Abstract
♦ Background: Since the mid-1990s, early dialysis initiation has dramatically increased in many countries. The Initiating Dialysis Early and Late (IDEAL) study demonstrated that, compared with late initiation, planned early initiation of dialysis was associated with comparable clinical outcomes and increased health care costs. Because residual renal function is a key determinant of outcome and is better preserved with peritoneal dialysis (PD), the present pre-specified subgroup analysis of the IDEAL trial examined the effects of early-compared with late-start dialysis on clinical outcomes in patients whose planned therapy at the time of randomization was PD.
♦ Methods: Adults with an estimated glomerular filtration rate (eGFR) of 10 - 15 mL/min/1.73 m2 who planned to be treated with PD were randomly allocated to commence dialysis at an eGFR of 10 - 14 mL/min/1.73 m2 (early start) or 5 - 7 mL/min/1.73 m2 (late start). The primary outcome was all-cause mortality.
♦ Results: Of the 828 IDEAL trial participants, 466 (56%) planned to commence PD and were randomized to early start (n = 233) or late start (n = 233). The median times from randomization to dialysis initiation were, respectively, 2.03 months [interquartile range (IQR):1.67 - 2.30 months] and 7.83 months (IQR: 5.83 - 8.83 months). Death occurred in 102 early-start patients and 96 late-start patients [hazard ratio: 1.04; 95% confidence interval (CI): 0.79 - 1.37]. No differences in composite cardiovascular events, composite infectious deaths, or dialysis-associated complications were observed between the groups. Peritonitis rates were 0.73 episodes (95% CI: 0.65 - 0.82 episodes) per patient-year in the early-start group and 0.69 episodes (95% CI: 0.61 - 0.78 episodes) per patient-year in the late-start group (incidence rate ratio: 1.19; 95% CI: 0.86 - 1.65; p = 0.29). The proportion of patients planning to commence PD who actually initiated dialysis with PD was higher in the early-start group (80% vs 70%, p = 0.01).
♦ Conclusion: Early initiation of dialysis in patients with stage 5 chronic kidney disease who planned to be treated with PD was associated with clinical outcomes comparable to those seen with late dialysis initiation. Compared with early-start patients, late-start patients who had chosen PD as their planned dialysis modality were less likely to commence on PD.
Keywords: Dialysis timing, mortality, outcomes, peritonitis
Since the mid-1990s, early dialysis initiation has increased dramatically in many countries. For example, between 1996 and 2008, the proportion of patients with end-stage kidney disease commencing dialysis in the United States with an estimated glomerular filtration rate (eGFR) greater than 10 mL/min/1.73 m2 increased to 55% from 25% in those with diabetes and to 48% from 16% in those without diabetes (1).
Recently, the Initiating Dialysis Early and Late (IDEAL) randomized controlled trial demonstrated, in 828 patients with stage 5 chronic kidney disease (CKD), that planned early initiation of any form of dialysis at a GFR between 10 mL/min/1.73 m2 and 14 mL/min/1.73 m2—compared with late dialysis commencement (GFR < 7 mL/min/1.73 m2 or when traditional clinical indicators of uremia supervene)—was not associated with improved survival, cardiovascular events, infectious events, dialysis-associated complications, or quality of life (2,3), but was associated with increased health care costs (3).
Because peritoneal dialysis (PD)—compared with hemodialysis (HD) (4-6) and, in one study, compared with the pre-dialysis period (7)—has been shown to be associated with a slower decline of residual renal function, and because residual renal function is a significant predictor of clinical outcome (8-12), it is conceivable that the IDEAL trial results may have differed in PD patients compared with the overall population. The aim of the present pre-planned subgroup analysis was to evaluate the effects of early- and late-start dialysis on clinical outcomes in patients whose planned therapy at the time of randomization was PD.
METHODS
The study design and methodology have previously been described (2,13). The study was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12609000266268), and the study protocol was approved by ethics committees at all participating centers. All patients provided written informed consent before trial participation.
PARTICIPANTS
The IDEAL trial included adult patients (≥18 years) with progressive CKD (including a failing kidney transplant) and a calculated GFR between 10 mL/min/1.73 m2 and 15 mL/min/1.73 m2 from 32 centers in Australia and New Zealand. The GFR was estimated from serum creatinine measured at local laboratories using the Cockcroft-Gault equation (14) corrected for body surface area (15). Patients were excluded if a kidney transplant from a live donor was planned for them within the subsequent 12 months, if they had a recent malignancy that was likely to affect survival, or if they were unable to provide written informed consent. The present pre-specified substudy included all participants in the IDEAL trial who were planned for PD initiation at the time of randomization, regardless of whether they actually commenced PD later in the study.
STUDY TREATMENT
Participants were randomized 1:1 either to commence dialysis at a GFR of 10 - 14 mL/min/1.73 m2 or to continue routine medical care and commence dialysis at a GFR of 5 - 7 mL/min/1.73 m2. Randomization was performed centrally by a computer-based randomization service (Clinical Trials Research Unit, University of Auckland, New Zealand) using a permuted block design stratified by centre, planned dialysis modality (HD or PD), and the presence or absence of diabetes mellitus. Planned dialysis modality was specified before randomization, although the dialysis modality and regimen ultimately prescribed remained the choice of the patient and the treating physician. It was recommended that all patients receive dietary advice, anemia and phosphate management, treatment of hypertension, and achievement of dialysis small-solute clearance targets as recommended by contemporary guidelines (16-20).
