Abstract
♦ Background: The angiogenic response is partly involved in the progression of encapsulating peritoneal sclerosis (EPS). However, the details of the angiogenic response, especially for lymphatic vessels in patients with EPS, remain unclear. In addition, because of technical limitations, morphology studies reported to date have examined only the parietal peritoneum. The morphologies of parietal and visceral lymphatic vessels in patients with EPS both need to be analyzed.
♦ Methods: We examined peritoneal samples from 18 patients with EPS who underwent enterolysis of the visceral peritoneum and compared them with samples from 17 autopsy cases (controls). To examine the angiogenic response, we performed immunohistochemistry for the endothelial markers CD34 (blood vessels) and podoplanin (lymphatic vessels) and for the cell proliferation marker Ki-67. Immunogold electron microscopy analysis for podoplanin was also performed. In 7 of 18 cases, we compared differences in the angiogenic response of the parietal and visceral peritoneal membranes.
♦ Results: Angiogenic responses were more frequent in the compact zone than in regenerated layers. The number of capillaries positive for anti-CD34 and anti-podoplanin monoclonal antibodies per unit area of visceral peritoneal tissue was, respectively, 41.1 ± 29.3/mm2 in EPS patients and 2.7 ± 4.4/mm2 in controls (p ≤ 0.01) and 48.1 ± 43.9/mm2 in EPS patients and 4.1 ± 5.4/mm2 in controls (p ≤ 0.01). The percentage of capillaries positive for anti-Ki-67, CD34, and podoplanin was 4.6% in EPS patients (p ≤ 0.01) and 0.8% in controls (p = 0.09). The immunogold electron microscopy analysis revealed that podoplanin was localized to endothelial cells with anchoring filaments, a specific feature of lymphatic vessels. Furthermore, compared with parietal peritoneal membrane, visceral peritoneal membrane had a more prominent podoplanin-positive capillary profile, but not a prominent CD34-positive capillary profile. In addition, fibroblast-like cells double-positive for podoplanin and smooth muscle actin were markedly increased in the degenerated layer, as previously reported.
♦ Conclusions: Our study demonstrated that lymphatic vessels are increased in the visceral peritoneum of patients with EPS.
Keywords: EPS, angiogenesis, lymphangiogenesis, CD34, podoplanin, Ki-67, visceral peritoneum, parietal peritoneum
Although peritoneal dialysis (PD) is one of the therapies for end-stage renal disease, long-term therapy with PD results in several morphologic and functional changes that often cause PD discontinuance (1). Among those changes, the most prominent are encapsulating peritoneal sclerosis (EPS) and ultrafiltration failure (UFF).
Histologically, EPS is characterized by fibrin deposition, mesothelial denudation, interstitial fibrosis, peritoneal fibroblast swelling, perivascular bleeding, capillary angiogenesis, microvascular sclerosis, and interstitial mononuclear cell infiltration (2). The pathogenesis of EPS is thought to involve glucose (because of advanced glycation end-products and glucose degradation products), low pH, hypoxia resulting from fibrosis, and inflammation (because of peritonitis). These factors are known to induce expression of vascular endothelial growth factor (3-7).
Recent work has emphasized the angiogenic response as a factor in EPS pathogenesis: anti-angiogenic therapy (anti-vascular endothelial growth factor neutralizing antibody) in an experimental EPS rat model ameliorated disease progression and was accompanied by a marked reduction in angiogenesis-associated factors (8). Indeed, studies have demonstrated that vascular formation or vascular density is markedly increased in EPS patients compared with control subjects (9,10). However, those studies raise two major concerns:
Vascular formation marked by commonly used antibodies such as factor VIII, anti-CD31, and anti-CD34 cannot distinguish between microvessels and lymphatic vessels.
Pathology evaluation usually considers the parietal, and not the visceral, peritoneal membrane at the time of catheter insertion or withdrawal.
In this context, detailed analyses of angiogenic responses in human EPS, including those of lymphatic vessels in both the visceral and the parietal peritoneal membrane, are required to better understand EPS.
Currently, anti-podoplanin antibodies are considered to be lymphatic and fibroblast markers (11-13). To assess angiogenesis and lymphangiogenesis in the visceral and the parietal peritoneal membrane in EPS, we applied immunohistochemistry for the endothelial markers CD34 (blood vessels) and podoplanin (lymphatic vessels) and the cell-proliferation marker Ki-67, and we performed immunogold microscopy analysis for podoplanin. We also evaluated the relationship between these histopathology findings and clinical data.
