Abstract
♦ Objective: We evaluated the ability of a recently developed peritoneal dialysis (PD) connector to prevent the risk of bacterial transfer to the fluid path after simulated touch and airborne contamination.
♦ Methods: Staphylococcus epidermidis ATCC1228 and Pseudomonas aeruginosa ATCC27853 strains were used. For touch contamination, 2 μL of a standardized inoculum [1×108 colony-forming units (CFU) per milliliter] were deposited on top of the pin closing the fluid path of the patient connector. For airborne contamination, the patient connector was exposed for 15 seconds to a nebulized standardized inoculum. To simulate the patient peritoneum and effluent, the patient connector was pre-attached to a 2-L bag of sterile PD solution. After contamination, the patient connector was attached to the transfer set, the pin was captured, flow control was turned to simulate “patient drain” into the empty bag, and then “patient fill” using the bag pre-attached to the connector. Finally, a new pin was recaptured. The PD solution collected in the bag pre-attached to the connector was run through a 0.20-μm filter for colony counts.
♦ Results: No infected connector transferred bacteria to the fluid path, regardless of the challenge procedure or the strain used.
♦ Conclusions: Our results show that the new PD connector may fully obviate the risk of bacterial infection, even in the presence of heavy contamination. Further studies are in progress to test our PD connector in a clinical setting.
Keywords: Peritonitis, peritoneal dialysis connector, microbial contamination
Peritonitis remains the most common acute complication of peritoneal dialysis (PD), ranking as the main cause of technique failure (1). High peritonitis rates are associated with mortality, either as a primary or a contributing factor (2-4). Technical improvements in PD systems and connectology, such as the double-bag system and the Y-set with a flush-before-fill technique, have been shown in randomized controlled studies to significantly reduce rates of peritonitis (5-7). Institution of a full range of clinical practices and protocols, combined with more careful patient selection for PD, seems at present the best approach to reducing the peritonitis risk (1,8,9). However, in large, unselected PD cohorts, which are likely to reflect the everyday experience of PD, the peritonitis rate is still disappointingly high (2,3,10). Thus, more effective methods of reducing the risk of peritonitis need to be found.
The routes of organism entry leading to peritonitis are touch, catheter-related, enteric, hematogenous, and gynecologic contamination. Touch contamination—that is, contamination at the time of exchange—is the most common source (11), and so safety in PD practice essentially relies on the patient rather than on the device. Indeed, the current PD procedure requires that patients
wash their hands at least 4 times daily,
dedicate a part of their living space at home to PD,
stock various accessories—caps, cap holders, soaps, disinfectants, masks, gowns, creams, and so on—for use in improving the sterility of the available connector system, and
periodically undergo retraining in safety procedures.
All these attempts to further improve safety increase the health care expenditure, although safety still relies on patient skills.
The challenge in designing a new connector is to succeed in completing a PD cycle by opening and closing the access of dialysis fluids to the peritoneum within a sterile environment. To accomplish that task, a capture-recapture mechanism (CRM) was engineered (Patent WO 2008/001234) and implemented in the connecting device (hereinafter “CRM device”). This CRM device should abolish the risk of peritonitis commonly derived from touch and aerosol contamination. In addition, the CRM device should relieve the patient or family members from the present burden of the PD procedure, shifting the responsibility for safety to the device from the patient’s skill and compliance.
In the present study, we assessed the efficiency of the CRM device in preventing the transfer of micro-organisms to the fluid path after deliberate touch and aerosol contamination of the connector.
METHODS
CRM PD SYSTEM
The solution delivery system used in our study was a twin-bag type, pre-attached to the CRM device, which includes online flow control (Figure 1).
Figure 1.
