Abstract
♦ Background: To understand how peritoneal dialysis (PD) was being used in Italy in 2005 and 2008, a census of all centers was carried out.
♦ Methods: In 2005 and 2008, data were collected from, respectively, 222 and 223 centers, with respect to 4432 and 4094 prevalent patients.
♦ Results: In the two periods, the PD incidence remained stable (24.3% vs 22.9%), varying from center to center. Continuous ambulatory PD (CAPD) was the main initial method (55%), but APD was more widespread among prevalent patients (53%). Among patients returning to dialysis from transplantation (Tx), PD was used in 10%. The use of incremental CAPD increased significantly from 2005 to 2008, in terms both of the number of centers (27.0% vs 40.9%) and of patients (13.6% vs 25.7%). Late referrals remained stable at 28%, with less use of PD. The overall drop-out rate (episodes/100 patient-years) remained unchanged (31.0 vs 32.8), with 13.1 and 12.9 being the result of death, and 11.8 and 12.4 being the result of a switch to hemodialysis, mainly after peritonitis. A dialysis partner was required by 21.8% of the PD patients. The incidence of peritonitis was 1 episode in 36.5 and 41.1 patient-months, with negative cultures occurring in 17.1% of cases in both periods. The incidence of encapsulating peritoneal sclerosis (episodes/100 patient-years) was 0.70, representing 1.26% of patients treated. The catheter types used and the sites and methods of insertion varied widely from center to center.
♦ Conclusions: These censuses confirm the good results of PD in Italy, and provide insight into little-known aspects such as the use of incremental PD, the presence of a dialysis partner, and the incidence of encapsulating peritoneal sclerosis.
Keywords: Nephrology referral, incremental dialysis, assisted PD, EPS, technique failure
In 2004, the Italian Society of Nephrology (SIN) census highlighted how only 17.8% of the incident patients in 611 adult dialysis centers in Italy were treated in the 46.8% of the centers that were private (286 patients, mainly located in central-southern regions). Peritoneal dialysis (PD) was used in only 0.4% of the incident patients in private centers, and 116 of the 325 public centers did not use PD at all (1). The use of PD in Italy was therefore limited to an incidence of 15.9% and a prevalence of 10.3% when all adult dialysis centers—including those that did not use PD—were taken into consideration. The percentages rose to 24.1% and 16.9% respectively if only the 209 adult public centers using PD were considered (1). Those percentages have remained substantially stable over recent years, although with considerable variation from region to region and from center to center (2).
The SIN PD Study Group organized two censuses—for the years 2005 (C05) and 2008 (C08)—of all the public Italian Nephrology and Dialysis Centers that use PD. The two censuses collected aggregate data per center on incidence, prevalence, change or interruption of modality, peritonitis, and nonrenal PD. For the year 2008, the census was also extended to collect data about encapsulating peritoneal sclerosis (EPS) and peritoneal catheters.
METHODS
The census data were collected using an online questionnaire published on the website of the SIN PD Study Group and aimed at all public centers using PD. This paper considers only data relating to non-pediatric public centers.
CENTERS
The list of centers surveyed during C05 (n = 222) was drawn from the SIN 2004 census (1); in 2008, C08 included the centers (n = 223) surveyed in the Choice of Dialysis Therapy Questionnaire conducted in 2006 - 2007 by the SIN PD Study Group (3,4). In this way, it was possible to ensure that C05 and C08 both considered all centers using PD in their respective years. Compared with C05, C08 found that 6 centers had discontinued using, and 7 had started using, PD.
PATIENTS
For C05 and C08, all patients who started dialysis [PD and hemodialysis (HD)] during the periods 1 January to 31 December in 2005 and 2008 respectively were considered “incident.” Among the incident patients, those performing 2 or fewer daily exchanges of continuous ambulatory PD (CAPD) or 4 or fewer weekly sessions of automated peritoneal dialysis (APD) were considered to be on “incremental PD (iPD)” [incremental CAPD (iCAPD) or incremental APD (iAPD)]. Patients with a pre-dialysis follow-up of 3 or fewer months were considered to be “late referrals.” For the assessment of iPD, reference was made to the 209 and 208 centers that had started incident patients on PD in the two census periods, and for the assessment of referral, reference was made to the 219 and 214 centers with congruent data available for the two census periods.
For both C05 and C08, “prevalence” refers to patients on dialysis at 31 December of the relevant year. For C05, the prevalence was calculated by subtracting the total drop-out from the SIN 2004 census prevalence and then by adding all patients started on PD during 2005. The need for assistance among prevalent patients was evaluated when the presence of a partner was considered essential for the performance of the dialysis procedures.
Patients treated with PD for nonrenal causes [glomerular filtration rate (GFR) ≥ 15 mL/min/1.73 m2] were excluded from the calculations of incidence and prevalence.
PERITONITIS
Peritonitis was defined as the presence of at least two of the following signs and symptoms: pain, turbid effluent or a white blood cell count greater than 100/mm3, and positive culture of effluent.
In C05, data on peritonitis were available only for 163 of the 222 included centers.
EPS
Only C08 assessed EPS, with reference to the 5-year period from 2004 to 2008. The centers were asked about the analyses used for diagnosis, the types of treatment undertaken, and the outcome of EPS. Diagnosis and outcome were assessed in 145 and 119 episodes of EPS in which the data were congruent.
PERITONEAL CATHETER
Peritoneal catheter data were assessed only in C08 and were available for only 124 of the 223 centers analyzed, involving 656 patients who started PD in 2008 as first treatment, after a transfer from HD, or after graft failure. Catheter re-implantations were not taken into consideration.
The types of catheters used, the sites, the operators, and the insertion techniques were investigated, considering the opening of the peritoneum to be a surgical technique (with local or general anesthetic), and the creation of an access with a trocar or cannula needle (or both) as semi-surgical (with local anesthetic).
