneal dialysis-related peritonitis from 1991 to 1998. Am J Kidney Dis 2000; 36:1009–13 [DOI] [PubMed] [Google Scholar]
- 13. Szeto CC, Chow VC, Chow KM, Lai RW, Chung KY, Leung CB, et al. Enterobacteriaceae peritonitis complicating peritoneal dialysis: a review of 210 consecutive cases. Kidney Int 2006; 69:1245–52 [DOI] [PubMed] [Google Scholar]
- 14. Jain AK, Blake PG. Non-Pseudomonas gram-negative peritonitis. Kidney Int 2006; 69:1107–9 [DOI] [PubMed] [Google Scholar]
- 15. Bernardini J, Holley JL, Johnston JR, Perlmutter JA, Piraino B. An analysis of ten-year trends in infections in adults on continuous ambulatory peritoneal dialysis (CAPD). Clin Nephrol 1991; 36:29–34 [PubMed] [Google Scholar]
- 16. Vas S. Changing picture of peritonitis in peritoneal dialysis. Am J Kidney Dis 2000; 36:1057–8 [DOI] [PubMed] [Google Scholar]
- 17. Topley N. In vitro biocompatibility of bicarbonate-based peritoneal dialysis solutions. Perit Dial Int 1997; 17:42–7 [PubMed] [Google Scholar]
- 18. Schambye HT. Effect of different buffers on the biocompatibility of CAPD solutions. Perit Dial Int 1996; 16(Suppl 1):S130–6 [PubMed] [Google Scholar]
- 19. Witowski J, Jörres A. Effects of peritoneal dialysis solutions on the peritoneal membrane: clinical consequences. Perit Dial Int 2005; 25(Suppl 3):S31–4 [PubMed] [Google Scholar]
- 20. Nau B, Schmitt CP, Almeida M, Arbeiter K, Ardissino G, Bonzel KE, et al. BIOKID: randomized controlled trial comparing bicarbonate and lactate buffer in biocompatible peritoneal dialysis solutions in children [IS-RCTN81137991]. BMC Nephrol 2004; 5:14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mortier S, De Vriese AS, McLoughlin RM, Topley N, Schaub TP, Passlick-Deetjen J, et al. Effects of conventional and new peritoneal dialysis fluids on leukocyte recruitment in the rat peritoneal membrane. J Am Soc Nephrol 2003; 14:1296–306 [DOI] [PubMed] [Google Scholar]
- 22. Jörres A, Bender TO, Finn A, Witowski J, Fröhlich S, Gahl GM, et al. Biocompatibility and buffers: effect of bicarbonate-buffered peritoneal dialysis fluids on peritoneal cell function. Kidney Int 1998; 54:2184–93 [DOI] [PubMed] [Google Scholar]
- 23. Boulanger E, Wautier MP, Wautier JL, Boval B, Panis Y, Wernert N, et al. AGEs bind to mesothelial cells via RAGE and stimulate VCAM-1 expression. Kidney Int 2002; 61:148–56 [DOI] [PubMed] [Google Scholar]
- 24. Mortier S, Faict D, Schalkwijk CG, Lameire NH, De Vriese AS. Long-term exposure to new peritoneal dialysis solutions: effects on the peritoneal membrane. Kidney Int 2004; 66:1257–65 [DOI] [PubMed] [Google Scholar]
- 25. Mortier S, Faict D, Lameire NH, De Vriese AS. Benefits of switching from a conventional to a low-GDP bicarbonate/lactate-buffered dialysis solution in a rat model. Kidney Int 2005; 67:1559–65 [DOI] [PubMed] [Google Scholar]
- 26. Mackenzie R, Holmes CJ, Jones S, Williams JD, Topley N. Clinical indices of in vivo biocompatibility: the role of ex vivo cell function studies and effluent markers in peritoneal dialysis patients. Kidney Int Suppl 2003; 64:S84–93 [DOI] [PubMed] [Google Scholar]
- 27. Johnson DW, Brown FG, Clarke M, Boudville N, Elias TJ, Foo MW, et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J Am Soc Nephrol 2012; 23:1097–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson DW, Clarke M, Wilson V, Woods F, Brown FG. Rationale and design of the balANZ trial: a randomised controlled trial of low GDP, neutral pH versus standard peritoneal dialysis solution for the preservation of residual renal function. BMC Nephrol 2010; 11:25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [DOI] [PubMed] [Google Scholar]
- 30. Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, Lye WC, et al. ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int 2011; 31:614–30 [DOI] [PubMed] [Google Scholar]
- 31. Burke M, Hawley CM, Badve SV, McDonald SP, Brown FG, Boudville N, et al. Relapsing and recurrent peritoneal dialysis-associated peritonitis: a multicenter registry study. Am J Kidney Dis 2011; 58:429–36 [DOI] [PubMed] [Google Scholar]
- 32. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31 [PubMed] [Google Scholar]