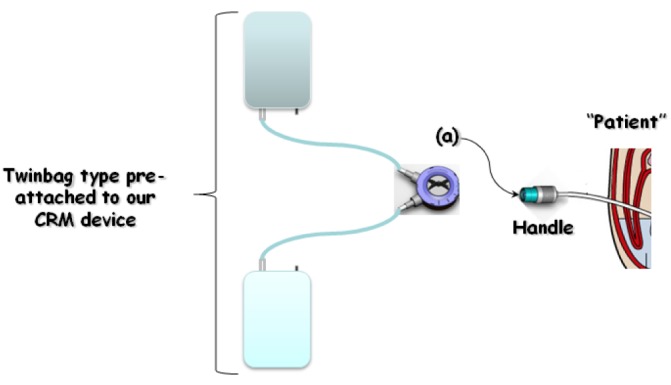
— Bacterial challenge and peritoneal dialysis exchange procedure using our “capture-recapture mechanism” (CRM) connecting device. 1) Touch or airborne contamination of the handle [patient side, (a)]. 2) Attach the transfer set catheter to the patient side. 3) Push the handle to capture the pin. 4) Turn the control switch clockwise to the drain position (°°). 5) After drain, turn the control switch clockwise to the fill position (°°). 6) After fill is complete, turn the control switch clockwise to the last position (°°°°). 7) Push the handle to capture the new pin and disconnect the patient side from the transfer set. 8) Filter the total fill volume through a 0.20 μm filter.
The patient side is closed with a pin, and once connection is made to the transfer set, the pin can be captured and safely sequestered in the transfer set by pushing the handle (patient side, Figure 2). A dial controls fluid flow, alternately opening and closing the lines connected to the drain and PD solution bags.
Figure 2.
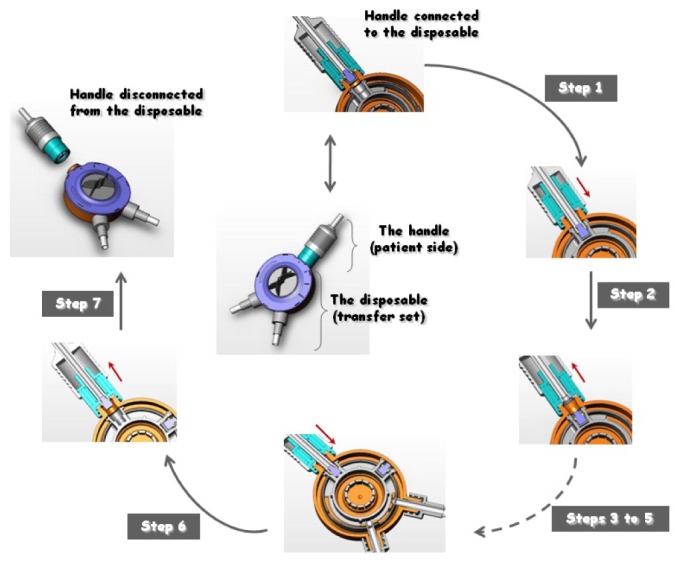
— Basic operating principle of our “capture-recapture mechanism” (CRM) connecting device. For a better appreciation of the mechanism of pin capture and recapture, cross-sections of the main CRM steps are shown. Center: A front view of the handle connected to the disposable unit before the start of the peritoneal dialysis cycle. Steps 1,2: The pin is captured and safely sequestered by the disposable unit. Pushing the handle toward the disposable unit (Step 1, directional arrow) and then releasing it (Step 2, directional arrow) removes the pin from the handle and secures it firmly to the housing of the disposable unit. Steps 3-5: Drain and fill procedures (described in detail in Figure 1). Step 6: Operating the handle (directional arrows) captures a new pin, present in the disposable unit. The cross-sections show the old pin being sequestered in the disposable unit and the new pin being captured. Step 7: A front view of the handle disconnected from the disposable unit at the end of the peritoneal dialysis cycle.
At the end of the fluid cycle and before the system is disconnected, a new pin present in the transfer set is ready to be recaptured by pushing the handle to close the patient side (Figure 2). When the PD solution and drain bags [continuous ambulatory PD (CAPD) twin bag with the CRM connector] were positioned in the way used by a PD patient (described in more detail shortly), the calculated flow rate was 6.5 cm3/s, which is comparable to that achieved with commercially available products. At that flow rate, it takes about 5 minutes for 2 L of fluid to traverse the system.
TOUCH AND AIRBORNE CONTAMINATION PROCEDURES
A series of in vitro microbiology tests were conducted to evaluate the risk of bacteria transfer to the fluid path by direct (touch) or indirect (airborne) contamination of the pin. To that end, we used Staphylococcus epidermidis ATCC1228 and Pseudomonas aeruginosa ATCC27853 reference strains. These species were chosen because they are the most common causative agents in accidental touch contamination leading to PD-associated peritonitis (12-14).