DATA ANALYSIS AND CALCULATIONS
Incongruent data were corrected by telephoning the center for clarification. Centers with incongruent and uncorrectable data were excluded from the C05 analysis of peritonitis and the C08 analysis of catheters.
Overall rates for death, Tx, and change of method from PD to HD were calculated and expressed in numbers of episodes per 100 patient-years using these formulas:
 |
The center effect on drop-out to HD, Tx, and death was assessed by dividing the centers into two groups: those with 20 or fewer prevalent patients, and those with more than 20 prevalent patients at 31 December 2008.
The incidence of peritonitis was calculated as the number of patient-months between episodes.
The EPS incidence was calculated in two ways: episodes per 100 patient-years, and percentage of cases in proportion to the number of patients treated in the 5-year period considered. For the first calculation, the average number of annual episodes observed in the 2004 - 2008 period, and the average follow-up years of prevalent patients in 2005 and 2008 divided by 100 were considered. For the second calculation, the number of treated patients was estimated by multiplying by 5 the average of the incident patients in C05 and in C08, and then by adding the average of the prevalent patients observed in C05 and in C08.
Data are expressed as mean ± standard deviation when distribution is normal and as median and interquartile range when distribution is asymmetric. The statistical comparisons between C05 and C08 were performed using the chi-square test.
RESULTS
PATIENTS
Incident: In C05 and in C08 respectively, 5945 and 6025 patients started dialysis as first treatment, with 1443 (24.3%) and 1379 (22.9%) of them starting on PD [p = nonsignificant (NS)]. Of the PD patients, 794 (55.0%, C05) and 759 (55.0%, C08) started with CAPD, and 649 (45.0%, C05) and 620 (45.0%, C08) started with APD.
Of the patients returning to dialysis after Tx (249 in C05 and 320 in C08), 25 (10.0%) and 32 (10.0%) respectively started on PD (p = NS). Another 89 (C05) and 82 (C08) patients were transferred to PD from HD.
Figure 1 shows the percentage use of PD across the various centers in C05 and C08: The mean and median use were, respectively, 30.8% ± 23.0% and 30.3% ± 24.5%, and 24.1% (range: 15.4% - 38.5%) and 23.3% (range: 13.8% - 36.6%).
Figure 1.
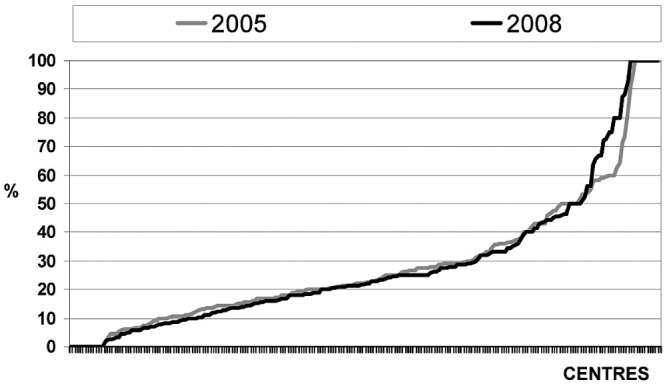
— Use of peritoneal dialysis among incident patients at the various centers in two observation periods.
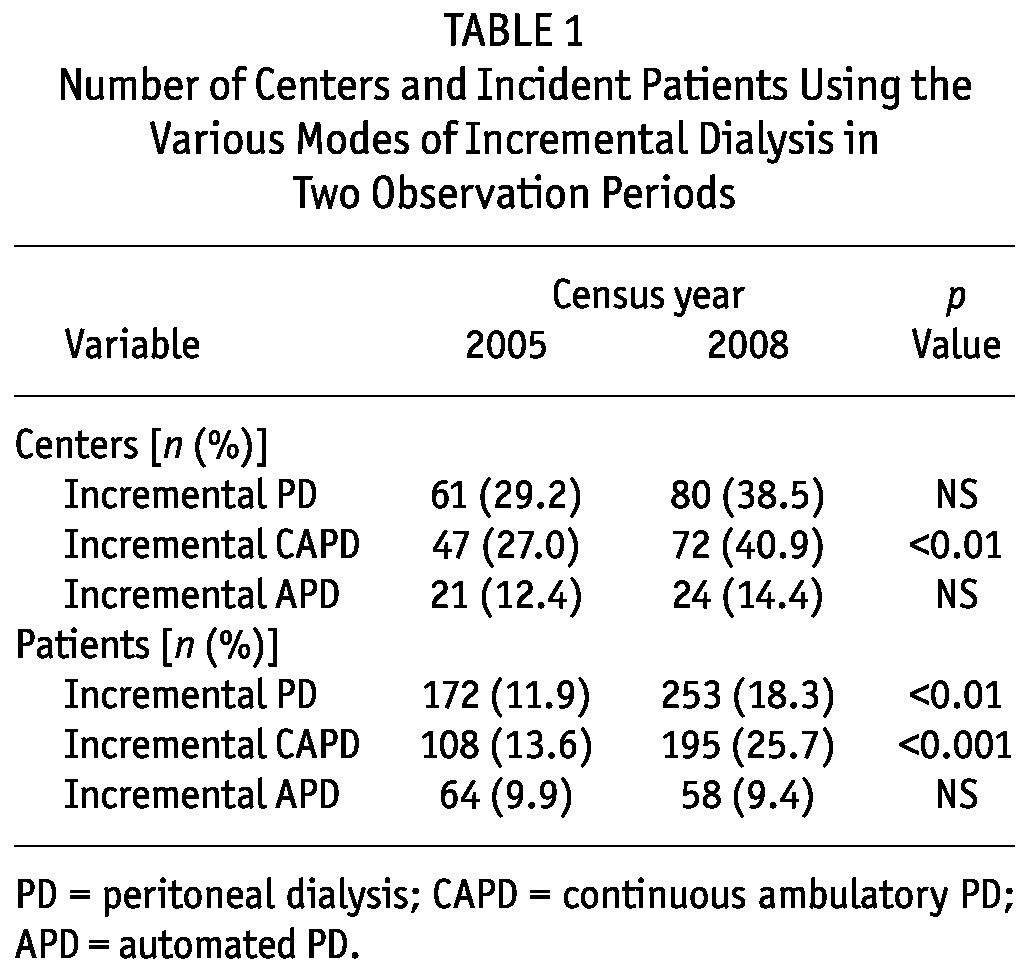
Table 1 shows the number of centers and incident patients using iPD in C05 and C08, both overall and by modality. Among centers that used CAPD in C05 (174 patients) and C08 (176 patients), the use of iCAPD increased significantly from 27.0% to 40.9% of centers (p < 0.01) and from 13.6% to 25.7% of patients (p < 0.001). The number of incident patients on PD was significantly higher in centers using iPD than in centers not using it, both in C05 [515 (30.7%) vs 929 (21.7%), p < 0.001] and in C08 [674 (29.1%) vs 705 (19.0%), p < 0.001].
