Abstract
♦ Background: Prophylactic gentamicin 0.1% cream has demonstrated efficacy in preventing both exit-site infection (ESI) and peritonitis attributable to gram-positive and gram-negative organisms; however, the effect of this practice on the gentamicin susceptibility patterns of bacterial pathogens isolated from such infections is unknown. We therefore examined the effect of a change in our prophylactic topical antibiotic exit-site protocol (from mupirocin 2% cream to gentamicin 0.1% cream) on infection rates and susceptibility patterns.
♦ Methods: This retrospective observational cohort study examined two periods of time: before and after the change in exit-site protocol. Each period was 30 months in duration, with a 2-month implementation period between, during which patient data were excluded. Demographic, clinical, and microbiology data were collected for each patient and episode of infection.
♦ Results: Overall, 377 patients were evaluated. In the mupirocin period (MUP), 145 infections occurred in 79 patients, and in the gentamicin period, 145 infections occurred in 93 patients. No significant effect was found either in overall episodes of infection (0.53 per year) or in episodes of peritonitis (0.429 vs 0.375 per year), but episodes of ESI increased significantly (0.098 vs 0.153 per year; p = 0.024; odds ratio: 1.55; 95% confidence interval: 1.05 to 2.28). Episodes of Staphylococcus aureus peritonitis increased by 38% (0.018 vs 0.025 per year), and episodes of S. aureus ESI increased significantly by 150% (0.022 vs 0.055 per year; p = 0.03; hazard ratio: 3.00; 95% confidence interval: 1.09 to 8.26). Episodes of pseudomonal peritonitis declined by 68% (0.022 vs 0.007 per year), and episodes of pseudomonal ESI increased by 150% (0.007 vs 0.018 per year). The gentamicin susceptibility for gram-positive isolates demonstrated no significant change; however, the gentamicin susceptibility for Enterobacteriaceae decreased by 12% and for Pseudomonas, by 14%.
♦ Conclusions: The significant increase in episodes of ESI and the decrease in susceptibility for both Enterobacteriaceae and Pseudomonas isolates represent a concerning trend. Centers should examine trends in infection rates and in bacterial susceptibilities to determine the most appropriate agent for ESI prophylaxis.
Keywords: Antibiotic cream, pathogen susceptibilities, peritonitis, exit-site infection
Peritonitis is one of the most common complications and reasons for hospitalization among patients receiving peritoneal dialysis (PD). Severe peritonitis can lead to scarring of the peritoneal membrane, with subsequent loss of the membrane for dialysis and even death (1). Exit site infections (ESIs) and tunnel infections, although less common than peritonitis, progress to peritonitis in up to 13% of cases (2). Peritonitis secondary to exit-site or tunnel infection is associated with a 74% treatment failure rate and a 72% catheter removal rate (2).
Updated guidelines on the treatment and prevention of PD-related infections have identified the application of antimicrobial cream such as that containing mupirocin (MUP) or gentamicin (GENT) to the catheter exit site as an effective measure to help prevent ESI (1). However, with the widespread use of prophylactic creams, concerns have arisen about the emergence of GENT and MUP resistance, as has been demonstrated by other authors (3-7).
In the months of November and December 2006, the PD program of the Wake Forest University Outpatient Dialysis centers and the inpatient PD program at Wake Forest Baptist Health initiated a conversion from MUP 2% cream to GENT 0.1% cream for routine ESI prophylaxis in all patients. The present study examines the effect of this conversion on the susceptibility patterns of bacterial pathogens causing PD-related infections. The rates of peritonitis, ESI, and peritonitis attributable to catheter exit-site and tunnel infections are also examined.
METHODS
This retrospective observational cohort study examined two periods of time: before and after the change in the exit-site protocol. The study was conducted both in the inpatient PD unit of Wake Forest Baptist Health and the affiliated outpatient PD units of the Wake Forest University Outpatient Dialysis centers. The study was approved by the institutional review board of Wake Forest University Health Sciences.
Patients were eligible for inclusion if they were adults (18 years or older) attending the Wake Forest University Outpatient Dialysis centers who had been receiving PD for at least 1 month. Patients received either MUP 2% cream (before November 2006) or GENT 0.1% cream (after November 2006) as a part of the standard exit-site care protocol. Data were collected on eligible patients who received PD during two 30-month study periods: the MUP period (May 2004 to October 2006) and the GENT period (January 2007 to June 2009). During a 2-month exclusion period (November 2006 to December 2006), no infection data were collected, allowing for the transition from one cream to the other.