Starting from overnight growth in Trypticase Soy Broth (Oxoid SpA, Milan, Italy), a standardized inoculum of each strain was spectrophotometrically adjusted at 550 nm to an optical density of about 0.150 in phosphate-buffered saline (Sigma-Aldrich, Milan, Italy).
For the touch contamination procedure, 2 μL of the standardized inoculum were deposited on top of the pin closing the fluid path of the patient connector. The pin was then allowed to dry for 15 minutes. For the airborne contamination procedure, the patient connector was exposed for 15 seconds to bacterial aerosols generated by nebulization of 10 mL of the standardized inoculum. The exposed connector was then allowed to dry for 15 minutes.
Colony counts were performed to assess
the size of the standardized bacterial inoculum,
the bacterial load transferred onto the pin by touch,
the bacterial load transferred onto both the pin and the handle by aerosol, and
the effect of air-drying on the viability of the bacterial inoculum.
To those ends, the contaminated pin (touch) or contaminated handle and pin (aerosol) were placed inside tubes containing sterile Trypticase Soy Broth, which were then spun at maximum speed for 1 minute. In each case, triplicate 100 μL samples from the broth were removed and submitted to serial dilution for colony counts.
To simulate the patient peritoneum and effluent, the patient side was pre-attached to a 2-L bag of sterile PD solution (Figure 1). The PD solution was a commercially available glucose-based, lactate-buffered solution (1.5% glucose, 75.5 mmol/L Dianeal: Baxter Healthcare SA, Castlebar, Ireland).
The position of the PD solution and drain bags (CAPD twin bag with the CRM connector) was similar to that typically used by a PD patient: the port tube of the solution bag being 125 cm from the floor, the bag simulating the peritoneum being 50 cm from the floor, and the drain bag being on the floor. Shortly after contamination, the patient side was connected to the transfer set, the pin was captured, the flow control was turned to simulate “patient drain” into the empty bag and then again to simulate “patient fill” from the bag pre-attached to the connector, and the new pin was recaptured.
After each complete simulated exchange, the PD solution collected in the bag pre-attached to the connector was entirely and aseptically passed through a 0.20-μm filter. Colony-forming units were then counted by applying the filter at the surface of a Trypticase Soy Agar medium (Oxoid), which was then incubated at 37°C for 24 hours.
For each contamination procedure (touch, aerosol) and for each strain (S. epidermidis, P. aeruginosa), 15 simulated exchanges were performed, each simulated exchange using a new CRM device.
To evaluate the efficiency of our recovery method, and as a positive control sample, the fluid path was inoculated with a known inoculum (1×103 CFU), which was flushed into the “patient” bag. That fluid was then filtered to determine CFUs. In addition, we suspended the P. aeruginosa and S. epidermidis inocula in the Dianeal PD fluid used in the study for up to at least 4 hours at room temperature to ensure that the viability of the inocula was not affected (data not shown).
RESULTS
The CFU counts carried out for both bacterial strains showed that
the standardized inoculum was 5.0 ± 0.8×108 CFU/mL in size,
the touch contamination procedure delivered 8.0 ± 1.0×105 CFU to the top of the pin,
the aerosol contamination procedure delivered 1.0 ± 0.2×105 CFU and 6.0 ± 1.5×105 CFU onto the pin and the handle respectively, and
air-drying did not significantly affect the viability of the bacterial inocula.
The efficiency of our recovery method was greater than 97% for both bacterial strains tested.
None of the infected connectors transferred contamination to the peritoneal washing fluid, regardless of the challenge technique (touch or aerosol) or the strain (S. epidermidis, P. aeruginosa) used.
DISCUSSION
The fear of peritonitis remains a major concern for many patients and physicians, and that fear may militate against use of PD as a replacement therapy in end-stage renal disease. Although peritonitis incidence rates have dramatically declined with antimicrobial prophylaxis (15), and despite the introduction of the flush-before-fill double-bag principle (6) and various technological improvements relating to disconnection systems (7), cases of refractory or even fatal PD-related peritonitis still occur (16), mainly because of P. aeruginosa—and, to a lesser extent, S. aureus—infection (2,6,17).