TABLE 1.
Number of Centers and Incident Patients Using the Various Modes of Incremental Dialysis in Two Observation Periods
Overall, late referrals in C05 and C08 numbered 1683 (28.4%) and 1691 (28.3%) respectively (p = NS). In both periods, the use of PD was significantly lower in late-referral than in early-referral patients (C05: 15.2% vs 28.0%, p < 0.0001; C08: 11.3% vs 27.2%, p < 0.0001), with a significant reduction in the percentage of late-referral patients treated with PD the later period (15.2% in C05 vs 11.3% in C08, p < 0.001).
Prevalent: Prevalent patients on PD numbered 4432 in C05 and 4094 in C08. In C08, 1926 patients (47%) were on CAPD, and 2168 (53%) were on APD. At 31 December 2008, 16.7% of all prevalent patients on dialysis were on PD.
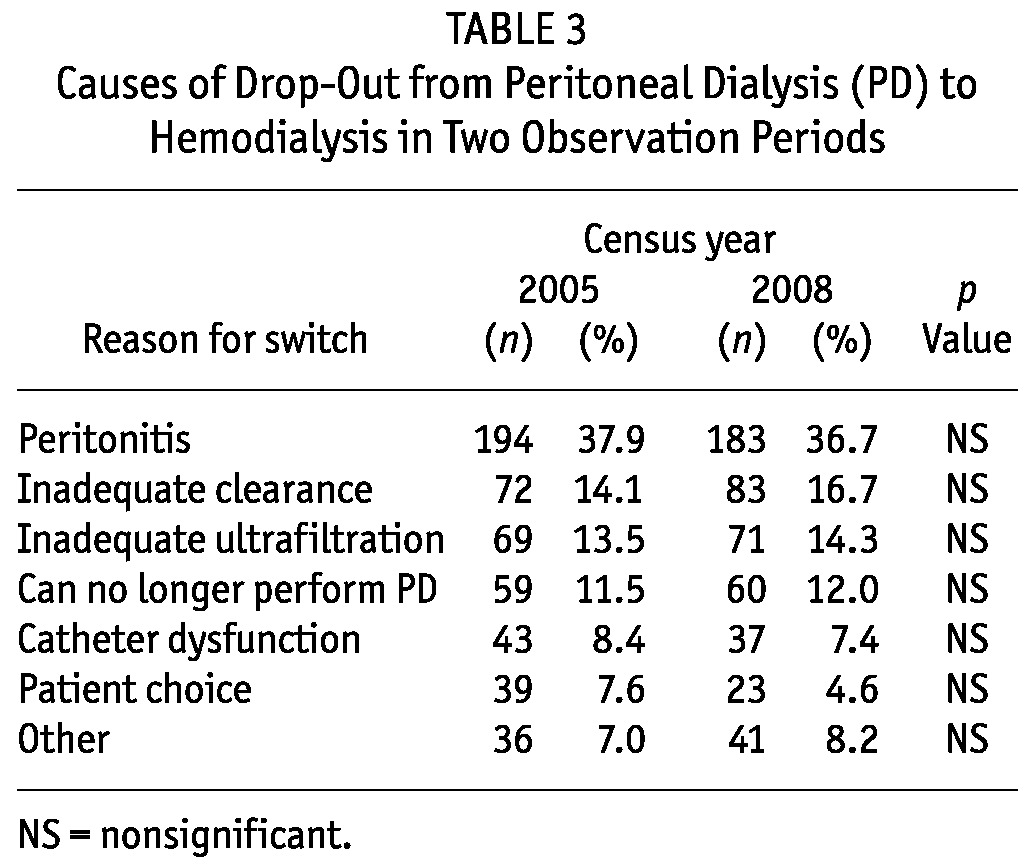
Table 2 shows the overall drop-out rate because of death, transfer to HD, or transplantation, expressed in episodes per 100 patient-years. In the 144 centers with 20 or fewer prevalent patients, drop-out to HD was significantly higher than it was in the 79 centers with a more extensive PD program (15.9 vs 10.7 episodes per 100 patient-years, p < 0.001). Drop-out rates attributable to mortality and transplant were not significantly different. Table 3 shows the various causes of drop-out from PD to HD.
TABLE 2.