Demographic and clinical data were collected for each patient. For patients with infections during either study period, the following information was also recorded: the appearance of the exit site, the peritoneal white blood cell count and differential, antibiotic treatments, and culture data. Exit-site infection was defined as the presence of purulent drainage from the exit site, with either erythema or tenderness at the site, or evidence of infection on ultrasonography. Peritonitis was defined as cloudy dialysate or abdominal pain, or both, with a peritoneal white blood cell count of 100 cells or more per cubic milliliter, with more than 50% being polymorphonuclear cells.
Microbiology data were also examined to determine the rates of GENT susceptibility for infection-related organisms. Isolates with unavailable susceptibility data were excluded from the susceptibility analysis. Recurrent and repeat infections were included in the analysis; relapsing infections were not. Multiple isolations of the same cultured organisms with different susceptibility patterns were evaluated separately. Isolate susceptibility testing was performed using microbroth dilution before June 2005, and using the automated MicroScan WalkAway system (Siemens Healthcare Diagnostics, Sacramento, CA, USA) afterward. Interpretation of the susceptibility results was based on guidelines from the Clinical and Laboratory Standards Institute (8). The rate of sterile cultures was also examined.
Descriptive statistics were calculated, including frequencies and proportions for categorical data, and sums, means, and standard deviations for continuous data. The chi-square approximation of the log-rank test was used to calculate the p values for failure-pattern comparisons of time to first infection between groups. The Kaplan-Meier method was used to estimate infection-free survival times for each study group (Figures 1, 2, 3). Poisson regression, with an adjustment for over-dispersion, was used to model the count data observed for each person and to model the rates of infection. For overall exit-site and peritonitis infections, the observed numbers of the infection types were modeled, with the offset parameter being the natural log of the time observed during the study period. Values of p < 0.05 were considered statistically significant.
Figure 1.
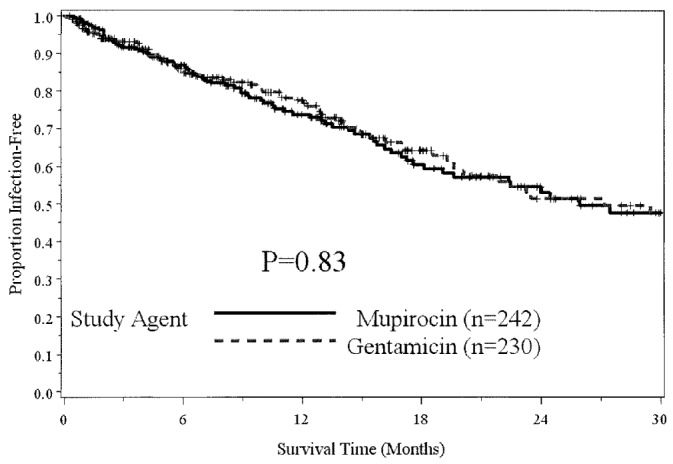
— Time to first peritonitis episode.
Figure 2.
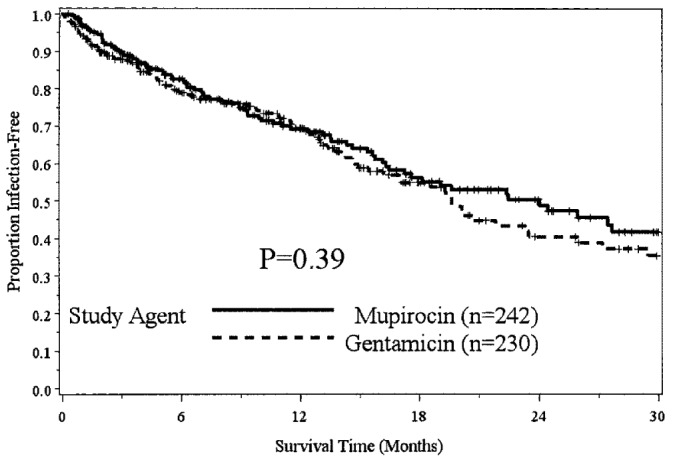
— Overall infection survival.
Figure 3.
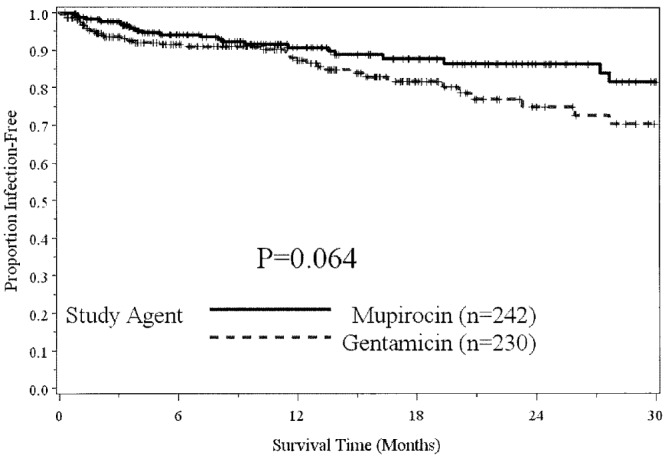
— Time to first exit-site infection.
