Abstract
♦ Background and Objectives: In automated peritoneal dialysis (APD), a patient’s peritoneal membrane is more intensively exposed to fresh dialysate than it is in continuous ambulatory peritoneal dialysis (CAPD). Our aim was to study, in incident peritoneal dialysis (PD) patients, the influence of APD—compared with that of CAPD—on peritoneal transport over 4 years.
♦ Design, Setting, Participants, and Measurements: Patients were included if at least 2 annual standard permeability analyses (SPAs) performed with 3.86% glucose were available while the patient was using the same modality with which they had started PD (APD or CAPD). Patients were followed until their first modality switch. Differences in the pattern of SPA outcomes over time were tested using repeated-measures models adjusted for age, sex, comorbidity, primary kidney disease, and year of PD start.
♦ Results: The 59 CAPD patients enrolled were older than the 47 APD patients enrolled (mean age: 58 ± 14 years vs 49 ± 14 years; p < 0.01), and they had started PD earlier (mean start year: 2000 vs 2002). Over time, no differences in solute (p > 0.19) or fluid transport (p > 0.13) were observed. Similarly, free water transport (p = 0.43) and small-pore transport (p = 0.31) were not different between the modalities. Over time, patients on APD showed a faster decline in effective lymphatic absorption rate (ELAR: p = 0.02) and in transcapillary ultrafiltration (TCUF: p = 0.07, adjusted p = 0.05). Further adjustment did not change the results.
♦ Conclusions: Compared with patients starting on CAPD, those starting on APD experienced a faster decline in ELAR and TCUF. Other transport parameters were not different over time between the groups.
Keywords: Automated peritoneal dialysis, continuous ambulatory peritoneal dialysis, effective lymphatic absorption rate, peritoneal transport, ultrafiltration, standard peritoneal permeability analysis
Automated peritoneal dialysis (APD) has become more and more popular as a peritoneal dialysis (PD) modality. Therefore, more attention is being paid to the possible disadvantages of APD. Several possible disadvantages have been studied: a faster decline in residual renal function, less sodium removal, more protein loss, and higher costs (1-11). However, the influence of APD on the time course of peritoneal transport in long-term PD is unknown.
Long-term alterations in peritoneal function have been studied in continuous ambulatory PD (CAPD) patients (12-17). From those studies, it is known that hypertonic glucose and its degradation products play a role in alterations of the peritoneal membrane, eventually causing alterations in peritoneal transport (12-17). Compared with CAPD, APD uses dwells that are shorter and more frequent. Given that the biocompatibility of a dialysis fluid increases during a dwell (18), the peritoneal membrane of an APD patient is more intensively exposed to bioincompatible dialysis solutions. Thus, compared with patients being treated with CAPD, those being treated with APD might have a higher risk of developing structural and functional changes of the peritoneum.
Peritoneal transport measured by means of a peritoneal function test has been compared between APD and CAPD in four studies that were either cross-sectional in nature or had a short follow-up (11,19-21). None of them compared the time course of peritoneal transport on the two modalities for longer than 16 months.
At the Academic Medical Center in Amsterdam, all PD patients undergo a standard permeability analysis (SPA) annually. This clinical practice made it possible to study the influence of APD compared with that of CAPD on peritoneal transport in incident PD patients over 4 years.
METHODS
PATIENTS
All incident PD patients at the Academic Medical Center in Amsterdam who started PD between June 1994 and August 2008 were eligible for inclusion. To be included, a patient had to have at least 1 SPA available within 2 years after the start of PD, and at least 2 SPAs available while using the same PD modality. Patients who had previously undergone kidney transplantation were excluded. Patient preference was the determinant for the choice between APD and CAPD (at our center, patients have a free choice of either modality). All patients were treated with continuous PD, and thus they had a peritoneal cavity containing dialysis solution during the entire follow-up period. No peritoneal resting was applied.