Drop-Out from Peritoneal Dialysis in Two Observation Periods
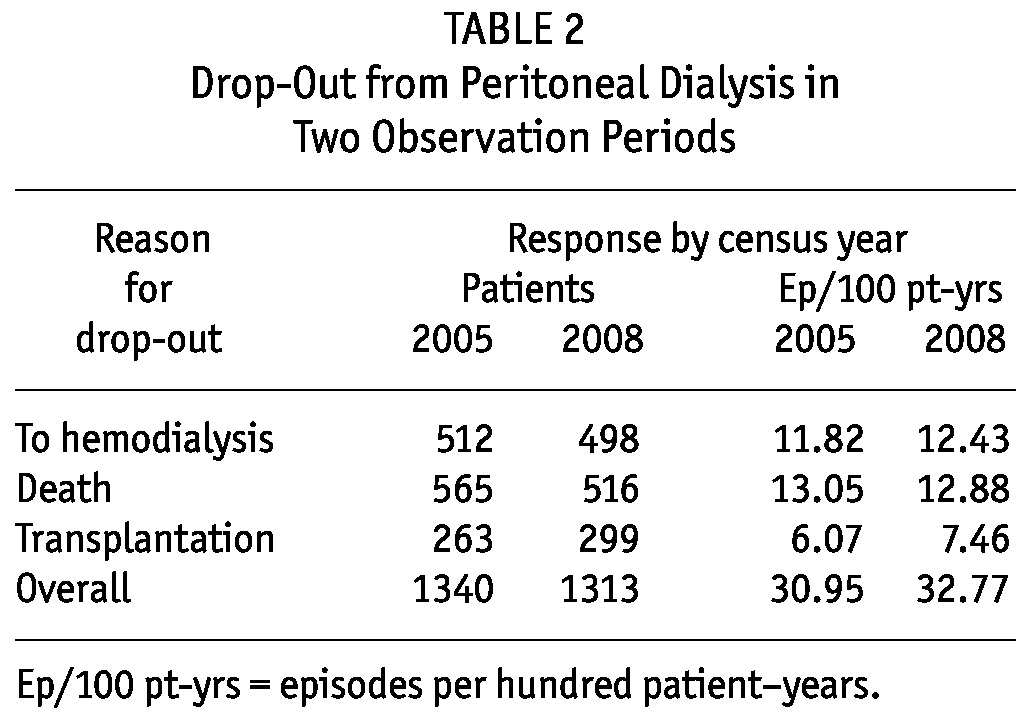
TABLE 3.
Causes of Drop-Out from Peritoneal Dialysis (PD) to Hemodialysis in Two Observation Periods
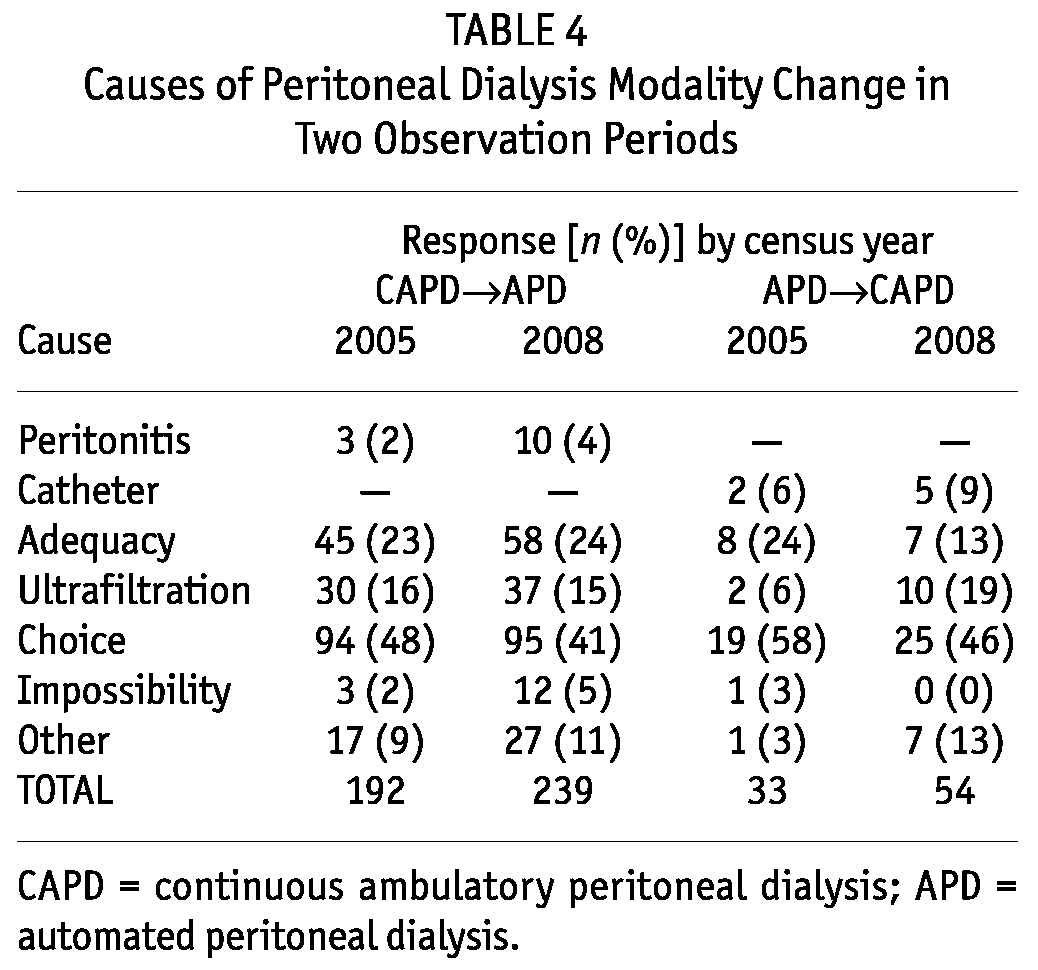
In C05, 225 (3.9%) of 5791 treated patients changed PD modality; in C08, 293 (5.4%) of 5413 treated patients made such a change (p < 0.001). Transfers from CAPD to APD were 192 and 239 in C05 and C08 respectively (p < 0.05); transfers from APD to CAPD were 33 and 54 respectively (p < 0.05). Table 4 details the causes of transfer between the two modalities in the two study periods.
TABLE 4.
Causes of Peritoneal Dialysis Modality Change in Two Observation Periods
In C08, 894 prevalent patients (21.8%) needed a partner. The partner was a family member in 80.6% of cases, a carer in 12.6%, and a nurse in 3.0%. The PD was performed in a facility for the elderly in 3.3% of cases.