STUDY OUTCOMES
The primary outcome measure was all-cause mortality. Secondary outcomes included cardiovascular events, infectious events, and dialysis complications.
STATISTICAL ANALYSIS
Results are expressed as frequencies and percentages for categorical variables, mean ± standard deviation for continuous normally distributed variables, and median with range for continuous non-normally distributed variables. Dichotomous and categorical data were compared using the chi-square test. Continuous normally distributed data were compared using the two-tailed unpaired t-test. Continuous non-normally distributed data were compared using the Mann-Whitney test. For the primary outcome of all-cause mortality, survival estimates and curves were generated according to the Kaplan-Meier method. All patients were followed until death or the trial end date, with censoring for transplantation and loss to follow-up. A Cox model adjusted for baseline covariates was estimated to investigate associations with outcome. All survival analyses were performed on an intention-to-treat basis. For the primary outcome measure of all-cause mortality, “per-protocol” sensitivity analyses (among patients planned to start with PD, only early-start patients who started dialysis early and late-start patients who started dialysis late were analyzed) and “as-treated” sensitivity analyses (patients started on PD in the early- and late-start groups were analyzed regardless of their original planned dialysis modality) were also performed. The analyses were performed using the SAS (version 9.1.3: SAS Institute, Cary, NC, USA) and R (version 2.8.1: The R Foundation for Statistical Computing, Vienna, Austria) software applications. Values of p less than 0.05 were considered statistically significant.
RESULTS
PATIENT CHARACTERISTICS
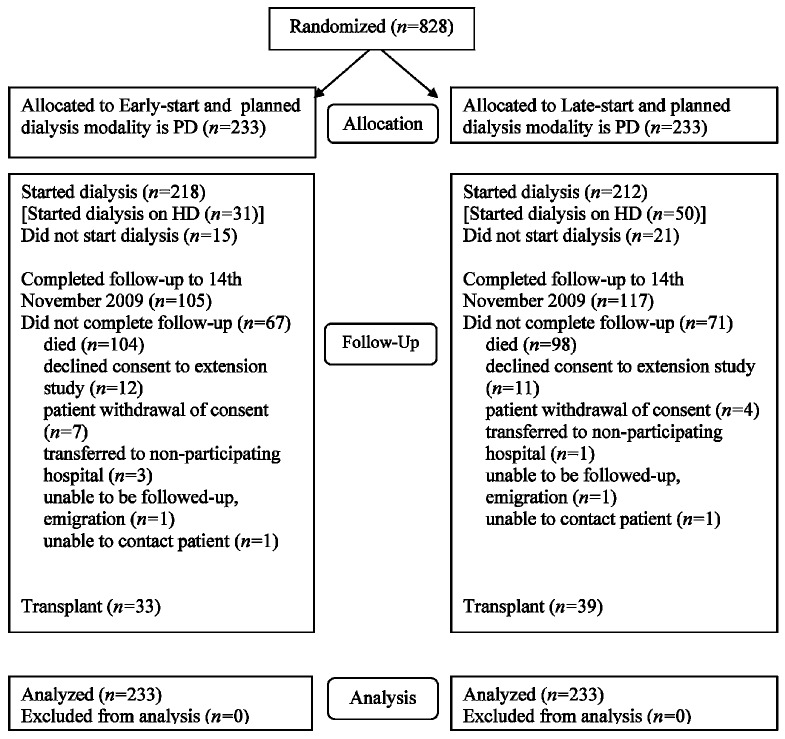
Of the 828 patients who participated in the IDEAL trial, 466 (56%) were planned to initiate with PD at the time of randomization. Those patients were randomized to receive either early-start (n = 233) or late-start (n = 233) dialysis between July 2000 and November 2008 and were followed until November 2009 (Figure 1). Most of the patients planning to commence PD (n = 349, 75%) actually commenced PD, although the proportion was significantly higher in the early-start group (n = 187, 80%) than in the late-start group (n = 162, 70%; p = 0.01). Of patients who started on PD, the proportions starting on continuous ambulatory PD and automated PD were identical in the early-start (n = 168, 90%, and n = 19, 10% respectively) and late-start (n = 146, 90%, and n = 16, 10%) groups (p = 0.93). The number of patients not starting dialysis in each group was 15 (6%) and 21 (9%) respectively (p = 0.4).
Figure 1.
— CONSORT diagram showing the number of patients with peritoneal dialysis (PD) as their planned dialysis modality recruited into the IDEAL study, randomized, followed, and analyzed. HD = hemodialysis.