SPA DETAILS
In our PD unit, SPAs are performed annually in stable patients who have been peritonitis-free at least 4 weeks leading up to the procedure. The SPAs are performed in an outpatient setting, using a 4-hour dwell, as previously described in detail (22,23). In brief: The abdomen is first rinsed with dialysate containing 1.36% glucose, which is drained immediately after inflow is complete. Thereafter, 3.86% glucose dialysate containing 1 g/L dextran 70 (Hyskon: Pharmacia AB, Uppsala, Sweden) is instilled. Dextran 70 is added to the test solution to determine peritoneal fluid kinetics. Before the SPA, a blood sample is drawn, and 20 mL dextran 1 (Promiten: Gynotec, Malden, Netherlands) is administered intravenously to prevent possible anaphylaxis stemming from the dextran 70 (24). Dialysate samples are drawn at time point 0 and at 10, 20, 30, 60, 120, 180, and 240 minutes after inflow of the test solution. “Dead space” effect is avoided by the temporarily drainage of 100 - 200 mL before dialysate sampling. After drainage of the test solution at 240 minutes, the peritoneal cavity is again rinsed with a 1.36% glucose solution.
MEASUREMENTS
The total dextran concentration in the dialysate was determined by high performance liquid chromatography (25). Creatinine, urea, and urate were measured using an enzymatic method on an automated analyzer (Hitachi H911: Boehringer Mannheim, Mannheim, Germany). Elec trolytes were determined using indirect ion-selective methods. Albumin, immunoglobulin G, and α2-macroglobulin were measured by nephelometry (BN100: Behring, Marburg, Germany). A microparticle enzyme immunoassay on an IMx system (Abbott Diagnostics, Chicago, IL, USA) was used to measure β2-microglobulin. And in the final effluent, cancer antigen 125 (CA125) was measured by microparticle enzyme immunoassay using a monoclonal antibody (Abbott Laboratories).
CALCULATIONS AND STATISTICS
The data obtained from the SPA provide information on fluid kinetics; on the transport of low molecular weight solutes, proteins, and sodium; and on the appearance rate of CA125. All calculations have been described previously (23,26-29). Net ultrafiltration was calculated as the difference between the transcapillary ultrafiltration (TCUF) and the effective lymphatic absorption (ELAR) at test end. The restriction coefficient was defined as the slope of the power relation between the clearances of macromolecules with various molecular weights and their free diffusion coefficients in water (30-32).
The date of the first available SPA was chosen as baseline. Patients were followed until their last SPA, with a maximum follow-up of 4 years. Baseline characteristics are presented as means and standard deviations or as absolute numbers with percentages. Differences at baseline were tested using a t-test, a chi-square test, or the Fisher exact test, as appropriate. Differences between the groups in the time courses of the SPA parameters were tested using linear repeated-measures models, because this model type gives valid results under the assumption of missing at random. An interaction term for time and modality was included in the model to test differences in the time courses between the modalities. Thus, a significant interaction term implies that such a difference is present. The covariance structure of the model was chosen on the best fit according to the Akaike information criterion, and therefore a fixed model was used. Time was included in the model as a factor. Because age, sex, comorbidity, primary kidney disease, and year of PD start are considered possible confounders, all analyses were also adjusted for those variables. To gain more insight into the factors influencing outcomes, sensitivity analyses were performed for nonusers of icodextrin and Physioneal (Baxter Healthcare BV, Utrecht, Netherlands), for patients with a follow-up of at least 3 years, and for patients who developed ultrafiltration failure. Ultrafiltration failure was defined as an ultrafiltration of less than 400 mL during the test dwell (30). We chose sensitivity analyses instead of adjustments because the foregoing factors are not considered possible confounders. They might have influenced the outcome, but not the choice for either modality. All analyses were performed using the SPSS application for Windows (versions 16.0 and 17.0: SPSS, Chicago, IL, USA).
RESULTS
Table 1 presents the characteristics at baseline (first SPA) of the 59 CAPD and 47 APD patients who met the study inclusion criteria. Table 2 presents the mean SPA values for the two groups at baseline. Among the study patients, 5 started on CAPD and switched to APD within 4 months. The first SPA was performed within 4 months of PD start in 26 CAPD patients (44%) and in 24 APD patients (51%). The first SPA was performed more than 6 months after PD start in 8 CAPD patients (14%) and in 11 APD patients (23%). After 2 years, 35 CAPD patients (59%) and 29 APD patients (62%) were still on their original PD modality; after 3 years, the numbers were 16 (27%) and 15 (32%); and after 4 years, they were 11 (19%) and 6 (13%).