METHODS
STUDY POPULATION
After informed consent and approval of the local ethics committee had been obtained, samples of visceral peritoneum were collected from 18 patients who underwent enterolysis for clinically diagnosed EPS. The clinical diagnosis of EPS was established based on the guidelines proposed by the International Society for Peritoneal Dialysis (14). In these PD patients with bowel obstruction symptoms, peritoneal hypertrophy and encapsulation were confirmed by abdominal computed tomography. We used the Nakamoto criteria (15)—stage 1, pre-EPS; stage 2, inflammation; stage 3, encapsulation or progression; stage 4, ileus or completion—to stage the patients. In the present study, all patients were stage 3 or 4 (operative cases). For the 17 autopsy cases [13 men, 4 women; age range: 33 - 90 years (mean: 70.9 - 13.2 years)] used as control subjects, the main causes of death were sepsis (n = 6), pneumonia (n = 4), cardiovascular disease (n = 3), disseminated intravascular coagulation (n =1), hepatocellular carcinoma (n =1), brain hemorrhage (n =1), and head trauma (n =1). Four autopsy cases had undergone hemodialysis. Table 1 shows clinical data for the 18 EPS patients and 17 control subjects.
TABLE 1.
Clinical Background of the Patients and Control Subjectsa

HISTOPATHOLOGY
The histologic evaluation was performed by 2 independent observers (TY, IY), who were blinded to the patient characteristics and clinical information. The enterolysis tissue obtained was cut vertically after the tissue had been clamped in every direction. The cut tissue was then embedded in paraffin in parallel strips, which permitted the length of the compact zone to be accurately measured. Formalin-fixed, paraffin-embedded sections were stained using hematoxylin and eosin, periodic acid-Schiff, and elastin-Masson trichrome and then classified as previously reported (2). Histopathologically, EPS sections showed fibrin deposition, mesothelial denudation, interstitial fibrosis, peritoneal fibroblast swelling, perivascular bleeding, capillary angiogenesis, microvascular sclerosis, and interstitial mononuclear cell infiltration as previously described (2). Fibrosis was defined as a submesothelial compact zone measuring more than 150 μm, and vasculopathy was graded according to reported methods (16). In all cases, mesothelial denudation and submesothelial thicknesses were confirmed. The visceral peritoneal histopathology was photographed (Olympus DP70: Olympus, Center Valley, PA, USA) under a light microscope (Olympus AX80).
IMMUNOHISTOCHEMISTRY (TRIPLE STAINING)
Formalin-fixed, paraffin-embedded blocks were sectioned at 4 μm, deparaffinized, and rehydrated. Antigen retrieval was performed by heating the sections in 0.01 mol/L citrate buffer (pH 6.0) in an autoclave at 121°C for 1 minute (Labo Autoclave MLS-3750F: Sanyo, Osaka, Japan). Thin sections were then blocked in 10% normal goat serum in 0.2 mol/L phosphate-buffered saline [PBS (pH 7.4)] and incubated with mouse antipodoplanin monoclonal antibody [mAb (AngioBio, Del Mar, CA, USA)] at 1:200 dilution for 60 minutes at room temperature. Slides were then stained using the Histofine Simple Stain Max AP (alkaline phosphatase) kit (Nichirei, Tokyo, Japan) for 30 minutes according to the manufacturer’s instructions. Reaction products were visualized by immersing sides in BCIP/NBT (Dako Corporation, Glostrup, Denmark) for 5 minutes. Next, sections were heated to 95°C in 0.01 mol/L citric acid buffer (pH 6.0) for 10 minutes and incubated in mouse anti-CD34 mAb (Novocastra Laboratories, Newcastle upon Tyne, UK) at 1:50 dilution for 60 minutes at room temperature. Slides were stained using the Histofine Simple Stain Max AP kit for 30 minutes. Reaction products were visualized by immersing slides in permanent red (Dako) for 5 minutes. Finally, the sections were again heated to 95°C in 0.01 mol/L citric acid buffer (pH 6.0), endogenous peroxidase in the sections was inactivated in 3% H2O2 for 10 minutes, and the sections were incubated in mouse anti-Ki-67 mAb (Zymed Laboratories, South San Francisco, CA, USA) at 1:5 dilution for 60 minutes at room temperature. Slides were then stained using anti-mouse Envision+/HRP (Dako) for 30 minutes at room temperature. Reaction products were visualized by immersing slides in diaminobenzidine tetrahydrochloride (Dako) for 5 minutes. As a podoplanin-positive control, we used intrarenal lymphatic vessels obtained from a renal graft biopsy after acute rejection; these vessels are normally located around interlobular arteries.
