Editor:
Treating hepatitis C virus (HCV) infection in patients on hemodialysis has become common (1,2), although according to current guidelines, the practice is supported only by low-quality evidence (3,4). In comparison, experience with HCV in patients on peritoneal dialysis (PD) has been very limited (5-7).
To our knowledge, there have been no reports on the pharmacokinetics of interferon (IFN) alpha in PD patients, nor on the ability of PD to prevent toxic IFN accumulation in the blood. Moreover, the relationship between blood IFN levels and treatment efficacy or toxicity (acute: doses up to 30×106 IU/kg subcutaneously, 250×106 IU/kg intravenously, and 500×106 IU/kg intramuscularly; subchronic: daily doses up to 25×106 IU/kg intramuscularly) has been evaluated mainly in studies using animal models (8,9), although IFN has been demonstrated to have species-restricted biologic activity and human IFN is antigenic for laboratory animals. Here, we report the case of a patient whose blood and peritoneal fluid IFN concentrations were monitored during conventional interferon monotherapy, to be used as an additional guide for dose modification.
A 55-year-old woman was referred for evaluation of chronic HCV infection before renal transplantation. Two years earlier, she had started automated PD (8 cycles with a fill volume of 1875 mL on a HomeChoice Pro cycler: Baxter Healthcare Corporation, Deerfield, IL, USA) for end-stage renal disease of undetermined origin (10). The patient had received blood transfusions at age 30 for complications of an ectopic pregnancy; 5 years later, HCV was diagnosed. Transaminases had always remained close to the upper limit of the reference range, and neither a liver biopsy nor antiviral treatment had ever been performed or suggested.
At physical examination, the patient’s appearance was of an obese woman in good general condition. The liver edge was palpable below the costal margin. Hemoglobin was 109 g/L, serum creatinine was 938 μmol/L with normal electrolytes, and liver biochemistry revealed no abnormalities. Viral studies demonstrated high viral load (serum HCV RNA 1 354 371 IU/mL), an easy-to-treat HCV genotype (2a/2c), and undetectable hepatitis B surface antigen.
At abdominal ultrasonography, the liver was enlarged, with a bright pattern consistent with steatosis. A liver biopsy, which may be hazardous in PD patients, was not performed; however, hepatic elastography was suggestive of severe fibrosis (liver stiffness: 14.1 kPa; METAVIR score: F4). The interleukin-28b rs12979860 genotype was C/T.
After approval was received from the local committee for off-label use of drugs and a written informed consent was signed, therapy with subcutaneous human recombinant IFN alpha-2a (Roferon-A: Roche, Milan, Italy) was started at an initial daily dose of 3×106 IU, for a scheduled duration of 24 weeks. That dose deliberately exceeded the usually recommended dose of standard IFN for the treatment of HCV-infected maintenance hemodialysis patients (3×106 IU thrice weekly) (3,4), because several predictors of viral nonresponse (high viral burden, advanced liver disease, obesity, menopause, and age > 50 years) were present (11).
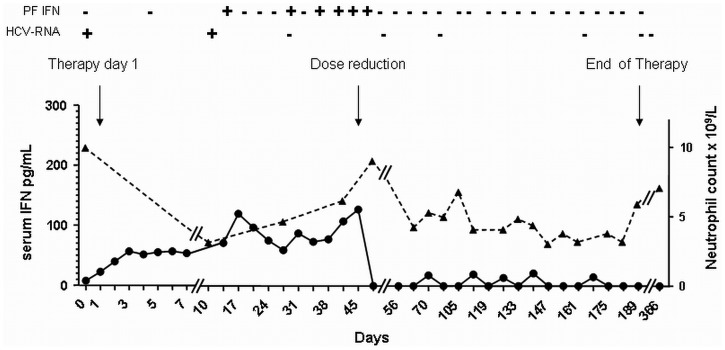
Concentrations of IFN alpha in blood and peritoneal fluid (collected in the morning, before the next IFN injection and after completion of the night dialysis session) were monitored using a commercially available enzyme immunoassay (Human IFN-alpha ELISA: Bender MedSystems, San Diego, CA, USA). As shown in Figure 1, IFN alpha was measurable in dialysate when trough serum levels exceeded 50 pg/mL. The percentage of the daily dose of IFN alpha-2a that was recovered in dialysate was on average less than 1%, assuming that 3×106 IU corresponded to 11.1 μg (information kindly provided by the manufacturer). Based on monitored data and tolerability [grade 3 fatigue at treatment week (TW) 6, hypotension with syncope at TW8, grade 3 anemia from TW11 to TW19], IFN was reduced to 3×106 IU on alternate days from TW7, according to the recommendations to tailor therapy based on patient response and tolerability (3).