TABLE 1.
Baseline Characteristics of the Study Patients
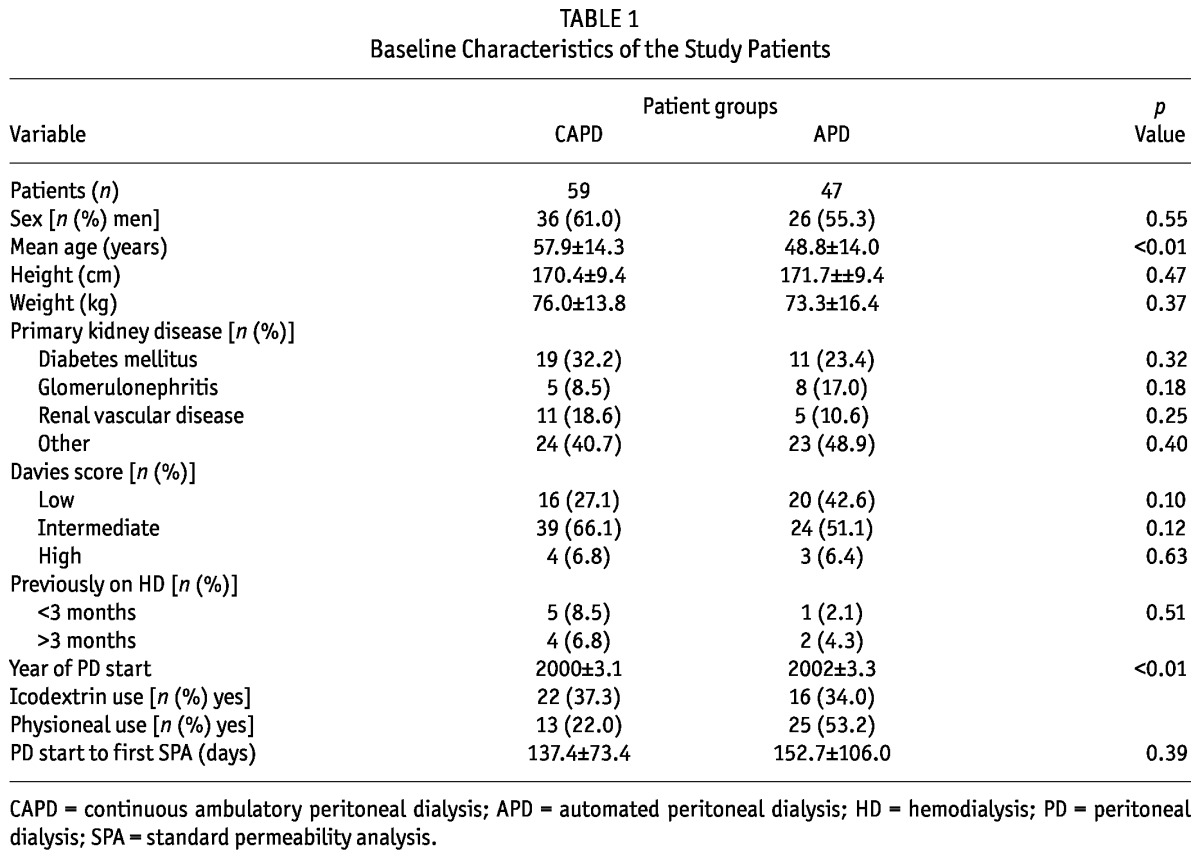
TABLE 2.