IMMUNOHISTOCHEMISTRY (DOUBLE STAINING)
Formalin-fixed, paraffin-embedded blocks were sectioned at 4 μm, deparaffinized, and rehydrated. Thin sections were then blocked in 10% normal goat serum in 0.2 mol/L PBS (pH 7.4) and incubated with mouse anti-podoplanin mAb (AngioBio) at 1:200 dilution for 60 minutes at room temperature. The slides were then stained using the Histofine Simple Stain Max AP kit for 30 minutes according to the manufacturer’s instructions. The reaction products were visualized by immersing the slides in permanent red (Dako) for 5 minutes. Next, the sections were heated to 95°C in 0.01 mol/L citric acid buffer (pH 6.0), the endogenous peroxidase in the sections was inactivated in 3% H2O2 for 10 minutes, and the sections were incubated in mouse anti-smooth muscle actin mAb (Dako) at 1:500 dilution for 60 minutes at room temperature. The slides were stained using the Histofine Simple Stain Max PO (peroxidase) kit (Nichirei) for 30 minutes at room temperature. The reaction products were visualized by immersing the slides in diaminobenzidine tetrahydrochloride (Dako) for 1 minute.
MORPHOMETRIC ANALYSIS
In each biopsy, we confirmed the compact zone using elastin-Masson trichrome stain. The serial sections of the triple-stained slides were then photographed at 40× magnification. Five randomly selected 1×1-mm areas of each compact zone were analyzed using an image analysis system (Lumina Vision: Mitani Corporation, Tokyo, Japan). Using simple staining for podoplanin, the lymphatic vessel density was calculated as the amount of the lumen that was surrounded by podoplanin-positive cells. Podoplanin-positive cells with no lumen were omitted because they were considered to be fibroblast-like cells, as previously reported (13). Among these lymphatic vessels, Ki-67-positive lumens were counted as newly organized lymphatic vessels. We applied the same calculation systems to the microvessels. Lumens double-positive for podoplanin and CD34 were counted as lymphatic vessels according to a previously reported method (17). Table 2 shows the average number of CD34- and podoplanin-positive vessels in the sections.
TABLE 2.
CD34- and Podoplanin-Positive Capillaries in Visceral Peritoneal Tissue from Patients and Control Subjects

IMMUNOELECTRON-MICROSCOPY ANALYSIS (PRE-EMBEDDED METHODS)
We used a reported method (18). Small pieces, 1 mm3 in size, taken from formalin-fixed, paraffin-embedded blocks from patients with EPS were deparaffinized and rehydrated. The pieces were then blocked in 10% normal bovine serum albumin and PBS (1:1) and incubated in mouse anti-podoplanin mAb (AngioBio) at 1:5 dilution overnight at room temperature. The sections were then blocked in 10% normal goat serum in 0.2 mol/L PBS (pH 7.4) and stained using 1.4 nm gold-conjugated goat anti-mouse immunoglobulin G (Nanoprobes, Stony Brook, NY, USA) at 1:20 dilution for 300 minutes. The pieces were then fixed with 1% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.4) for 30 minutes. For low-power work, the pieces were silver-enhanced using a method previously reported, followed by post-fixation in 1% osmium tetroxide in 0.1 mol/L cacodylate buffer (pH 7.4). After dehydration through an ethanol series, the pieces were embedded in an Epon resin mixture (Polysciences, Warrington, PA, USA) according to a method previously described (19). Ultrathin sections (80-90 nm) were cut with an ultramicrotome, stained with 0.2% oolong tea extract (Suntory, Osaka, Japan), uranyl acetate, and lead citrate, and observed under a transmission electron microscope (JEM-2000 FX II: Jeol, Tokyo, Japan) operating at 100 kV accelerating voltage.
STATISTICAL ANALYSIS
All data are presented as mean ± standard deviation. The Mann-Whitney U-test and chi-square test were used to evaluate statistical differences between the groups, as indicated. Spearman rank correlations were used to evaluate the statistical differences between two variables. All p values were two-sided, and values below 0.05 were considered to indicate statistical significance.
RESULTS
CLINICAL CHARACTERISTICS
Table 1 shows the clinical characteristics of the patients with EPS [age range: 35 - 70 years (mean: 56.1 ± 8.9 years); 14 men (77.7%)]. The average duration of continuous ambulatory PD was 98.8 ± 29.7 months, and UFF was recognized in 80% of the patients. The dialysate-to-plasma concentration ratio for creatinine was 0.62 - 1.15 (mean: 0.84 ± 0.15). No patient had diabetes mellitus, but all had hypertension. Only 2 patients used icodextrin. The frequency of previous peritonitis was 56% [10 of 18 (range: 0 - 6 episodes; mean: 1.4 ± 1.8 episodes)].