PERITONITIS
The incidence of peritonitis was 1 episode in 36.5 patient-months in C05 and 1 episode in 41.1 patient-months in C08. The rate of negative cultures was 17.1% in both periods.
EPS
In the 2004 - 2008 period, 146 episodes of EPS were reported in 81 centers, equivalent to an incidence of 0.70 episodes in 100 patient-years, or 1.26% of treated patients. At the time of diagnosis, 89 patients were on PD, 45 were on HD, and 12 had undergone Tx. The diagnosis of EPS was made using computed tomography or nuclear magnetic resonance in 82.9% of cases, abdominal echography in 36.6%, histologic examination in 35.9%, and intestinal transit in 23.4%.
Surgery was undertaken in 54 cases (37.0%).
Among 120 reported EPS treatments, steroids were used in 109 cases (90.8%), tamoxifen in 78 (65.0%), and immunosuppression in 19 (15.8%). Steroids were used on their own in 37 cases (30.8%), in association with tamoxifen in 56 (46.7%), and in association with tamoxifen and immunosuppression in 14 (11.7%). Tamoxifen alone was used in 8 cases (6.7%), and immunosuppression alone in 3 (2.5%).
Among 119 reported EPS evolutions, the clinical profile was recovery in 22 cases (18.49%), stabilization in 37 (31.09%), and deterioration in 9 (7.56%); 51 patients (42.86%) died.
CATHETERS
The catheter types used included the straight double-cuff Tenckhoff in 233 patients (35.5%), the curved double-cuff Tenckhoff in 76 (11.6%), the straight double-cuff swan-neck in 37 (5.6%), the curved double-cuff swan-neck in 142 (21.6%), the Vicenza catheter in 59 (9.0%), the Di Paolo catheter in 99 (15.1%), and other catheter types in 10 cases (1.5%).
The placement technique was surgical in 575 cases (87.7%), 41.4% of which were performed by a surgeon, 26.3% by a nephrologist, and 32.3% by both. General anesthesia was used in 9.9% of the cases of surgical insertion. A semi-surgical technique managed by a nephrologist was used in 30 cases (4.6%), videolaparoscopy in 35 (5.3%), and another technique in 16 (2.4%).
The insertion site was the linea alba in 222 patients (33.8%), the rectal muscle in 392 (59.8%), the suprapubic region in 38 (5.8%), and another unspecified site in 4 (0.6%).
DISCUSSION
INCIDENCE
The two censuses confirm that the use of PD in non-pediatric public dialysis centers is substantially stable. Indeed, in the two periods, the number of centers and the percentage of incident patients using PD (24.3% vs 22.9%) remained substantially unchanged. This stability shows that the use of PD in Italy continues to be limited (2).
In the centers using PD, we noted considerable variability in the use of that modality from center to center in both periods, confirming that the infrequent use of PD is not linked to clinical or social grounds, but rather to other factors analyzed previously—for example, the status of the center as public or private, the availability of HD beds, and the size of the center (1,5).
No change was observed in the percentage use of either CAPD or APD among incident patients. In such patients, CAPD remains the most widely used method, as in North America (6,7), Australia and New Zealand (8), and Europe, except for Belgium, Finland and Denmark (9).
The two census periods also confirm that PD is used in a marginal percentage of patients returning to dialysis after Tx (10.0%), despite the fact that the survival of these patients on PD and on HD has recently been demonstrated to be nearly identical (10) and that 44.7% use of PD in patients returning from Tx has been observed in Australia (8). Moreover, it is notable that, in Italy, this percentage would be even lower if all dialysis centers were taken into consideration.
The 2008 census highlights a significant increase in the use of iPD, mostly as iCAPD (Table 1) because of an increase in the number of centers using it as the initial modality. The increase in iCAPD compared to iAPD can be explained by a greater liking for the manual modality until the number of daily exchanges exceeds 3 (11,12).
The overall percentage of late referrals among incident patients remains high and unchanged in the two periods (C05: 28.4%; C08: 28.3%), higher than the 22.0% reported in Australia (8) and the 22.2% reported in the United Kingdom (13), but still decidedly lower than the 43.7% reported in the United States (6). The use of PD in late- and early-referral patients is almost identical to that seen in the British Registry (13), and significantly lower in late-referral than in early-referral patients, as widely documented in literature.
PREVALENCE AND DROP-OUT
The total number of prevalent patients on PD declined by 7.6% from C05 to C08. Given that the overall dropout rate remained substantially unchanged in the two periods (Table 2), this reduction can be attributed to a lower incidence of PD in the period examined. Compared with rates in Australia and New Zealand, the drop-out rates attributable to Tx and death were similar, but the rate attributable to transfer to HD was considerably lower (8). In relation to the number of patients on treatment, the rate for drop-out to HD confirms observations by other authors (14-16) that the results of the PD modality are conditioned by the size of the center (“center effect”).
The main cause of drop-out from PD in the two census periods remains peritonitis, which caused more than one third of all drop-outs (Table 3), as was also recently reported by Netherlands Cooperative Study on the Adequacy of Dialysis (17). Despite the greater attention paid in the literature to ultrafiltration (18), we observed in C08 that drop-out to HD because of ultrafiltration deficiency remained unchanged (Table 3). The reduction in the percentage of late referrals to PD in the more recent period could explain the significant reduction in drop-out to HD linked with choice (Table 3). The lower rate of drop-out for that cause—compared with rates in other countries such as 29.6% in Holland (17) and 39.9% in Australia and 25.9% in New Zealand (8)—is probably a result of a more selective choice of the patients admitted to a PD program.