Baseline Values and Differences in Pattern Over Time Between the Study Groups
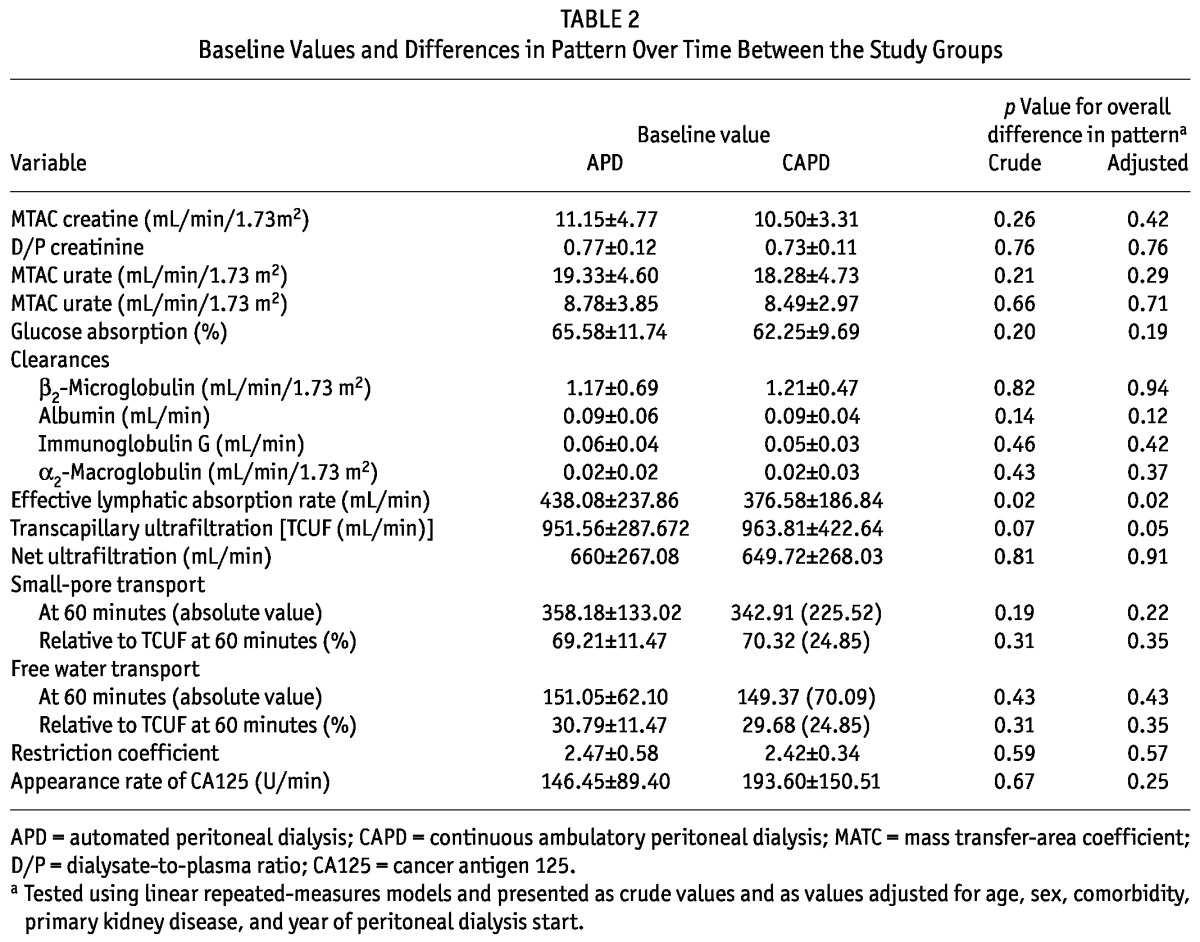
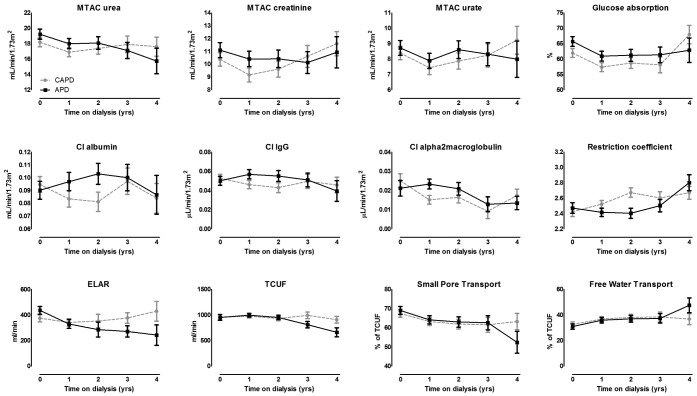
Figure 1 presents, for both modalities, the crude time courses of solute transport, fluid transport, ELAR, TCUF, small-pore transport, and free water transport. Table 2 presents the associated crude and adjusted p values representing differences in the pattern of the course of peritoneal transport between APD and CAPD.