RESULTS
The study evaluated 377 patients, accounting for 275 patient-years in each period. Baseline demographic information (Table 1) was similar in the two groups, except for weight at start of PD (81 kg vs 91 kg, p = 0.017) and median total PD vintage (18 months vs 27 months, p = 0.03). During the study period, 28 patients died (12 in the MUP period and 16 in the GENT period). Infections led to catheter removal in 28 episodes in the MUP period and 29 episodes in the GENT period. The use of intraperitoneal or intravenous GENT in treating peritonitis also decreased from 18% of all episodes during the MUP period to 7% of all episodes during the GENT period.
TABLE 1.
Baseline Demographics in Patients with Infections

During the MUP period, 145 infections occurred in 79 patients, and during the GENT period, 145 infections occurred in 93 patients. The overall infection rate was 0.53 infections per year for each period (Table 2). During the study period, 208 patients were free of infection.
TABLE 2.
Comparison of Infection Rates

In a Poisson regression model with the number of infections for each individual being the outcome, study arm being the independent variable, and the offset being time observed, no significant effect for overall infections (p = 0.99) or for peritonitis (p = 0.35) was evident, but there was a significant difference in ESIs for GENT relative to MUP (p = 0.024; odds ratio: 1.55; 95% confidence interval: 1.05 to 2.28).
The total rate of ESI increased 56% to 0.153 episodes per year (ep/y) during the GENT period from 0.098 ep/y during the MUP period. The rate of gram-positive ESI increased 83% to 0.087 ep/y from 0.047 ep/y after the conversion. The rate of gram-negative ESI increased to 0.033 ep/y from 0.025 ep/y. This increase was most affected by a 150% increase in the rate of Pseudomonas ESI (0.007 ep/y vs 0.018 ep/y). The rate of fungal ESI increased to 0.007 ep/y during the GENT period from 0.004 ep/y during the MUP period, and the rate of fungal peritonitis increased from 0.011 ep/y to 0.036 ep/y.
The o verall peritonitis rate declined by 13% to 0.375 ep/y in the GENT period from 0.429 ep/y in the MUP period. The rate of overall gram-positive peritonitis exhibited a small increase of 12% (to 0.200 ep/y from 0.178 ep/y); however the rate of gram-negative peritonitis declined 43% to 0.058 ep/y from 0.102 ep/y. The rate of S. aureus peritonitis increased by 38% (0.018 ep/y vs 0.025 ep/y), and Pseudomonas peritonitis cases declined by 68% (0.022 ep/y vs 0.007 ep/y). Concurrent ESI and peritonitis occurred in 6 patients; however, the same organisms (S. aureus and Candida) were cultured from both sites in only 2 concurrent episodes.
A total of 221 isolates were examined: 101 in the MUP period, and 120 in the GENT period. Polymicrobial infections accounted for 6% of infections in the MUP period and 11% in the GENT period. The susceptibility evaluation excluded 11 infections because of the unavailability of susceptibility information. Of the remaining 210 isolates, 98 were obtained in the MUP period (54 gram-positive, 40 gram-negative, 4 fungal), and 114 in the GENT period (69 gram-positive, 33 gram-negative, 12 fungal). Table 3 shows the percentages of isolates susceptible to GENT.
TABLE 3.