The leading cause of peritonitis continues to be poor aseptic technique at the time of PD exchange, because during the procedure to attach and detach connectors, micro-organisms can inadvertently be transferred via the lumen of the peritoneal catheter into the peritoneal space, causing peritonitis.
Currently, catheter connecting systems are of three main types:
The standard or straight system, in which a new connection is made at each exchange
The Y-set or disconnect system, in which the patient disconnects from the bags between exchanges
The double- or twin-bag system, in which the connection with the fresh dialysis solution bag is already made, so that the patient has to perform one less connection procedure
Observational studies and systematic reviews have clearly demonstrated that disconnect systems (double-bag and Y-connection) are superior to conventional spike (or Luer-lock) connect systems in terms of the prevention of peritonitis (5,11,18). The most likely reason for that observation is the reduction of inadvertent peritoneal microbial contamination because of the flush-before-fill maneuver during connections with the Y-set and twin-bag systems (19). The elimination of one connection procedure entailed by the twin-bag system should theoretically further reduce peritonitis episodes beyond what is achieved by the Y-connection (5). The design of the CAPD delivery system could therefore affect the incidence of both peritonitis and CAPD technique failure.
In the present study, we assessed the efficiency of a new CRM device in preventing the transfer of micro-organisms to the fluid path after deliberate contamination. This CRM device was conceived and designed to forestall the peritonitis risk deriving from touch and airborne contamination. From an engineering standpoint, that objective was successfully achieved by devising a mechanism which, at the beginning of a PD fluid exchange, captures the pin that closes the fluid path between PD exchanges (patient side, Figure 2). At the end of the PD fluid exchange, a new pin is recaptured from the transfer set and closes off the fluid path (patient side). All the steps occur in a fully automated manner and with the lumen of the catheter always protected from bacterial access to the fluid path during the PD fluid exchange. Furthermore, unlike currently available PD connecting systems, the pin is not equipped with a sponge ring loaded with povidone iodine, thereby preventing the side effects of peritoneal inflammation and fibrous thickening (which may trigger encapsulating peritoneal sclerosis) associated with povidone iodine flowing into the peritoneal cavity (20,21).
Our results show that none of the bacteria contaminating the pin were transferred to the fluid path, ruling out the possibility of bacteria gaining access to the peritoneum during PD fluid exchanges. These results are all the more impressive considering the level of contamination generated by the challenge procedures (1.6×105 CFU), which is considerably higher than might be expected in the clinical setting—from 102 CFU by unprepared hands, up to 4.5×103 CFU if hands are washed but not dried (22)—or than has been reported as the worst-case touch contamination scenario in previous studies (23-25) evaluating the ability of several commercially available PD connectors to prevent fluid path contamination (Table 1).
TABLE 1.
Published Studies Evaluating the Microbiological Safety of Peritoneal Dialysis Systems
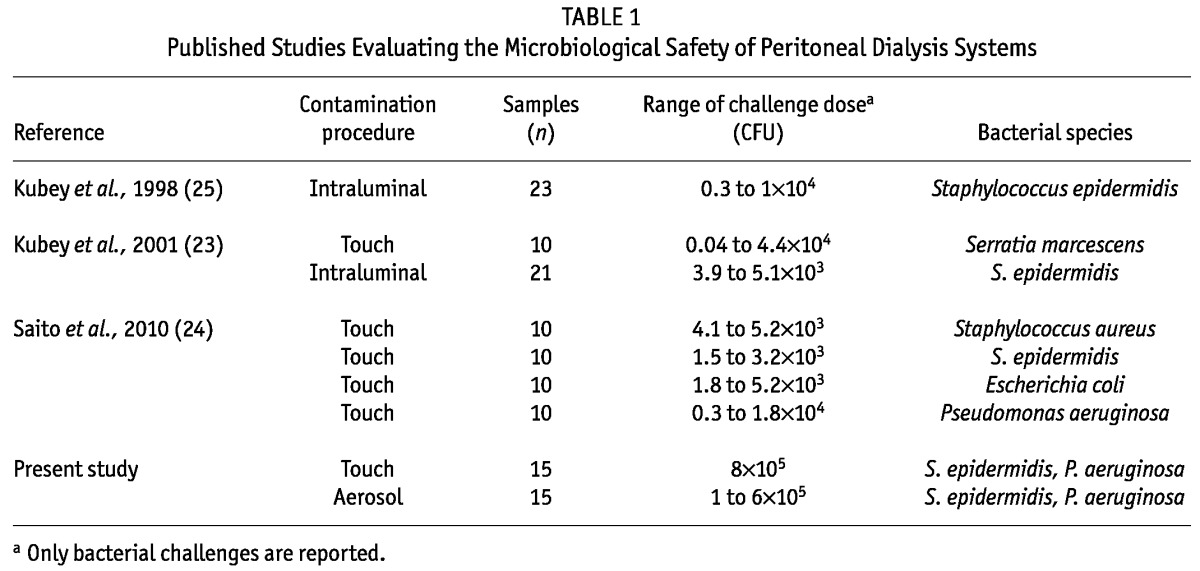
Moreover, our study is the first to use an airborne contamination procedure, which is a much worse contamination scenario than touch contamination alone. However, the present study does not allow for the possibility of biofilm formation on the part of the connection that is not discarded to be unequivocally ruled out. Although the chances of such an occurrence are low, that possibility deserves future investigation.