Figure 1.
— Estimated means over time from the linear repeated-measures model for the various parameters of a standard permeability analysis in patients treated with continuous ambulatory peritoneal dialysis (CAPD, dashed gray lines) or automated peritoneal dialysis (APD, solid black lines). MTAC = mass transfer-area coefficient; Cl = clearance; IgG = immunoglobulin G; ELAR = effective lymphatic absorption rate; TCUF = transcapillary ultrafiltration.
The time course of most SPA parameters was not different between the modalities. However, patients on APD experienced a decline in ELAR over time, and those on CAPD remained at the same level (Figure 1, Table 2). Likewise, TCUF declined over time in the APD patients, but held at a continuous level over time in the CAPD patients (Figure 1, Table 2).
To study the influence of icodextrin and Physioneal use, we performed sensitivity analyses in nonusers of both PD fluids. In non-users of icodextrin, the analysis included 33 CAPD (56%) and 30 APD (64%) patients. After 3 years, 8 patients on CAPD (14%) and 8 patients on APD (17%) were still included in the analysis. As in the main analysis, only the ELAR (crude and adjusted p = 0.003) and TCUF (crude p = 0.01, adjusted p = 0.02) showed differences over time between the modalities. In nonusers of Physioneal, the analysis included 42 CAPD patients (71%) and 21 APD patients (45%). Here, the crude analyses showed differences in the time courses of ELAR (p = 0.04) and TCUF (p = 0.004), but statistical significance was lost after adjustment (ELAR: p = 0.06; TCUF: p = 0.07). For the remainder of the SPA parameters, we observed no differences over the time course between the modalities.
Another sensitivity analysis was performed in patients who survived at least 3 years on the same PD modality, which was the case for 18 CAPD patients (31%), and 17 APD patients (36%). Here, the crude analysis of the difference in the time course of ELAR between the modalities had a p value of 0.07, which became 0.03 after adjustment. Testing for differences in the time course of TCUF resulted in a p value of 0.03 in the crude model and a p value of 0.07 in the adjusted model. Again, only those SPA parameters showed a difference over time between APD and CAPD.
Finally, a separate analysis that excluded patients after the development of ultrafiltration failure looked at 43 CAPD patients (73%) and 32 APD patients (68%). After 3 years, 9 CAPD patients (15%) and 9 APD patients (19%) were still on their original PD modality. In that group, the ELAR in APD and CAPD patients did not have a different time course (crude p = 0.65, adjusted p = 0.25). In the crude model, TCUF showed a significant difference in time course between the modalities (p = 0.01), but significance was lost after adjustment (p = 0.26).
DISCUSSION
To our knowledge, ours is the first study to compare the long-term time course of peritoneal transport in patients treated with APD and with CAPD. We found no differences between the modalities in the time course of solute removal or of net ultrafiltration, but we did find significant differences with respect to TCUF and ELAR.
The faster decline of TCUF in APD patients in the present study confirms the results of two earlier studies showing lower net ultrafiltration rates in APD patients than in CAPD patients over time (11,21). However, those studies compared net ultrafiltration volumes instead of TCUF. Two other studies using peritoneal equilibration test data did not find a difference in ultrafiltration (19,20). Those results could be a result of the short follow-up in one study and the cross-sectional design in the other (19,20). Figure 1 shows that the difference between the modalities became apparent after 2 years of follow-up in patients still being treated with their original PD modality. The decline of TCUF in APD patients conforms with the hypothesis that higher exposure to bioincompatible dialysis solution could lead to more peritoneal damage; however, the difference between the modalities was very small. It did not result in a difference in net ultrafiltration, thereby not causing earlier technique failure in patients treated with APD (33-35).