Gentamicin Susceptibility for Select Isolates Causing Peritoneal Dialysis-Related Infections
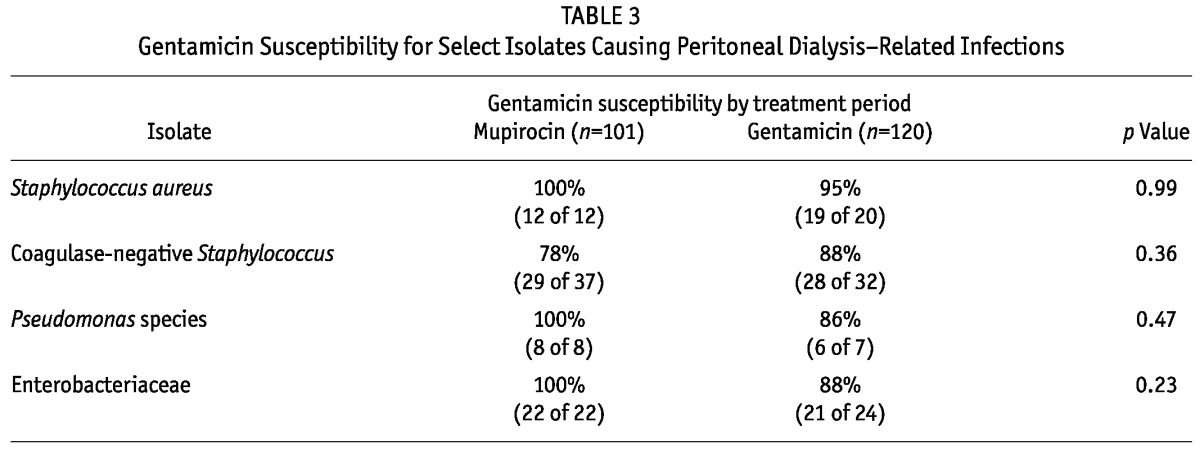
Although the GENT susceptibility of coagulase-negative Staphylococcus isolates improved to 88% from 78%, GENT susceptibility of S. aureus declined slightly from 100% to 96%. Among isolates from gram-negative organisms, those for Pseudomonas species and Enterobacteriaceae showed declining GENT susceptibility (by 14%, to 86% from 100%, and by 12%, to 88% from 100%, respectively).
DISCUSSION
The MUP and GENT creams both previously demonstrated efficacy in preventing ESIs attributable to gram-positive organisms (9-10). However, the spectrum of activity against gram-negative organisms is broader for GENT than for MUP. That activity is likely the reason that GENT was shown to be superior to MUP in preventing PD-related infections caused by gram-negative organisms (10).
The present investigation demonstrated a small decline in the rate of peritonitis and a 56% increase in the overall rate of ESI after conversion from MUP to GENT. Those findings are consistent with increased ESI rates seen by Chu and colleagues, who demonstrated a 90% increase in the ESI rate (11). However, our 14% decline in the rate of peritonitis is less than that found by Bernardini and colleagues, who reported a 35% decline in the rate of peritonitis (10). The change in our peritonitis rate reflects both a small increase in the rate of gram-positive infections and a larger decline in the rate of gram-negative infections. The increase in ESI overall was most likely influenced by the 83% increase in gram-positive ESI, which included a significant 150% increase in S. aureus ESI. Increases in gram-positive and S. aureus ESI could indicate inferior efficacy against S. aureus for GENT compared with MUP. Resistance to GENT is not likely to be the reason for this trend, as evidenced by the increase in coagulase-negative Staphylococcus susceptibility and the relatively small decrease in S. aureus susceptibility over the 5-year study period. Notably, the number of coagulase-negative Staphylococcus isolates was 15% lower after the protocol conversion.
The decline in gram-negative peritonitis might be expected, given the broader anti-gram-negative spectrum of GENT compared with MUP (12). However, that supposition is not consistent with the increase in gram-negative ESI in the present study. The rate of fungal infections (3 yeasts vs 9 yeasts and 1 mold) more than doubled from the MUP period to the GENT period, which is not consistent with the observations of either Sheth and colleagues (13) or Bernardini and colleagues (10). It might be speculated that the increase is the result of the selection of fungi in the presence of inhibition by broad-spectrum antimicrobials of both gram-positive and gram-negative organisms.
The decline in intravenous and intraperitoneal GENT use from the MUP period to the GENT period makes our results more impressive, given that the lesser use of GENT by other routes of administration may have prevented a further decline in GENT susceptibility.
The GENT susceptibility rate increased for coagulase-negative Staphylococcus, while it declined to 88% from 100% for Enterobacteriaceae and to 86% from 100% for Pseudomonas. While not statistically significant, those changes in susceptibility are concerning, given the other reports of antimicrobial resistance to prophylactic GENT use both in the burn population (5,6) and in hemodialysis patients using GENT in catheter lock solutions (7). A trend toward greater resistance could limit the usefulness of systemic aminoglycosides in the treatment of peritonitis.