MICROVESSELS AND LYMPHATIC VESSELS IN CONTROL VISCERAL PERITONEUM
Figure 1 shows normal small intestine from an autopsy subject. The visceral peritoneal tissue was a very thin layer, less than 50 μm above the longitudinal layer of muscle. The parietal peritoneum was morphologically identical to the visceral peritoneal tissue (data not shown). Very few CD34- and podoplanin-positive capillaries were observed in the visceral peritoneal tissue (2.7 ± 4.4/mm2 and 4.1 ± 5.4/mm2 respectively, Table 2). Anti-Ki-67 mAb staining was totally negative in the nuclei of both endothelial cells (intestinal mucosal cells were used as the positive control), as shown in Figure 1.
Figure 1.
— Normal small-intestine tissue histology from a representative autopsy case. (A,B) Very thin visceral membrane layer, longitudinal layer of muscle, circular layer of muscle, submucosa, and mucosa (Masson trichrome). 100× and 400× magnification respectively. (C,D) Triple-staining for CD34 (red), podoplanin (blue), and Ki-67 (brown). 100× and 400× magnification respectively. In normal small-intestine tissue, microvessels (white arrow) and lymphatic vessels are rarely observed in the visceral membrane. In the longitudinal layer of muscle, microvessels (black arrowhead) are observed focally and are accompanied by lymphatic vessels (white arrowhead). These vessels were negative for Ki-67. The proliferative cell zone (asterisk) was used as a positive control.
ANGIOGENESIS AND LYMPHANGIOGENESIS IN VISCERAL PERITONEUM IN EPS VERSUS CONTROL
The independent observers were in almost perfect agreement regarding the numbers of CD34- and podoplanin-positive vessels counted with single staining. As shown in Table 2, CD34-positive capillaries per unit area of visceral peritoneal tissue were 41.1 ± 29.3/mm2 (range: 11.4 - 128.8/mm2) in patients with EPS and 2.7 ± 4.4/mm2 (0 - 15/mm2) in control subjects (p ≤ 0.01). Of those capillaries, 4.6% (0 - 8.4/mm2) in patients with EPS and 0% in control subjects were positive for Ki-67 (p ≤ 0.01). With respect to lymphangiogenesis, we observed 48.1 ± 43.9/mm2 (range: 3.2 - 155.2/mm2) podoplanin-positive capillaries in EPS patients and 4.1 ± 5.4/mm2 (range: 0 - 15/mm2) in control subjects (p ≤ 0.01), of which 0.8% (0 - 3/mm2) and 0% were positive for Ki-67 respectively (nonsignificant).
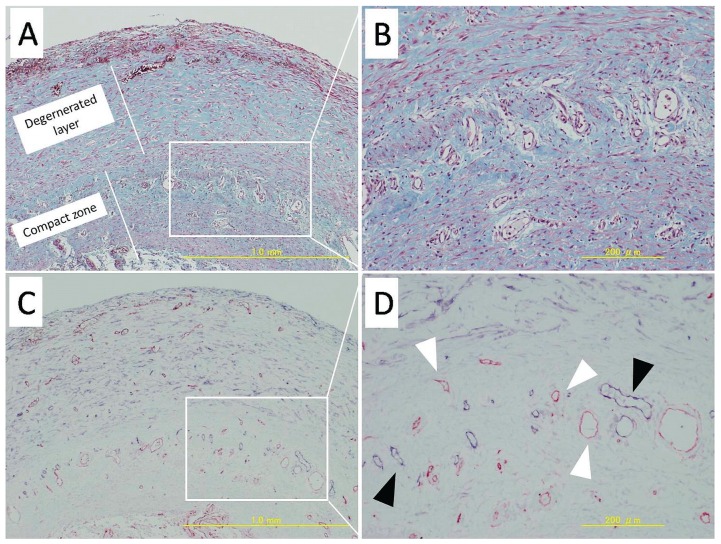
Figure 2 shows representative features of angiogenesis and lymphangiogenesis in visceral tissues from patients with EPS. In the compact zone, proliferation of blood vessels is marked.
Figure 2.