We also found a faster decline of ELAR in APD patients, which conforms with a cross-sectional study showing that, compared with CAPD patients, APD patients had a lower ELAR (19). The authors hypothesized that an increase in ELAR over time could be a result of long-term exposure to high peritoneal pressure. Such pressure potentially has a dilating effect on the peritoneal lymphatic gaps (19). A cross-sectional study in CAPD patients showed intraperitoneal pressure to be lower in the supine position, indeed leading to a lower ELAR (36). Following that line of reasoning, APD patients could be protected from the dilating effect because intraperitoneal pressures are lower during APD. The latter could explain the findings in the present study. However, the current results contrast with earlier findings by our group, which showed no change in ELAR over time—an effect that might have been a result of the inclusion mainly of CAPD patients in that study (37).
Given that the present study is observational in nature and compares outcomes of therapies, confounding by indication cannot be ruled out. Despite the fact that the choice for a particular PD modality was not related to peritoneal membrane function, some differences between the modality groups were present at baseline. We were able to adjust for those confounders, but residual confounding might still be present. The limited number of patients included might be another drawback of the study. However, by including only patients from our own center, we could be sure that the SPAs were performed in a standardized setting, resulting in high-quality data. The limited number of patients could give rise to a concern about drop-out over time, because bias might occur if more of the best or worst patients dropped out. That concern was the reason that only incident PD patients were included; by using that group, major differences at the start of dialysis were avoided. In addition, use of a repeated-measures model takes drop-out into account. Furthermore, differences that might appear during treatment are attributable to the treatment under study and are thus part of the study question. Repeated-measures models also diminish the problem of possible survival bias. In the models, dropouts are taken into account under the assumption of missing at random. Therefore, this study was the first to compare free water transport, solute-coupled water transport, and protein transport in APD and CAPD patients over time. The present study tested many variables, and the small differences in the decline of TCUF and ELAR between the PD modalities should therefore be interpreted with caution. Most of parameters were shown not to be different over time for patients starting dialysis with either APD or CAPD.
CONCLUSIONS
Compared with patients starting PD on CAPD, those starting on APD might have a faster decline of TCUF and ELAR. Other transport parameters are not different over time. More studies are needed to confirm these results.
DISCLOSURES
The present study was supported by an unrestricted grant from Baxter Healthcare. The funding source was involved in neither the collection, interpretation, and analysis of the data, nor the decision to write and submit this report for publication. The authors otherwise have no financial conflicts of interest to declare.
Acknowledgments
The authors thank M. Langedijk, N. Glas, N. Scheper, and S. Duis for performing the SPA tests. Portions of the data presented in this manuscript were used in an oral presentation at the annual conference of the International Society for Peritoneal Dialysis in 2010.
REFERENCES
- 1. Bro S, Bjorner JB, Tofte-Jensen P, Klem S, Almtoft B, Danielsen H, et al. A prospective, randomized multicenter study comparing APD and CAPD treatment. Perit Dial Int 1999; 19:526–33 [PubMed] [Google Scholar]
- 2. Gallar P, Ortega O, Carreno A, Vigil A. Rate of decline in residual renal function is equal in CAPD and automated peritoneal dialysis patients. Perit Dial Int 2000; 20:803–5 [PubMed] [Google Scholar]