Although our study examined 550 patient-years of experience with PD, it has several limitations, including co-intervention bias and recall bias. This single-center retrospective cohort study could not control for multiple factors. For example, the use of systemic aminoglycosides in the treatment of peritonitis, and other possible sources of co-intervention bias such as other changes in exit-site care intervention over time, could not be practically controlled for. Although the protocol recommended the use of prophylactic cream (MUP or GENT) with daily exit-site care, the actual documentation of prescription for both MUP and GENT creams was available in only about 60% of patients in each period, and compliance with exit-site care could not be evaluated. Given that our study depended on a review of medical records, there may have been instances of incomplete records causing underreporting or overreporting of infections. Our investigation also used a more stringent definition of ESI and peritonitis than may have been used clinically at the time of diagnosis. The change from microbroth dilution to the automated MicroScan susceptibility system occurred 6 months into a 60-month study; but because both methods are validated for susceptibility testing, it is unlikely that the change would have had a significant effect on the study results. Also, routine MUP susceptibility testing was not conducted; the baseline MUP resistance rate is therefore unknown, as is the rate of MUP resistance after conversion. Nonsignificant findings could have been a result of the study being underpowered to detect differences. To achieve adequate statistical power for these analyses, a multicenter study would have been needed.
CONCLUSIONS
Compared with the routine use of MUP cream, use of GENT cream was shown to be at least as safe and efficacious in preventing peritonitis, but significant increases in the ESI rate and declines in the GENT susceptibility for both Enterobacteriaceae and Pseudomonas are concerning trends. Centers should examine trends in infection rates and bacterial susceptibilities to determine the most appropriate agent for ESI prophylaxis.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
Acknowledgments
The authors acknowledge the contributions of Angela Graham, RN; Joan Gustufson, RN; and Michelle Wilson of Wake Forest University Outpatient Dialysis centers.
REFERENCES
- 1. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31 [PubMed] [Google Scholar]
- 2. Gupta B, Bernardini J, Piraino B. Peritonitis associated with exit site and tunnel infections. Am J Kidney Dis 1996; 28:415–19 [DOI] [PubMed] [Google Scholar]
- 3. Pérez-Fontán M, Rosales M, Rodríguez-Carmona A, Falcón TG, Valdés F. Mupirocin resistance after long-term use for Staphylococcus aureus colonization in patients undergoing chronic peritoneal dialysis. Am J Kidney Dis 2002; 39:337–41 [DOI] [PubMed] [Google Scholar]
- 4. Annigeri R, Conly J, Vas S, Dedier H, Prakashan KP, Bargman JM, et al. Emergence of mupirocin-resistant Staphylococcus aureus in chronic peritoneal dialysis patients using mupirocin prophylaxis to prevent exit-site infection. Perit Dial Int 2001; 21:554–9 [PubMed] [Google Scholar]
- 5. Ramirez AT, Melendres FA, Capili CA. Pseudomonas resistance to gentamicin. Scand J Plast Reconstr Surg 1979; 13:69–71 [DOI] [PubMed] [Google Scholar]
- 6. Bridges K, Kidson A, Lowbury EJ, Wilkins MD. Gentamicin- and silver-resistant Pseudomonas in a burns unit. Br Med J 1979; 1:446–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Landry DL, Braden GL, Gobeille SL, Haessler SD, Vaidya CK, Sweet SJ. Emergence of gentamicin-resistant bacteremia in hemodialysis patients receiving gentamicin lock catheter prophylaxis. Clin J Am Soc Nephrol 2010; 5:1799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. Twenty-first informational supplement. CLSI document M100-S21 Wayne, PA: CLSI; 2011. [PubMed] [Google Scholar]
- 9. Thodis E, Bhaskaran S, Pasadakis P, Bargman JM, Vas SI, Oreopoulos DG. Decrease in Staphylococcus aureus exit-site infections and peritonitis in CAPD patients by local application of mupirocin ointment at the catheter exit site. Perit Dial Int 1998; 18:26–70 [PubMed] [Google Scholar]
- 10. Bernardini J, Bender F, Florio T, Sloand J, Palmmontalbano L, Fried L, et al. Randomized, double-blind trial of antibiotic exit site cream for prevention of exit site infection in peritoneal dialysis patients. J Am Soc Nephrol 2005; 16:539–45 [DOI] [PubMed] [Google Scholar]
- 11. Chu KH, Choy WY, Cheung CC, Fung KS, Tang HL, Lee W, et al. A prospective study of the efficacy of local application of gentamicin versus mupirocin in the prevention of peritoneal dialysis catheter-related infections. Perit Dial Int 2008; 28:505–8 [PubMed] [Google Scholar]
- 12. Sutherland R, Boon RJ, Griffin KE, Masters PJ, Slocombe B, White AR. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicob Agents Chemother 1985; 27:495–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheth HS, Bernardini J, Bender F, Piraino B. Follow-up evaluation of gentamicin cream prophylaxis for peritoneal dialysis catheter infection (Abstract). Perit Dial Int 2007; 27(Suppl 3):S26 [Google Scholar]