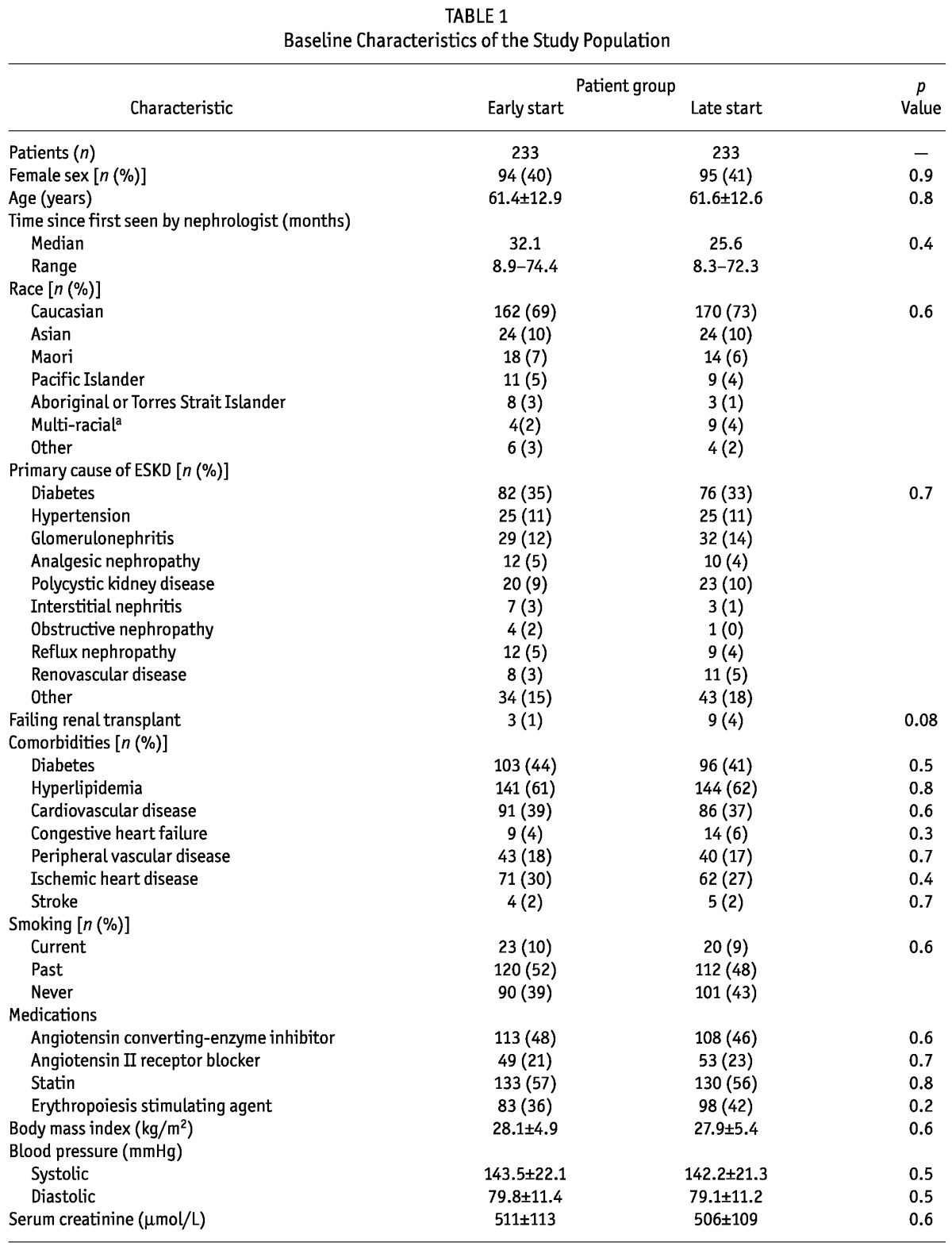
The early- and late-start groups were well matched with respect to all baseline characteristics (Table 1). The median length of follow-up was 3.36 years overall [interquartile range (IQR): 1.82 - 4.91 years], 3.42 years (IQR: 1.69 - 4.90 years) in the early-start group, and 3.12 years (IQR: 2.02 - 4.94 years) in the late-start group. The duration spent on PD was 2.53 ± 1.74 years in the early-start group and 2.2 ± 1.33 years in the late-start group (p = 0.06).
TABLE 1.
Baseline Characteristics of the Study Population
DIALYSIS COMMENCEMENT
The median time to dialysis initiation after randomization was 2.03 months (IQR: 1.67 - 2.30 months) in the early-start group and 7.83 months (IQR: 5.83 - 8.83 months) in the late-start group (p < 0.001, Figure 2). At the start of dialysis, the mean Cockcroft-Gault eGFR was 11.86 ± 3.17 mL/min/1.73 m2 in the early-start group and 9.86 ± 3.09 mL/min/1.73 m2 in the late-start group (p < 0.001). Those values equate to MDRD (Modification of Diet in Renal Disease) eGFR values of 9.07 ± 3.31 mL/min/1.73 m2 in the early-start group and 7.44 ± 2.91 mL/min/1.73 m2 in the late-start group (p < 0.001).
Figure 2.
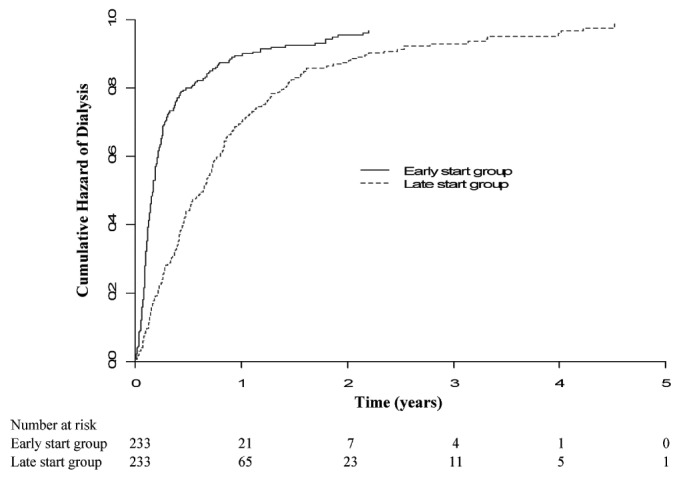
— Kaplan-Meier cumulative hazard plot of time to dialysis initiation after randomization in the early- and late-start groups. The difference between the groups was statistically significant (log rank score: 58.3; p < 0.001).