- 33. Montenegro J, Saracho R, Gallardo I, Martínez I, Muñoz R, Quintanilla N. Use of pure bicarbonate-buffered peritoneal dialysis fluid reduces the incidence of CAPD peritonitis. Nephrol Dial Transplant 2007; 22:1703–8 [DOI] [PubMed] [Google Scholar]
- 34. Furkert J, Zeier M, Schwenger V. Effects of peritoneal dialysis solutions low in GDPs on peritonitis and exit-site infection rates. Perit Dial Int 2008; 28:637–40 [PubMed] [Google Scholar]
- 35. Tranæus A. A long-term study of a bicarbonate/lactate-based peritoneal dialysis solution—clinical benefits. The Bicarbonate/Lactate Study Group. Perit Dial Int 2000; 20:516–23 [PubMed] [Google Scholar]
- 36. Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, et al. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (Balance) on the peritoneal membrane. Kidney Int 2004; 66:408–18 [DOI] [PubMed] [Google Scholar]
- 37. le Poole CY, van Ittersum FJ, Weijmer MC, Valentijn RM, ter Wee PM. Clinical effects of a peritoneal dialysis regimen low in glucose in new peritoneal dialysis patients: a randomized crossover study. Adv Perit Dial 2004; 20:170–6 [PubMed] [Google Scholar]
- 38. Szeto CC, Chow KM, Lam CW, Leung CB, Kwan BC, Chung KY, et al. Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose-degradation products—a 1-year randomized control trial. Nephrol Dial Transplant 2007; 22:552–9 [DOI] [PubMed] [Google Scholar]
- 39. Choi HY, Kim DK, Lee TH, Moon SJ, Han SH, Lee JE, et al. The clinical usefulness of peritoneal dialysis fluids with neutral pH and low glucose degradation product concentration: an open randomized prospective trial. Perit Dial Int 2008; 28:174–82 [PubMed] [Google Scholar]
- 40. Fan SL, Pile T, Punzalan S, Raftery MJ, Yaqoob MM. Randomized controlled study of biocompatible peritoneal dialysis solutions: effect on residual renal function. Kidney Int 2008; 73:200–6 [DOI] [PubMed] [Google Scholar]
- 41. Weiss L, Stegmayr B, Malmsten G, Tejde M, Hadimeri H, Siegert CE, et al. Biocompatibility and tolerability of a purely bicarbonate-buffered peritoneal dialysis solution. Perit Dial Int 2009; 29:647–55 [PubMed] [Google Scholar]
- 42. Kim S, Oh J, Kim S, Chung W, Ahn C, Kim SG, et al. Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: a 1-year study. Nephrol Dial Transplant 2009; 24:2899–908 [DOI] [PubMed] [Google Scholar]
- 43. Haag-Weber M, Krämer R, Haake R, Islam MS, Prischl F, Haug U, et al. Low-GDP fluid (Gambrosol Trio) attenuates decline of residual renal function in PD patients: a prospective randomized study. Nephrol Dial Transplant 2010; 25:2288–96 [DOI] [PubMed] [Google Scholar]
- 44. Srivastava S, Hildebrand S, Fan SL. Long-term follow-up of patients randomized to biocompatible or conventional peritoneal dialysis solutions show no difference in peritonitis or technique survival. Kidney Int 2011; 80:986–91 [DOI] [PubMed] [Google Scholar]
- 45. Hoff CM. In vitro biocompatibility performance of Physioneal. Kidney Int Suppl 2003; (88):S57–74 [DOI] [PubMed] [Google Scholar]
- 46. Himmele R, Jensen L, Fenn D, Ho CH, Sawin DA, Diaz-Buxo JA. A new neutral-pH low-GDP peritoneal dialysis fluid. Perit Dial Int 2012; 32:444–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park SH, Lee EG, Kim IS, Kim YJ, Cho DK, Kim YL. Effect of glucose degradation products on the peritoneal membrane in a chronic inflammatory infusion model of peritoneal dialysis in the rat. Perit Dial Int 2004; 24:115–22 [PubMed] [Google Scholar]
- 48. Wieczorowska-Tobis K, Brelinska R, Witowski J, Passlick-Deetjen J, Schaub TP, Schilling H, et al. Evidence for less irritation to the peritoneal membrane in rats dialyzed with solutions low in glucose degradation products. Perit Dial Int 2004; 24:48–57 [PubMed] [Google Scholar]
- 49. Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT on behalf of the NECOSAD Study Group. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. Am J Kidney Dis 2003; 41:1293–302 [DOI] [PubMed] [Google Scholar]
- 50. Han SH, Lee SC, Ahn SV, Lee JE, Kim DK, Lee TH, et al. Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transplant 2007; 22:2653–8 [DOI] [PubMed] [Google Scholar]