It is important to note that, although the CRM device can also operate the flush-before-fill step, we simulated PD exchange in the present study without including that step. Without such convective removal of bacteria contaminating the fluid path, commercially available connector systems would not be very efficient in reducing bacterial contamination (23,25).
CONCLUSIONS
Prevention is the most important step in managing peritonitis and is therefore still a matter of great concern. For prevention, a good connection technique is essential to avoid transfer of microbes to the peritoneum. The CRM device described here is the first PD connecting system fully able to remove the risk of bacterial infection, even in the presence of heavy contamination.
Randomized clinical studies will be needed to demonstrate a relationship between our in vitro results and clinical performance of the CRM device in terms of reducing peritonitis rates.
DISCLOSURES
FA and AA are employees of Glomeria Therapeutics (San Giovanni Teatino, Chieti, Italy).
Acknowledgments
The authors thank Reto Miozzari for critical revision of the manuscript.
REFERENCES
- 1. Mactier R. Peritonitis is still the Achilles’ heel of peritoneal dialysis. Perit Dial Int 2009; 29:262–6 [PubMed] [Google Scholar]
- 2. Kavanagh D, Prescott GJ, Mactier RA. Peritoneal dialysis associated peritonitis in Scotland 1999-2002. Nephrol Dial Transplant 2004; 19:2584–91 [DOI] [PubMed] [Google Scholar]
- 3. Davenport A. Peritonitis remains the major clinical complication of peritoneal dialysis: the London, UK, peritonitis audit 2002-2003. Perit Dial Int 2009; 29:297–302 [PubMed] [Google Scholar]
- 4. Pérez Fontan M, Rodríguez-Carmona A, García-Naveiro R, Rosales M, Villaverde P, Valdés F. Peritonitis-related mortality in patients undergoing peritoneal dialysis. Perit Dial Int 2005; 25:274–84 [PubMed] [Google Scholar]
- 5. Daly CD, Campbell MK, MacLeod AM, Cody DJ, Vale LD, Grant AM, et al. Do the Y-set and double-bag systems reduce the incidence of CAPD peritonitis? A systematic review of randomized controlled trials. Nephrol Dial Transplant 2001; 16:341–7 [DOI] [PubMed] [Google Scholar]
- 6. Peritonitis in continuous ambulatory peritoneal dialysis (CAPD): a multi-centre randomized clinical trial comparing the Y connector disinfectant system to standard systems. Canadian CAPD Clinical Trials Group. Perit Dial Int 1989; 9:159–63 [PubMed] [Google Scholar]
- 7. Harris DC, Yuill EJ, Byth K, Chapman JR, Hunt C. Twin- versus single-bag disconnect systems: infection rates and cost of continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 1996; 7:2392–8 [DOI] [PubMed] [Google Scholar]
- 8. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31 [PubMed] [Google Scholar]
- 9. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [DOI] [PubMed] [Google Scholar]
- 10. Johnson D, Chang S, Excell L, Livingston B, Bannister K, McDonald S. Chapter 6: Peritoneal dialysis. In: McDonald S, Chang S, Excell L, eds. The Thirtieth Report: Australia and New Zealand Dialysis and Transplant Registry, 2007. Adelaide, South Australia: The ANZDATA Registry; 2007. [Available online at: http://www.anzdata.org.au/anzdata/AnzdataReport/30thReport/Ch06PeritonealDialysis.pdf; accessed 21 December 2011] [Google Scholar]