PRIMARY OUTCOME
A total of 198 deaths occurred during the follow-up period (102 in the early-start group, 96 in the late-start group). Overall survival was comparable between the early-start and late-start groups [hazard ratio (HR): 1.04; 95% CI: 0.79 - 1.37 (late-start as reference); Figure 3]. In the per-protocol sensitivity analysis, whereby only early-start patients who started dialysis early and late-start patients who started dialysis late were analyzed, survival was not significantly different between the groups (HR: 1.49; 95% CI: 0.80 - 2.75). An as-treated sensitivity analysis was also performed using 195 patients in the early-start group and 176 patients in the late-start group who started PD after randomization regardless of their original planned dialysis modality. Again, no difference in survival was observed (HR: 1.14; 95% CI: 0.83 - 1.58).
Figure 3.
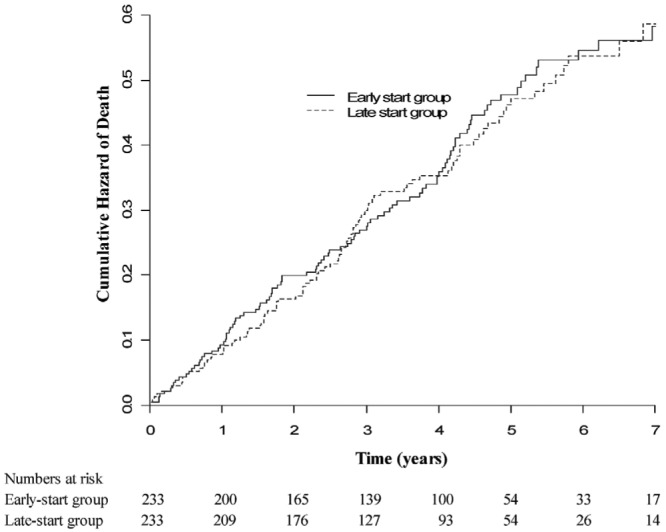
— Kaplan-Meier curves for overall patient survival in the early- and late-start groups. No significant difference was observed between the groups (log rank score: 0.07; p = 0.8).
SECONDARY OUTCOMES
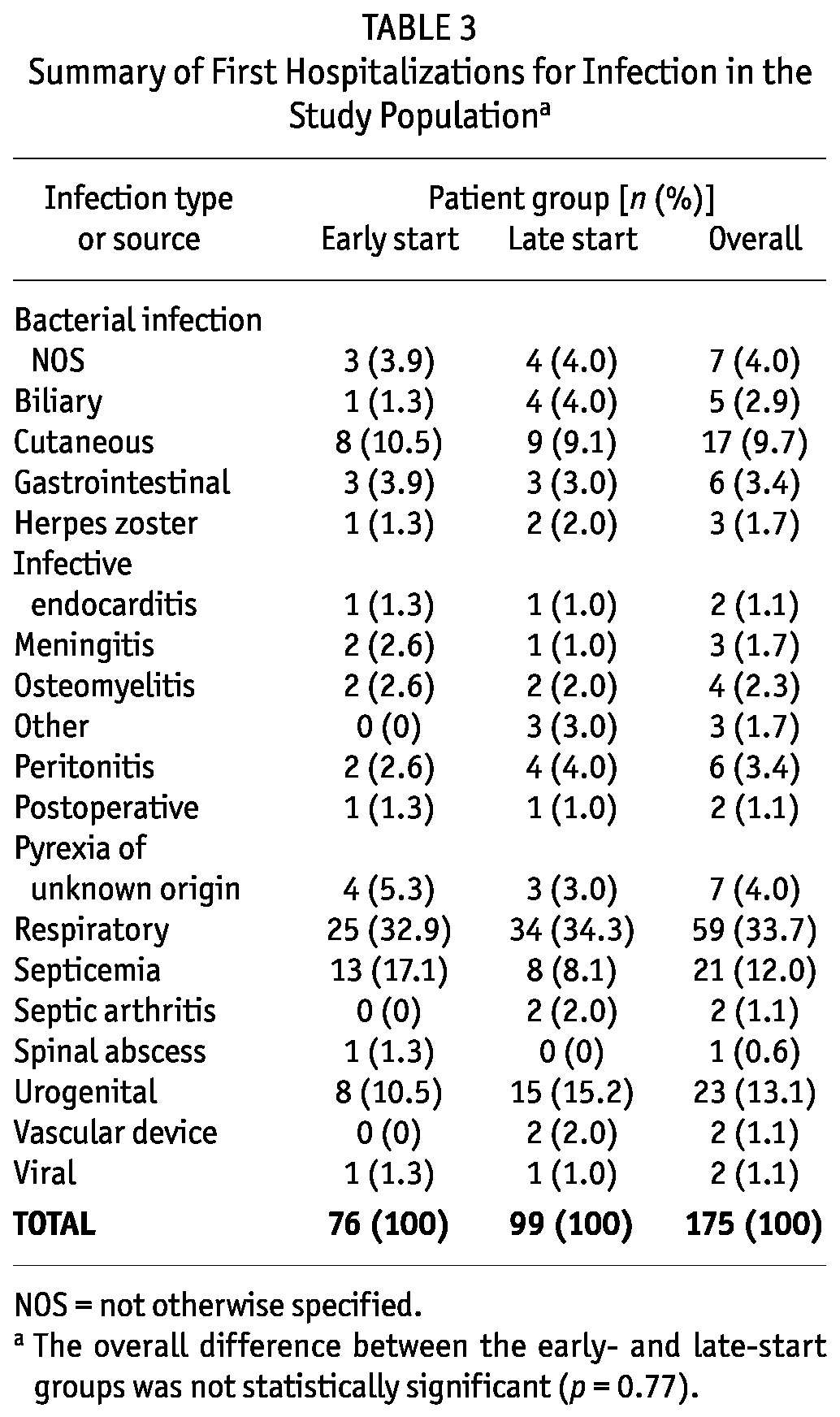
No differences in time to first cardiovascular events, composite infectious events, infectious deaths, or dialysis-associated complications were observed between the early-start and late-start groups (Table 2). However, patients in the early-start group had a significant 27% lower risk of hospitalization from infection (HR: 0.73; 95% CI: 0.54 - 0.98). No difference was observed in the risk of access infection between the groups (HR: 1.19; 95% CI: 0.68 - 2.07). The causes of hospitalization for infection in the early- and late-start groups were not significantly different (Table 3).