Change in the type of PD was significantly higher in C08, with a significantly higher switchover from CAPD to APD, which could be interpreted as greater flexibility so as to reduce the PD drop-out rate. This change of PD type increased the use of APD in prevalent patients, as has been seen in all registries (6-9), resulting in greater use of APD in prevalent patients than in incident patients (53% vs 45%). Choice (41%) represented the main cause of transfer from CAPD to APD, with insufficient adequacy (clearance and ultrafiltration combined) being second at 39% (Table 4).
Data from C08 show how, for about one fifth of the population, a partner is essential for the performance of the dialysis procedure. That finding can be explained by the aging of the dialysis population as found in various registries, and it is evidence of a greater need for dialysis assistance in elderly patients (19).
PERITONITIS
The incidence of peritonitis was found to be less than half the minimum threshold (1 episode in 18 patient-months) suggested by the International Society for Peritoneal Dialysis (20) and lower than rates recently reported in registers from France [1 episode in 33.7 patient-months (8)], Andalusia [1 episode in 24.0 patient-months (19)], and Australia [1 episode in 19.2 patient-months (21)], or in Canada [1 episode in 33 patient-months (22)], demonstrating the good quality of dialysis treatment in the centers where PD is performed in Italy. The percentage of peritonitis episodes with negative cultures is also below the 20% suggested by the International Society for Peritoneal Dialysis guidelines (20).
EPS
The incidence of EPS in Italy is very similar to the 0.87 episodes in 100 patient-years reported in the Scottish registry for 1638 prevalent and incident patients in 2000 - 2007 (23). The percentage of EPS in treated patients (1.26%) lies between the percentages reported in the literature, which vary from a minimum of 0.8% to a maximum of 3.33% (23-28). Most cases were diagnosed by computed tomography and nuclear magnetic resonance, with only slightly more than one third being diagnosed by histologic examination.
The drugs most frequently used for EPS therapy were steroids and tamoxifen, which were used together in approximately half the cases. In slightly more than one third of cases, treatment was surgical, and the high mortality rate is comparable to rates reported in the literature (23-28).
CATHETERS
A straight or curved double-cuff Tenckhoff catheter was used in about half the patients, the former remaining the most widely used catheter. Straight or curved swan-neck catheters were used in more than one quarter of patients. The high percentage of use for the self-locating catheter compared with percentages found in the literature can be attributed to the Italian origins of that type of catheter.
Surgical placement under local anesthesia was the most commonly used method of insertion, and in a quarter of cases, it was still performed by a nephrologist. Videolaparoscopy was used only in selected patients. The abdominal rectus muscle is the most frequent implantation site, although the linea alba is still used in one third of patients.
The wide variation in catheter type and in site and method of placement can be explained by the fact that the literature provides no clear grounds for choosing one catheter type over another (29,30).
DISCLOSURES
The authors declare that no financial conflict of interest exists.
Acknowledgments
We thank the contacts at the centers that took part in the censuses; their dedication made the data collection and this paper possible: E. Alberghini (Cinisello Balsamo), G. Amici (Treviso), F. Amoroso (Formia), P. Ancarani (Sestri Levante), C. Ariaudo (Torino), S. Aterini (Prato), V. Autuly (Città Di Castello), S. Ballocchi (Piacenza), M. Barattini (Carrara), M.T. Barone (Milano), V. Barone (Napoli), V. Bellizzi (Salofra), R. Bergia (Biella), G. Berlingo’ (Bassano Del Grappa), F. Bermond (Torino), M. Bertoli (Padova), L. Bertoncini (Magenta), V. Bertuzzi (Urbino), M. Biagioli (Siena), R. Boccadoro (Rimini), F. Boier (Nuoro), M. Bonaduce (Teramo), C. Bonesso (San Dona’Di Piave), F. Bonello (Ivrea), L. Bonfante (Padova), A.M. Bongi (Torino), F. Bonvegna (Verbania), M.A. Boschetti (Borgomanero), M. Bosco (Gorizia), P. Botti (Mantova), S. Brandi (Andria), M. Brigante (Campobasso), F. Bruni (San Benedetto Del Tronto), F. Budetta (Salerno), M.C. Cadoni (Sanluri), G. Campus (Perugia), F. Cappelletti (Siena), F. Caputo (Palermo), S. Caria (Olbia), A. Carlini (Lucca), A.M. Caselli (Ascoli Piceno), G.M. Caselli (Firenze), T. Casuscelli Di Tocco (Messina), B. Catania (Pisa), G. Cataldi (Altamura), G. Cavaliere (Salerno), P. Cervini (Varese), G. Cesano (Asti), C. Cherubini (Roma), D. Chimienti (Acquaviva Delle Fonti), F. Ciabattini (Fabriano), M. Ciabattoni (Genova), A. Cioni (Livorno), D. Ciurlino (Sesto San Giovanni), P. Colombo (Vercelli), F. Conte (Vizzolo Predabissi), M. Conte (Molfetta), B. Corradi (Lodi), S. Costa (Voghera), G. Cuzzocrea (Crotone), G. D’Adamo (Roma), G. D’Aloya (Milano), A. Dal Col (Milano), F.P. Damiani (San Giovanni Rotondo), P. David (Novara), C. Del Corso (Pescia), R.F. Dell’Aquila (San Severo), C. Della Gatta (Nola), E.D. De Lucia (La Spezia), A. De Martino (Rieti), F. De Padova (Martina Franca), O. De Prisco (Ceva), N. Del Negro (Vasto), P. Delli Carri (Foggia), N. Di Piazza (Enna), A. Di Somma (Castrovillari), C. Distratis (Manduria), L. Donello (Thiene), G. Enia (Reggio Calabria), R. Estivi (Tolentino), G.M. Fabozzi (Anzio), E. Falvo (Lamezia Terme), V. Fanelli (Sassari), V. Farina (Vibo Valentia), F. Fasciolo (Montichiari), P. Fatuzzo (Catania), L. Fattori (Senigallia), M. Feriani (Mestre), C. Ferrando (Cuneo), F. Ferazzoli (Frosinone), A. Ferretti (Napoli), C. Figliola (Gallarate), V. Finato (Empoli), C. Fregonese (Udine), R. Gaggi (Bologna), E. Galli (Treviglio), S. Galli (Imperia), M.A. Gallieni (Milano), D. Garofalo (Fermo), V. Gattullo (Andria), A. Gazo (Vigevano), P. Ghiringhelli (Cremona), V. Giancaspro (Bari), B. Gianoglio (Torino), P. Gianoli (Collegno), E. Giovannetti (Lido Di Camaiore), A. Giudicissi (Cesena), F. Grandinetti (Soverato), L. Guarda (Venezia), G.M. Iadarola (Torino), V. La Milia (Lecco), R. La Rosa (Putignano), R. Lanzetta (Roma), A. Laudon (Trento), D.G. Licciardello (Catania), G. Lindner (Reggio Emilia), E. Lisi (Macerata), C. Lubatti (Alessandria), L. Lucchi (Modena), M. Maccarone (Lanciano), S. Maffei (Torino), C. Magarini (Terni), F. Malacarne (Ferrara), S. Mangano (Como), R. Marcon (Castelfranco Veneto), A. Marini (Popoli), F.K. Martino (Vicenza), L. Massanti (Grosseto), M. Matalone (Catania), M.M. Mauro (Albano L.), T. Mauro (Rossano), A. Mazzotta (Casale Monferrato), B. Memoli (Napoli), A. Mencherini (Arezzo), G. Mennella (Chioggia), G. Merico (Casarano), A. Micheli (Manerbio), M. Minari (Parma), N. Miranda (Napoli), A. Mollica (Cosenza), G. Montagna (Pavia), S. Mostacci (Matera), D. Mulas (Genova), R. Nacca (Cassino), S. Oliva (Fano), E. Orazi (Vizzolo Predabissi), A. Paglialunga (Modica), D. Palmarini (Pisa), A. Pappani (Foggia), G.M. Passaghe (Olbia), G. Paternoster (Aosta), G. Perez (Dolo), M. Piva (Rovigo), M.T. Porri (Milano), P. Presta (Catanzaro), G. Priasca (Chieri), F. Puricelli (Crema), G. Quattrocchi (Caltagirone), C. Raimondi (Bologna), S. Randone (Siracusa), D. Ricciardi (Castiglione Del Lago), A.M. Ricciatti (Ancona), A. Rizzuto (Pinerolo), A.R. Rocca (Roma), V. Rondanini (Palmi), M. Rosa (Roma), D. Rossi (Pesaro), A. Rucci (L’Aquila), C. Rugiu (Verona), F. Russo (Lecce), F.S. Russo (Castellammare Di Stabia), G.E. Russo (Roma), S. Saffioti (Genova), F. Sama’ (Sondrio), S. Santarelli (Jesi), A. Santoboni (Colleferro), A. Saraniti (Messina), A. Scalamogna (Milano), B.I. Scalzo (Cirie’), R. Scanziani (Desio), O. Schillaci (Palermo), D. Schinella (Pordenone), V. Sepe (Pavia), S. Sisca (Firenze), P. Sorino (Bari), E. Sozzo (Galatina), E. Staffolani (Roma), R. Stanziale (Pescara), A. Stefoni (Civitanova Marche), D. Stellato (Benevento), A. Stingone (Ortona), P. Strippoli (Brindisi), A. Sturniolo (Roma), M. Tancredi (Atri), C. Tessarin (Belluno), G. Tiraboschi (Bergamo), A. Tirotta (Savona), I. Todaro (Piazza Armerina), A. Tommasi (Vimercate), R. Torpia (Busto Arsizio), A. Torre (Nocera Inferiore), G. Torti (Monza), L. Toscani (Montepulciano), G.B. Traverso (Genova), C. Turri (Legnano), N. Ungaro (Cerignola), R. Vecchi (Mercato San Severino), S. Venanzi (Foligno), G. Vezzoli (Milano), S. Vianello (Trieste), S. Vinciguerra (Caltanissetta), G. Virga (Camposampiero), M. Viscione (Avellino), L.A. Vitelleschi (Sulmona), V. Vizzardi (Brescia), L. Zambianchi (Forlì), M.B. Zani (Tradate), A. Zuccalà (Imola).