Figure 1.
— Changes of serum interferon (IFN) alpha concentration (filled circles) in relation to variations in peritoneal fluid IFN concentration, serum hepatitis C virus (HCV) RNA. Modifications of IFN dose (arrows) and neutrophil count (filled triangles) during IFN treatment are also shown. + = detectable; - = undetectable; PF = peritoneal fluid.
Circulating HCV RNA turned negative at TW4 and remained undetectable thereafter. Liver stiffness was reduced to 6.1 kPa 24 weeks after treatment completion, and the patient was accepted to the renal transplantation waiting list.
This case report is the first to clearly document successful treatment of HCV infection by conventional IFN alpha-2a monotherapy in a PD patient. We chose to use conventional instead of pegylated IFN (whose longer half-life reduces flexibility for dose modification), delivering instead high IFN doses by daily subcutaneous administration. In our approach, symptoms of toxicity remained the pivotal clues for tailoring therapy. However, measurements of IFN alpha concentrations in blood and dialysate were intended to further increase patient safety, because IFN pharmacokinetics during automated PD could not be accurately predicted. Moreover, we aimed to document the ability of PD to clear IFN. The present case shows that IFN becomes detectable in peritoneal fluid only once high serum levels are reached, indicating that PD may clear some IFN alpha-2a, but only a very small amount. Monitoring IFN alpha concentration in dialysate may be a useful additional element in finding the right balance between efficacy and tolerability.
DISCLOSURES
All authors declare they have had no support or competing financial interests in relation to the work described.
REFERENCES
- 1. Terrault NA, Adey DB. The kidney transplant recipient with hepatitis C infection: pre- and posttransplantation treatment. Clin J Am Soc Nephrol 2007; 2:563–75 [DOI] [PubMed] [Google Scholar]
- 2. Rostaing L, Chatelut E, Payen JL, Izopet J, Thalamas C, Ton-That H, et al. Pharmacokinetics of alphaIFN-2b in chronic hepatitis C virus patients undergoing chronic hemodialysis or with normal renal function: clinical implications. J Am Soc Nephrol 1998; 9:2344–8 [DOI] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl 2008; (109):S1–99 [DOI] [PubMed] [Google Scholar]
- 4. Ghany MG, Strader DB, Thomas DL, Seeff LB. on behalf of the American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009; 49:1335–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan TM, Ho SK, Tang CS, Tse KC, Lam MF, Lai KN, et al. Pilot study of pegylated interferon-alpha 2a in dialysis patients with chronic hepatitis C virus infection. Nephrology (Carlton) 2007; 12:11–17 [DOI] [PubMed] [Google Scholar]
- 6. Mimura I, Ishibashi Y, Tateishi R, Kaname S, Fujita T. Pegylated interferon alpha-2a monotherapy in a peritoneal dialysis patient with chronic hepatitis C. NDT Plus 2008; 1:233–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tae HJ, Jun DW, Choi JW, Kim WJ, Kim BY, Sohn JH, et al. A case of pegylated interferon alpha-2a monotherapy in a peritoneal dialysis patient with chronic hepatitis C [Korean]. Korean J Gastroenterol 2011; 58:107–10 [DOI] [PubMed] [Google Scholar]
- 8. Gresser I. Biologic effects of interferons. J Invest Dermatol 1990; 95:66S–71S [DOI] [PubMed] [Google Scholar]
- 9. Trown PW, Wills RJ, Kamm JJ. The preclinical development of Roferon-A. Cancer 1986; 57:1648–56 [DOI] [PubMed] [Google Scholar]
- 10. Chou CY, Wang IK, Liu JH, Lin HH, Wang SM, Huang CC. Comparing survival between peritoneal dialysis and hemodialysis treatment in ESRD patients with chronic hepatitis C infection. Perit Dial Int 2010; 30:86–90 [PubMed] [Google Scholar]
- 11. Kau A, Vermehren J, Sarrazin C. Treatment predictors of a sustained virologic response in hepatitis B and C. J Hepatol 2008; 49:634–51 [DOI] [PubMed] [Google Scholar]