TABLE 2.
Primary and Secondary Outcomes
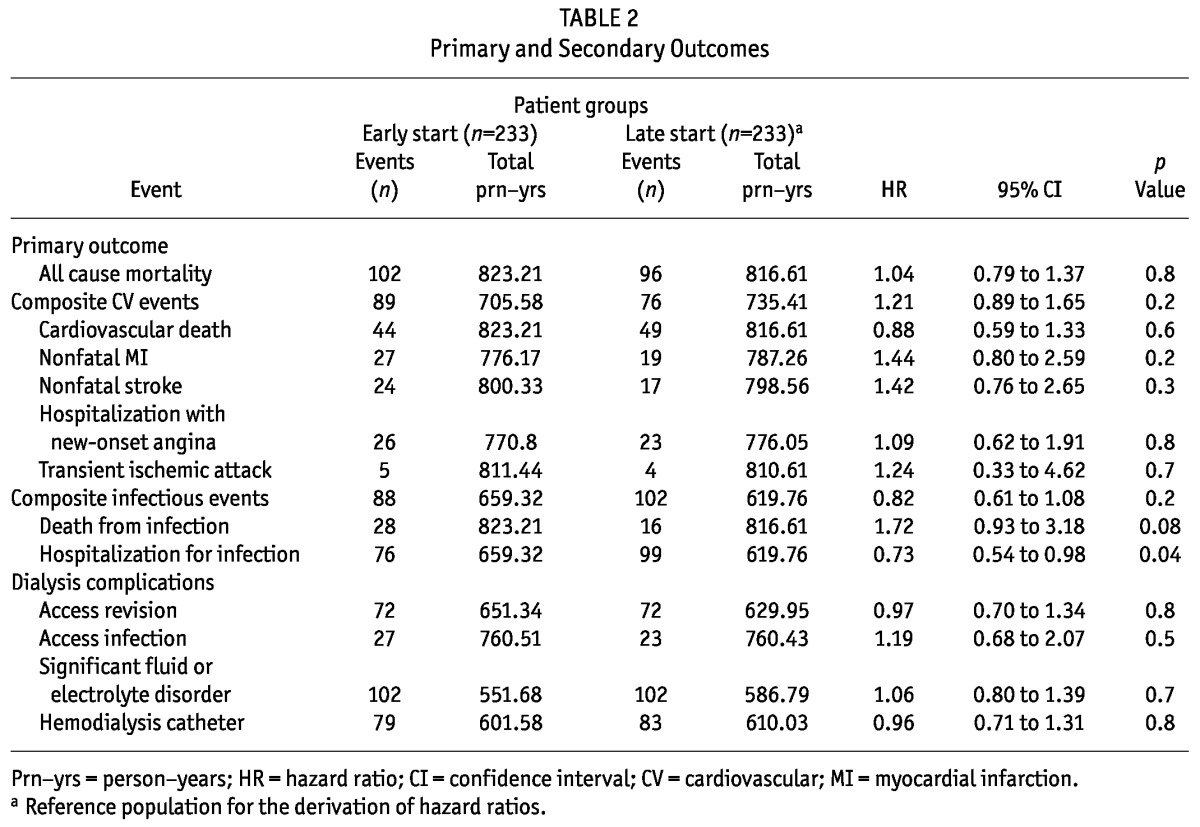
TABLE 3.
Summary of First Hospitalizations for Infection in the Study Populationa
In the as-treated sensitivity analysis, patients in the early-start group had a lower incidence of first hospitalization for infection than did patients in the late-start group (HR: 0.70; 95% CI: 0.50 - 0.99), although no differences were observed between the groups with respect to first composite infectious events (HR: 0.80; 95% CI: 0.58 - 1.10), infectious deaths (HR: 1.52; 95% CI: 0.77 - 3.00), or first dialysis access infections (HR: 1.36; 95% CI: 0.69 - 2.67). The hazard of first composite cardiovascular events was higher in the early-start group (HR: 1.52; 95% CI: 1.05 - 2.18). No differences were observed between the groups for dialysis access revision (HR: 0.92; 95% CI: 0.63 - 1.34) or fluid and electrolyte disorders (HR: 1.08; 95% CI: 0.80 - 1.47).