REFERENCES
- 1. Viglino G, Neri L, Alloatti S, Cabiddu G, Cocchi R, Limido A, et al. Analysis of the factors conditioning the diffusion of peritoneal dialysis in Italy. Nephrol Dial Transplant 2007; 22:3601–5 [DOI] [PubMed] [Google Scholar]
- 2. Società Italiana di Nefrologia (SIN). Registro Italiano di Dialisi e Trapianto. Italy: SIN; 2011. [data for 2009]: 14,19 [Available online at: http://www.sin-ridt.org/Italia/Report2009/REPORT%20RIDT%202009.pdf; accessed 11 January 2012] [Google Scholar]
- 3. Società Italiana di Nefrologia (SIN), Gruppo di Studio di Dialisi Peritoneale. La Scelta della Terapia Dialitica [da dicembre 2006 a luglio 2007]. Italy: SIN; 2006. [Available online at: http://www.dialisiperitoneale.org/files/questionario/scelta_trattamento_dialitico.pdf; accessed 11 January 2012] [Google Scholar]
- 4. Cabiddu G, Viglino G, Neri L, Filippini A, Limido A, Luchi L, et al. La scelta del trattamento dialitico. Risultati di un questionario del Gruppo di Studio di Dialisi Peritoneale. G Ital Nefrol 2008; 25(Suppl 39):S40 [Google Scholar]
- 5. Viglino G, Neri L. Theory and reality in the selection of peritoneal dialysis. Perit Dial Int 2008; 28:480–3 [PubMed] [Google Scholar]
- 6. United States Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, US Renal Data System (USRDS). 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD; USRDS; 2010. [Google Scholar]
- 7. Canadian Institute for Health Information (CIHI). Treatment of End-Stage Organ Failure in Canada, 1999 to 2008. 2010 Annual Report. Ottawa, ON: CIHI; 2010. [Google Scholar]
- 8. Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). The 32nd Report: 2009. Adelaide, Australia: ANZDATA; 2009. [Google Scholar]
- 9. European Renal Association (ERA) - European Dialysis and Transplant Association (EDTA). ERA-EDTA Registry Annual Report 2008. Amsterdam, Netherlands: Academic Medical Center, Department of Medical Informatics; 2010. [Google Scholar]
- 10. Perl J, Hasan O, Bargman JM, Jiang D, Na Y, Gill JS, et al. Impact of dialysis modality on survival after kidney transplant failure. Clin J Am Soc Nephrol 2011; 6:582–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Vecchi AF, Scalamogna A, Finazzi S, Colucci P, Ponticelli C. Preliminary evaluation of incremental peritoneal dialysis in 25 patients. Perit Dial Int 2000; 20:412–17 [PubMed] [Google Scholar]
- 12. Viglino G, Neri L, Barbieri S. Incremental peritoneal dialysis: effects on the choice of dialysis modality, residual renal function and adequacy. Kidney Int Suppl 2008; (108):S52–5 [DOI] [PubMed] [Google Scholar]
- 13. Ansell D, Feehally J, Fogarty D, Inward C, Tomson CRV, Warwick G, et al. , eds. UK Renal Registry 12th Annual Report (December 2009). Nephron Clin Pract 2010; 115(Suppl 1):c1–c319 [DOI] [PubMed] [Google Scholar]
- 14. Schaubel DE, Blake PG, Fenton SS. Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int 2001; 60:1517–24 [DOI] [PubMed] [Google Scholar]
- 15. Huisman RM, Nieuwenhuizen MG, Th de Charro F. Patient-related and centre-related factors influencing technique survival of peritoneal dialysis in the Netherlands. Nephrol Dial Transplant 2002; 17:1655–60 [DOI] [PubMed] [Google Scholar]
- 16. Afolalu B, Troidle L, Osayimwen O, Bhargava J, Kitsen J, Finkelstein FO. Technique failure and center size in a large cohort of peritoneal dialysis patients in a defined geographic area. Perit Dial Int 2009; 29:292–6 [PubMed] [Google Scholar]
- 17. Kolesnyk I, Dekker FW, Boeschoten EW, Krediet RT. Time-dependent reasons for peritoneal dialysis technique failure and mortality. Perit Dial Int 2010; 30:170–7 [DOI] [PubMed] [Google Scholar]
- 18. Lo WK, Bargman JM, Burkart J, Krediet RT, Pollock C, Kawanishi H, et al. Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit Dial Int 2006; 26:520–2 [PubMed] [Google Scholar]
- 19. Le Registre de Dialyse Péritonéale de Langue Française (RDPLF). Profils (Adultes, France Métropolitaine) - Année 2010: Pourcentage des Patients Autonomes ou Aidés par un Tiers en Fonction de l’Âge [web page]. France: RDPLF; 2011. [Available at: http://www.rdplf.org/profils/515-profil-2010.html; accessed 11 January 2012] [Google Scholar]
- 20. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [DOI] [PubMed] [Google Scholar]
- 21. Remon Rodriguez C, Quiros Ganga PL, Gil Cunquero JM, Ros Ruiz S, Aresté Fosalba N, Ruiz Fernández A, et al. Ten years of peritoneal dialysis in Andalusia (1999-2008): epidemiologic data, types of treatment, peritonitis, comorbidity and survival patients and technique. Nefrologia 2010; 30:46–53 20098470 [Google Scholar]
- 22. Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV. Predictors of peritonitis in patients on peritoneal dialysis: results of a large, prospective Canadian database. Clin J Am Soc Nephrol 2009; 4:1195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown MC, Simpson K, Kerssens JJ, Mactier RA. Encapsulating peritoneal sclerosis in the new millennium: a national cohort study. Clin J Am Soc Nephrol 2009; 4:1222–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson DW, Cho Y, Livingston BE, Hawley CM, McDonald SP, Brown FG, et al. Encapsulating peritoneal sclerosis: incidence, predictors, and outcomes. Kidney Int 2010; 77:904–12 [DOI] [PubMed] [Google Scholar]
- 25. Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A, Kubo H, et al. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis 2004; 44:729–37 [PubMed] [Google Scholar]
- 26. Summers AM, Clancy MJ, Syed F, Harwood N, Brenchley PE, Augustine T, et al. Single-center experience of encapsulating peritoneal sclerosis in patients on peritoneal dialysis for end-stage renal failure. Kidney Int 2005; 68:2381–8 [DOI] [PubMed] [Google Scholar]
- 27. Lee HY, Kim BS, Choi HY, Park HC, Kang SW, Choi KH, et al. Sclerosing encapsulating peritonitis as a complication of long-term continuous ambulatory peritoneal dialysis in Korea. Nephrology (Carlton) 2003; 8(Suppl):S33–9 [DOI] [PubMed] [Google Scholar]
- 28. Trigka K, Dousdampanis P, Chu M, Khan S, Ahmad M, Bargman JM, et al. Encapsulating peritoneal sclerosis: a single-center experience and review of the literature. Int Urol Nephrol 2011; 43:519–26 [DOI] [PubMed] [Google Scholar]
- 29. Gokal R, Alexander S, Ash S, Chen TW, Danielson A, Holmes C, et al. Peritoneal catheters and exit-site practices toward optimum peritoneal access: 1998 update. Perit Dial Int 1998; 18:11–33 [PubMed] [Google Scholar]
- 30. Figueiredo A, Goh BL, Jenkins S, Johnson DW, Mactier R, Ramalakshmi S, et al. Clinical practice guidelines for peritoneal access. Perit Dial Int 2010; 30:424–9 [DOI] [PubMed] [Google Scholar]