- 3. Hidaka H, Nakao T. Preservation of residual renal function and factors affecting its decline in patients on peritoneal dialysis. Nephrology (Carlton) 2003; 8:184–91 [DOI] [PubMed] [Google Scholar]
- 4. Hiroshige K, Yuu K, Soejima M, Takasugi M, Kuroiwa A. Rapid decline of residual renal function in patients on automated peritoneal dialysis. Perit Dial Int 1996; 16:307–15 [PubMed] [Google Scholar]
- 5. Holley JL, Aslam N, Bernardini J, Fried L, Piraino B. The influence of demographic factors and modality on loss of residual renal function in incident peritoneal dialysis patients. Perit Dial Int 2001; 21:302–5 [PubMed] [Google Scholar]
- 6. Hufnagel G, Michel C, Queffeulou G, Skhiri H, Damieri H, Mignon F. The influence of automated peritoneal dialysis on the decrease in residual renal function. Nephrol Dial Transplant 1999; 14:1224–8 [DOI] [PubMed] [Google Scholar]
- 7. Johnson DW, Mudge DW, Sturtevant JM, Hawley CM, Campbell SB, Isbel NM, et al. Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit Dial Int 2003; 23:276–83 [PubMed] [Google Scholar]
- 8. Liao CT, Shiao CC, Huang JW, Hung KY, Chuang HF, Chen YM, et al. Predictors of faster decline of residual renal function in Taiwanese peritoneal dialysis patients. Perit Dial Int 2008; 28(Suppl 3):S191–5 [PubMed] [Google Scholar]
- 9. Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 2000; 11:556–64 [DOI] [PubMed] [Google Scholar]
- 10. Singhal MK, Bhaskaran S, Vidgen E, Bargman JM, Vas SI, Oreopoulos DG. Rate of decline of residual renal function in patients on continuous peritoneal dialysis and factors affecting it. Perit Dial Int 2000; 20:429–38 [PubMed] [Google Scholar]
- 11. Ortega O, Gallar P, Carreño A, Gutierrez M, Rodriguez I, Oliet A, et al. Peritoneal sodium mass removal in continuous ambulatory peritoneal dialysis and automated peritoneal dialysis: influence on blood pressure control. Am J Nephrol 2001; 21:189–93 [DOI] [PubMed] [Google Scholar]
- 12. Davies SJ, Phillips L, Naish PF, Russell GI. Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol 2001; 12:1046–51 [DOI] [PubMed] [Google Scholar]
- 13. Di Paolo N, Sacchi G. Peritoneal vascular changes in continuous ambulatory peritoneal dialysis (CAPD): an in vivo model for the study of diabetic microangiopathy. Perit Dial Int 1989; 9:41–5 [PubMed] [Google Scholar]
- 14. Nakayama M, Kawaguchi Y, Yamada K, Hasegawa T, Takazoe K, Katoh N, et al. Immunohistochemical detection of advanced glycosylation end-products in the peritoneum and its possible pathophysiological role in CAPD. Kidney Int 1997; 51:182–6 [DOI] [PubMed] [Google Scholar]
- 15. Rippe B, Simonsen O, Heimbürger O, Christensson A, Haraldsson B, Stelin G, et al. Long-term clinical effects of a peritoneal dialysis fluid with less glucose degradation products. Kidney Int 2001; 59:348–57 [DOI] [PubMed] [Google Scholar]
- 16. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 2002; 13:470–9 [DOI] [PubMed] [Google Scholar]
- 17. Selgas R, Fernandez-Reyes MJ, Bosque E, Bajo MA, Borrego F, Jimenez C, et al. Functional longevity of the human peritoneum: how long is continuous peritoneal dialysis possible? Results of a prospective medium long-term study. Am J Kidney Dis 1994; 23:64–73 [DOI] [PubMed] [Google Scholar]
- 18. Breborowicz A, Martis L, Oreopoulos DG. Changes in biocompatibility of dialysis fluid during its dwell in the peritoneal cavity. Perit Dial Int 1995; 15:152–7 [PubMed] [Google Scholar]
- 19. Fusshöller A, zur Nieden S, Grabensee B, Plum J. Peritoneal fluid and solute transport: influence of treatment time, peritoneal dialysis modality, and peritonitis incidence. J Am Soc Nephrol 2002; 13:1055–60 [DOI] [PubMed] [Google Scholar]
- 20. La Milia V, Limardo M, Cavalli A, Crepaldi M, Locatelli F. Transport of peritoneal membrane assessed before and after the start of peritoneal dialysis. Nephrol Dial Transplant 2009; 24:2894–8 [DOI] [PubMed] [Google Scholar]