PERITONITIS
Overall, 293 episodes of peritonitis occurred in 117 individuals in the early-start group, and 237 episodes of peritonitis occurred in 111 individuals in the late-start group. Peritonitis rates were 0.73 episodes (95% CI: 0.65 - 0.82 episodes) per patient-year in the early-start group and 0.69 episodes (95% CI: 0.61 - 0.78 episodes) per patient-year in the late-start group. The incident rate ratio for peritonitis in the early-start group compared with the late-start group was 1.19 (95% CI: 0.86 - 1.65; p = 0.29).
DISCUSSION
This substudy analysis of the IDEAL trial demonstrated that, in patients with stage 5 CKD who planned to commence PD at the time of randomization, early commencement of dialysis (compared with late commencement) was not associated with a significant difference in survival. We also observed no significant differences between the groups with respect to the secondary outcomes of composite cardiovascular events, infectious events, and dialysis complications. Peritonitis rates were also not significantly different between the groups. Those findings mirror the findings of the main IDEAL trial (2) and suggest that early commencement of dialysis in patients planning to start on PD does not confer a significant clinical benefit.
The findings of the present study differ from those of previous observational cohort studies, which suggested that early dialysis commencement was associated with important clinical benefits (21-28). For example, in the CANUSA study (29), the 2-year survival of PD patients with a measured weekly GFR of at least 38 L (average of urinary urea and creatinine clearances) at dialysis commencement was significantly superior to that of PD patients with a measured weekly GFR of less than 38 L (82.1% vs 73.6%, p = 0.01). When the CANUSA investigators applied a multivariate Cox proportional hazards model analysis, adjusting for age, diabetes, cardiovascular disease, country of treatment, and serum albumin concentration, each 5-L increase in weekly GFR at dialysis initiation was associated with a 5% reduction in the hazard for death (95% CI: 1% - 9%) (29). The CANUSA study also reported that a higher level of renal function at PD commencement was associated with improved nutrition status (30). In contrast, more recent studies have suggested that early dialysis commencement is associated with harm (31-35). However, all of the foregoing studies were potentially limited by selection, lead-time, and referral biases, which were not operative in the IDEAL randomized controlled trial.
One observed difference between the present substudy and the main IDEAL trial was that, compared with late-start patients, early-start patients in the present analysis had a significant 27% lesser risk of first hospitalization for infection (p = 0.04). That finding was not accounted for by differences in rates of peritonitis, access infection, or HD catheter insertions between the groups. Moreover, the groups showed no significant differences with respect to the causes of infection necessitating first hospitalization or the composite outcome measure of infection-related deaths and hospitalization. It is conceivable that the finding of decreased first hospitalizations for infection in the early-start group occurred by random chance (type 1 statistical error), given that no adjustment was made for multiple statistical comparisons in the secondary outcome analyses.
Another interesting finding of the present study is that early initiation of dialysis in patients originally planning to start on PD was associated with a higher probability of actually commencing PD (80% vs 70% in late-start patients, p = 0.01). That result did not appear to be accounted for by an increased probability of emergent dialysis initiation without permanent PD access in the late-start group, because the occurrence of HD catheter insertions in the two groups was comparable. All study participants had been referred to renal units at an early stage (GFR > 10 mL/min/1.73 m2), had received pre-dialysis education about renal replacement therapy options, and were able to specify PD as their planned dialysis option at the time of randomization. However, the participants randomly allocated to the late-start group were less likely than their early-start counterparts to initiate PD, either because of changes in patient or clinician preference over the longer course of the pre-dialysis phase or because of a reduced propensity for units to proceed with PD training in late-start patients who were symptomatically uremic. Those results contrast with the results in IDEAL participants planning to start HD at randomization, whereby the proportions actually starting HD were comparable and high in the early- and the late-start group alike (92% and 86% respectively). Indeed, the proportion of patients planning to start HD at the time of randomization who subsequently changed their minds and started on PD instead was only 5% in both the early-start and late-start groups (p = 0.76). Although previous studies have reported that 30% - 50% of end-stage renal failure patients who receive pre-dialysis education will choose PD (36-38), only a fraction of such patients will actually start on PD (39,40). Those results suggest that greater effort may be required to fully support and resource a patient’s decision to undertake PD, particularly during the more protracted pre-dialysis phase associated with late-start dialysis.
To maintain the intention-to-treat analysis, the present investigation focused on planned PD treatment at the time of randomization rather than actual PD commencement. Randomization was stratified according to the planned dialysis modality so that the numbers of patients intending to commence PD were balanced between the early- and late-start groups. The per-protocol and as-treated sensitivity analyses of early and late dialysis initiation both showed similar results.
The strengths of the present study are that it included a variety of centers (metropolitan, regional, and rural; general and university) across two countries with long experience in PD and relatively high PD penetration rates. Those strengths should be balanced against the study’s limitations, which include use of the Cockcroft-Gault equation rather than the MDRD formula for estimating GFR. The Cockcroft-Gault equation was in more widespread use at the time the IDEAL trial was designed. Protocol violations, in which most late-start patients commenced dialysis at a GFR above 7 mL/min/1.73 m2, reduced the mean GFR difference between the early- and late-start groups to 2 mL/min/1.73 m2, thereby potentially biasing the trial results in favor of the null hypothesis. Nevertheless, that difference translated into a statistically significant and clinically meaningful average delay in dialysis initiation of 6 months between the early- and late-start groups. Finally, 25% of planned PD initiations actually culminated in HD starts.
CONCLUSIONS
Early initiation of dialysis in IDEAL trial participants with stage 5 CKD who planned to commence PD at the time of randomization was associated with clinical outcomes—including survival, composite cardiovascular events, composite infectious events, and dialysis complications—comparable to those in a late-start group. Compared with early-start patients, late-start patients were significantly more likely to change their planned dialysis modality from PD to HD.