- 11. Piraino B. Peritonitis as a complication of peritoneal dialysis. J Am Soc Nephrol 1998; 9:1956–64 [DOI] [PubMed] [Google Scholar]
- 12. Barretti P, Montelli AC, Batalha JE, Caramori JC, Cunha Mde L. The role of virulence factors in the outcome of staphylococcal peritonitis in CAPD patients. BMC Infect Dis 2009; 9:212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta S, Muralidharan S, Gokulnath, Srinivasa H. Epidemiology of culture isolates from peritoneal dialysis peritonitis patients in southern India using an automated blood culture system to culture peritoneal dialysate. Nephrology (Carlton) 2011; 16:63–7 [DOI] [PubMed] [Google Scholar]
- 14. Piraino B, Bernardini J, Florio T, Fried L. Staphylococcus aureus prophylaxis and trends in gram-negative infections in peritoneal dialysis patients. Perit Dial Int 2003; 23:456–9 [PubMed] [Google Scholar]
- 15. Strippoli GF, Tong A, Johnson D, Schena FP, Craig JC. Antimicrobial agents for preventing peritonitis in peritoneal dialysis patients. Cochrane Database Syst Rev 2004; (4):CD004679 [DOI] [PubMed] [Google Scholar]
- 16. Troidle L, Finkelstein F. Treatment and outcome of CPD-associated peritonitis. Ann Clin Microbiol Antimicrob 2006; 5:6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holley JL, Bernardini J, Piraino B. Infecting organisms in CAPD patients on the Y-set. Am J Kidney Dis 1994; 23:569–73 [DOI] [PubMed] [Google Scholar]
- 18. Strippoli GF, Tong A, Johnson D, Schena FP, Craig JC. Catheter-related interventions to prevent peritonitis in peritoneal dialysis: a systematic review of randomized, controlled trials. J Am Soc Nephrol 2004; 15:2735–46 [DOI] [PubMed] [Google Scholar]
- 19. Bazzato G, Landini S, Fracasso A, Morachiello P, Righetto F, Scanferla F, et al. Why the double-bag system still remains the best technique for peritoneal fluid exchange in continuous ambulatory peritoneal dialysis. Perit Dial Int 1993; 13(Suppl 2):S152–5 [PubMed] [Google Scholar]
- 20. Kashiwagi T, Iino Y, Miyahara H, Katayama Y. Microbicidal efficacy of povidone-iodine in a noncontact manner applied to a continuous ambulatory peritoneal dialysis connection system. J Nihon Med Sch 2010; 77:86–92 [DOI] [PubMed] [Google Scholar]
- 21. Keating JP, Neill M, Hill GL. Sclerosing encapsulating peritonitis after intraperitoneal use of povidone iodine. Aust N Z J Surg 1997; 67:742–4 [DOI] [PubMed] [Google Scholar]
- 22. Miller TE, Findon G. Touch contamination of connection devices in peritoneal dialysis—a quantitative microbiologic analysis. Perit Dial Int 1997; 17:560–7 [PubMed] [Google Scholar]
- 23. Kubey W, Straka P, Holmes CJ. An in vitro bacterial touch contamination risk assessment of two CAPD twinbag systems. Blood Purif 2001; 19:62–7 [DOI] [PubMed] [Google Scholar]
- 24. Saito SK, Kashiwagi T, Miyahara H, Iino Y, Katayama Y. A comparative study of sterility levels in continuous ambulatory peritoneal dialysis system. J Nihon Med Sch 2010; 77:306–11 [DOI] [PubMed] [Google Scholar]
- 25. Kubey W, Straka P, Holmes CJ. Importance of product design on effective bacterial removal by fluid convection in Y set and twinbag systems. Blood Purif 1998; 16:154–61 [DOI] [PubMed] [Google Scholar]