- 21. Rodriguez-Carmona A, Pérez-Fontán M, Garca-Naveiro R, Villaverde P, Peteiro J. Compared time profiles of ultrafiltration, sodium removal, and renal function in incident CAPD and automated peritoneal dialysis patients. Am J Kidney Dis 2004; 44:132–45 [DOI] [PubMed] [Google Scholar]
- 22. Smit W, van Dijk P, Langedijk MJ, Schouten N, van den Berg N, Struijk DG, et al. Peritoneal function and assessment of reference values using a 3.86% glucose solution. Perit Dial Int 2003; 23:440–9 [PubMed] [Google Scholar]
- 23. Pannekeet MM, Imholz AL, Struijk DG, Koomen GC, Langedijk MJ, Schouten N, et al. The standard peritoneal permeability analysis: a tool for the assessment of peritoneal permeability characteristics in CAPD patients. Kidney Int 1995; 48:866–75 [DOI] [PubMed] [Google Scholar]
- 24. Renck H, Ljungström KG, Hedin H, Richter W. Prevention of dextran-induced anaphylactic reactions by hapten inhibition. III. A Scandinavian multicenter study on the effects of 20 mL dextran 1, 15%, administered before dextran 70 or dextran 40. Acta Chir Scand 1983; 149:355–60 [PubMed] [Google Scholar]
- 25. Koomen GC, Krediet RT, Leegwater AC, Struijk DG, Arisz L, Hoek FJ. A fast reliable method for the measurement of intraperitoneal dextran 70, used to calculate lymphatic absorption. Adv Perit Dial 1991; 7:10–14 [PubMed] [Google Scholar]
- 26. Smit W, Struijk DG, Ho-Dac-Pannekeet MM, Krediet RT. Quantification of free water transport in peritoneal dialysis. Kidney Int 2004; 66:849–54 [DOI] [PubMed] [Google Scholar]
- 27. Westra WM, Smit W, Zweers MM, Struijk DG, Krediet RT. Diffusion correction of sodium sieving applicable in a peritoneal equilibration test. Adv Perit Dial 2003; 19:6–9 [PubMed] [Google Scholar]
- 28. Ho-dac-Pannekeet MM, Hiralall JK, Struijk DG, Krediet RT. Longitudinal follow-up of CA125 in peritoneal effluent. Kidney Int 1997; 51:888–93 [DOI] [PubMed] [Google Scholar]
- 29. Akman S, van Westrhenen R, De Waart DR, Hiralall JK, Zweers MM, Krediet RT. The effect of dwell time on dialysate cancer antigen 125 appearance rates in patients on continuous ambulatory peritoneal dialysis. Adv Perit Dial 2003; 19:24–7 [PubMed] [Google Scholar]
- 30. Imholz AL, Koomen GC, Struijk DG, Arisz L, Krediet RT. Effect of dialysate osmolarity on the transport of low-molecular weight solutes and proteins during CAPD. Kidney Int 1993; 43:1339–46 [DOI] [PubMed] [Google Scholar]
- 31. Krediet RT, Zemel D, Struijk DG, Koomen GC, Arisz L. Individual characterization of the peritoneal restriction barrier to macromolecules. Adv Perit Dial 1991; 7:15–20 [PubMed] [Google Scholar]
- 32. Zemel D, Krediet RT, Koomen GC, Struijk DG, Arisz L. Day-to-day variability of protein transport used as a method for analyzing peritoneal permeability in CAPD. Perit Dial Int 1991; 11:217–23 [PubMed] [Google Scholar]
- 33. Badve SV, Hawley CM, McDonald SP, Mudge DW, Rosman JB, Brown FG, et al. Automated and continuous ambulatory peritoneal dialysis have similar outcomes. Kidney Int 2008; 73:480–8 [DOI] [PubMed] [Google Scholar]
- 34. Mehrotra R, Chiu YW, Kalantar-Zadeh K, Vonesh E. The outcomes of continuous ambulatory and automated peritoneal dialysis are similar. Kidney Int 2009; 76:97–107 [DOI] [PubMed] [Google Scholar]
- 35. Michels WM, Verduijn M, Boeschoten EW, Dekker FW, Krediet RT. Similar survival on automated peritoneal dialysis and continuous ambulatory peritoneal dialysis in a large prospective cohort. Clin J Am Soc Nephrol 2009; 4:943–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Imholz AL, Koomen GC, Voorn WJ, Struijk DG, Arisz L, Krediet RT. Day-to-day variability of fluid and solute transport in upright and recumbent positions during CAPD. Nephrol Dial Transplant 1998; 13:146–53 [DOI] [PubMed] [Google Scholar]
- 37. Michels WM, Zweers MM, Smit W, Korevaar J, Struijk DG, van Westrhenen R, et al. Does lymphatic absorption change with the duration of peritoneal dialysis? Perit Dial Int 2004; 24:347–52 [PubMed] [Google Scholar]