DISCLOSURES
DWJ has received consulting fees from Baxter Healthcare, Amgen, Roche, Abbott, Boehringer Ingelheim, Lilley, and AstraZeneca; grant support from Baxter Healthcare and Fresenius Medical Care; speaker’s honoraria from Baxter Healthcare, Fresenius Medical Care, Amgen, Shire, Roche, Merck Sharpe and Dohme, and Janssen-Cilag; payment for development of educational presentations from Baxter Healthcare, Shire, and Janssen-Cilag; and travel support from Amgen, Baxter Healthcare, Fresenius Medical Care, and Roche. DCH has received consulting fees and travel support from Amgen Australia. CAP has received consulting fees from Amgen, lecture fees from Amgen and Baxter Healthcare, payment for development of educational presentations from Amgen and Baxter Healthcare, and travel support from Amgen. The remaining authors have no financial conflicts of interest to declare.
Acknowledgments
The invaluable assistance provided by Avinesh Pillai with regard to all statistical analyses in this study is gratefully acknowledged.
The IDEAL study was an investigator-initiated and conducted study, funded by the following sources: National Health and Medical Research Council of Australia (grant numbers 211146 and 465095); Australian Health Ministers Advisory Council (grant number PDR 2001/10); Royal Australasian College of Physicians/Australian and New Zealand Society of Nephrology (Don and Lorraine Jacquot Fellowship), 2001; National Heart Foundation (Australia), 2003; National Heart Foundation (New Zealand), 2003; and unrestricted grants from Baxter Healthcare Corporation, the Health Funding Authority New Zealand (Te Mana Putea Hauora O Aotearoa), the International Society for Peritoneal Dialysis, Amgen Australia Pty Ltd., and Janssen Cilag Pty Ltd.
REFERENCES
- 1. Rosansky S, Glassock RJ, Clark WF. Early start of dialysis: a critical review. Clin J Am Soc Nephrol 2011; 6:1222–8 [DOI] [PubMed] [Google Scholar]
- 2. Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363:609–19 [DOI] [PubMed] [Google Scholar]
- 3. Harris A, Cooper BA, Li JJ, Bulfone L, Branley P, Collins JF, et al. Cost-effectiveness of initiating dialysis early: a randomized controlled trial. Am J Kidney Dis 2011; 57:707–15 [DOI] [PubMed] [Google Scholar]
- 4. Lysaght MJ, Vonesh EF, Gotch F, Ibels L, Keen M, Lindholm B, et al. The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans 1991; 37:598–604 [PubMed] [Google Scholar]
- 5. Canacarini G, Bunori G, Camerini C, Brasa S, Manili L, Maiorca R. Renal function recovery and maintenance of residual diuresis in CAPD and hemodialysis. Perit Dial Bull 1986; 6:77–9 [Google Scholar]
- 6. Holley JL, Aslam N, Bernardini J, Fried L, Piraino B. The influence of demographic factors and modality on loss of residual renal function in incident peritoneal dialysis patients. Perit Dial Int 2001; 21:302–5 [PubMed] [Google Scholar]
- 7. Berlanga JR, Marrón B, Reyero A, Caramelo C, Ortiz A. Peritoneal dialysis retardation of progression of advanced renal failure. Perit Dial Int 2002; 22:239–42 [PubMed] [Google Scholar]
- 8. Rumpsfeld M, McDonald SP, Johnson DW. Peritoneal small solute clearance is nonlinearly related to patient survival in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int 2009; 29:637–46 [PubMed] [Google Scholar]
- 9. Maiorca R, Brunori G, Zubani R, Cancarini GC, Manili L, Camerini C, et al. Predictive value of dialysis adequacy and nutritional indices for mortality and morbidity in CAPD and HD patients: a longitudinal study. Nephrol Dial Transplant 1995; 10:2295–305 [DOI] [PubMed] [Google Scholar]
- 10. Fung L, Pollock CA, Caterson RJ, Mahony JF, Waugh DA, Macadam C, et al. Dialysis adequacy and nutrition determine prognosis in continuous ambulatory peritoneal dialysis patients. J Am Soc Nephrol 1996; 7:737–44 [DOI] [PubMed] [Google Scholar]
- 11. Bargman JM, Thorpe KE, Churchill DN, and the CANUSA Peritoneal Dialysis Study Group. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12:2158–62 [DOI] [PubMed] [Google Scholar]
- 12. Davies SJ, Phillips L, Russell GI. Peritoneal solute transport predicts survival on CAPD independently of residual renal function. Nephrol Dial Transplant 1998; 13:962–8 [DOI] [PubMed] [Google Scholar]
- 13. Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Dempster J, et al. on behalf of the IDEAL Study Steering Committee. The Initiating Dialysis Early and Late (IDEAL) study: study rationale and design. Perit Dial Int 2004; 24:176–81 [PubMed] [Google Scholar]
- 14. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41 [DOI] [PubMed] [Google Scholar]
- 15. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 1916; 17:863–71 [PubMed] [Google Scholar]
- 16. Johnson D, Brown F, Lammi H, Walker R. on behalf of the Caring for Australians with Renal Impairment (CARI). The CARI guidelines. Dialysis adequacy (PD) guidelines. Nephrology (Carlton) 2005; 10(Suppl 4):S81–107 [DOI] [PubMed] [Google Scholar]
- 17. NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy. National Kidney Foundation. Am J Kidney Dis 1997; 30(Suppl 2):S67–136 [DOI] [PubMed] [Google Scholar]
- 18. Churchill DN, Blake PG, Jindal KK, Toffelmire EB, Goldstein MB. Clinical practice guidelines for initiation of dialysis. Canadian Society of Nephrology. J Am Soc Nephrol 1999; 10(Suppl 13):S289–91 [PubMed] [Google Scholar]
- 19. Pollock C, McMahon L. on behalf of Caring for Australians with Renal Impairment (CARI). The CARI guidelines. Biochemical and haematological targets guidelines. Haemoglobin. Nephrology (Carlton) 2005; 10(Suppl 4):S108–15 [DOI] [PubMed] [Google Scholar]
- 20. Pollock C, Voss D, Hodson E, Crompton C. on behalf of Caring for Australasians with Renal Impairment (CARI). The CARI guidelines. Nutrition and growth in kidney disease. Nephrology (Carlton) 2005; 10(Suppl 5):S177–230 [DOI] [PubMed] [Google Scholar]
- 21. Bonomini V, Baldrati L, Stefoni S. Comparative cost/benefit analysis in early and late dialysis. Nephron 1983; 33:1–4 [DOI] [PubMed] [Google Scholar]
- 22. Bonomini V, Albertazzi A, Vangelista A, Bortolotti GC, Stefoni S, Scolari MP. Residual renal function and effective rehabilitation in chronic dialysis. Nephron 1976; 16:89–102 [DOI] [PubMed] [Google Scholar]
- 23. Bonomini V, Feletti C, Scolari MP, Stefoni S. Benefits of early initiation of dialysis. Kidney Int Suppl 1985; 17:S57–9 [PubMed] [Google Scholar]
- 24. Jungers P, Zingraff J, Albouze G, Chauveau P, Page B, Hannedouche T, et al. Late referral to maintenance dialysis: detrimental consequences. Nephrol Dial Transplant 1993; 8:1089–93 [PubMed] [Google Scholar]
- 25. Jungers P, Zingraff J, Page B, Albouze G, Hannedouche T, Man NK. Detrimental effects of late referral in patients with chronic renal failure: a case-control study. Kidney Int Suppl 1993; 41:S170–3 [PubMed] [Google Scholar]
- 26. McCusker FX, Teehan BP, Thorpe KE, Keshaviah PR, Churchill DN. How much peritoneal dialysis is required for the maintenance of a good nutritional state? Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Kidney Int Suppl 1996; 56:S56–61 [PubMed] [Google Scholar]
- 27. Sesso R, Belasco AG. Late diagnosis of chronic renal failure and mortality on maintenance dialysis. Nephrol Dial Transplant 1996; 11:2417–20 [DOI] [PubMed] [Google Scholar]
- 28. Tattersall J, Greenwood R, Farrington K. Urea kinetics and when to commence dialysis. Am J Nephrol 1995; 15:283–9 [DOI] [PubMed] [Google Scholar]
- 29. Churchill DN. An evidence-based approach to earlier initiation of dialysis. Am J Kidney Dis 1997; 30:899–906 [DOI] [PubMed] [Google Scholar]
- 30. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol 1996; 7:198–207 [DOI] [PubMed] [Google Scholar]
- 31. Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Ramkumar N, Pappas LM, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol 2003; 14:2305–12 [DOI] [PubMed] [Google Scholar]
- 32. Kazmi WH, Gilbertson DT, Obrador GT, Guo H, Pereira BJ, Collins AJ, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis 2005; 46:887–96 [DOI] [PubMed] [Google Scholar]
- 33. Lassalle M, Labeeuw M, Frimat L, Villar E, Joyeux V, Couchoud C, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int 2010; 77:700–7 [DOI] [PubMed] [Google Scholar]
- 34. Stel VS, Dekker FW, Ansell D, Augustijn H, Casino FG, Collart F, et al. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant 2009; 24:3175–82 [DOI] [PubMed] [Google Scholar]
- 35. Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol 2002; 13:2125–32 [DOI] [PubMed] [Google Scholar]
- 36. Jager KJ, Korevaar JC, Dekker FW, Krediet RT, Boeschoten EW. on behalf of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group. The effect of contraindications and patient preference on dialysis modality selection in ESRD patients in the Netherlands. Am J Kidney Dis 2004; 43:891–9 [DOI] [PubMed] [Google Scholar]
- 37. Korevaar JC, Feith GW, Dekker FW, van Manen JG, Boeschoten EW, Bossuyt PM, et al. on behalf of NECOSAD Study Group. Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: a randomized controlled trial. Kidney Int 2003; 64:2222–8 [DOI] [PubMed] [Google Scholar]
- 38. Marrón B, Martínez Ocaña JC, Salgueira M, Barril G, Lamas JM, Martín M, et al. on behalf of the Spanish Group for CKD. Analysis of patient flow into dialysis: role of education in choice of dialysis modality. Perit Dial Int 2005; 25(Suppl 3):S56–9 [PubMed] [Google Scholar]
- 39. Liebman SE, Bushinsky DA, Dolan JG, Veazie P. Differences between dialysis modality selection and initiation. Am J Kidney Dis 2012; 59:550–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jose MD, Johnson DW, Mudge DW, Tranæus A, Voss D, Walker R, et al. Peritoneal dialysis practice in Australia and New Zealand: a call to action. Nephrology (Carlton) 2011; 16:19–29 [DOI] [PubMed] [Google Scholar]