— Pathology in small-intestine tissue from a representative patient with encapsulating peritoneal sclerosis. (A,B) Very thick degenerated layer and compact zone (Masson trichrome). The degenerated layer shows a large increase in fibroblast-like cells. 50× and 200× magnification respectively. (C,D) Staining for CD34 (red) and podoplanin (blue). 50× and 200× magnification respectively. Microvessels (white arrowhead) and lymphatic vessels (black arrowhead) are prominent in the compact zone.
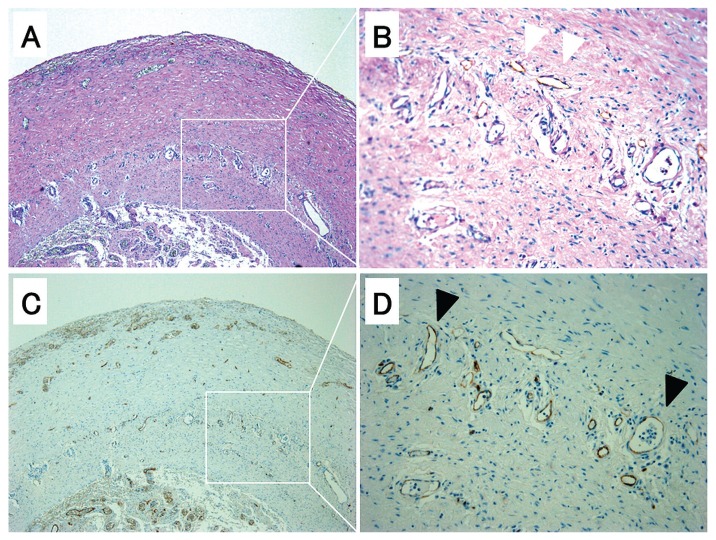
Figure 3 shows single staining for podoplanin, with after-staining using periodic acid-Schiff [Figure 3 (A,B)] and CD34 [Figure 3 (C,D)]. The immunoreactivity of CD34 in podoplanin-positive lumens was variable, but mostly low to absent. With respect to angiogenesis, CD34- and Ki-67-positive capillaries are both seen in Figure 4(B-1). With respect to lymphangiogenesis, podoplanin- and Ki-67-positive capillaries are both seen in Figure 4(B-2). In the degenerated layer, fibroblast-like cells double-positive for podoplanin and smooth-muscle actin are observed in Figure 5(B-1). By contrast, only podoplanin-positive lumens are observed in the compact zone [Figure 5(B-2)]. Figure 6 shows the CD31 [Figure 6(A)] and CD34 [Figure 6(C)] positivity of the podoplanin-positive capillaries [Figure 6(D)]. The vessels double-positive for CD34 and podoplanin [Figure 6(B)] were counted as lymphatic vessels in the present study.
Figure 3.
— Pathology in small-intestine tissue from a representative patient with encapsulating peritoneal sclerosis. (A,B) Very thick degenerated layer and compact zone [periodic acid-Schiff and podoplanin (white arrowhead) staining]. 50× and 200× magnification respectively. (C,D) Staining for CD34. Microvessels (black arrowhead) are prominent in the compact zone. 50× and 200× magnification respectively. Views (B) and (D) distinguish microvessels and lymphatic veins.
Figure 4.
— Angiogenic response in small-intestine tissue from a representative patient with encapsulating peritoneal sclerosis. (A) Very thick degenerated layer and compact zone (Masson trichrome). 100× magnification. (B) Triple-staining of CD34 (red), podoplanin (blue), and Ki-67 (blown) shows a more prominent angiogenic response in the compact zone than in the degenerated layer. 100× magnification. (B-1) Angiogenesis: CD34-positive lumens with Ki-67-positive cells (white arrowhead). 400× magnification. (B-2) Lymphangiogenesis: podoplanin-positive lumens with Ki-67-positive cells (black arrowhead). 400× magnification.
Figure 5.
— Pathology of fibroblast-like cells in small-intestine tissue from a representative patient with encapsulating peritoneal sclerosis. (A) Staining for CD34 (red) and podoplanin (blue). 50× magnification. (B) Double-staining for smooth-muscle actin and podoplanin shows a very thick degenerated layer and compact zone. The degenerated layer shows numerous increases in fibroblast-like cells. 50× magnification. (B-1) The fibroblast-like cells in the degenerated layer are positive for podoplanin (white arrowhead) and for smooth-muscle actin (black arrowhead). Fibroblast-like cells positive for both podoplanin and smooth-muscle actin (black arrow) are prominent in the degenerated layer. 400× magnification. (B-2) Podoplanin-positive lumens (white arrow). 200× magnification.
Figure 6.
— Pathology of CD31- and CD34-positive microvessels and podoplanin-positive lymphatic veins in small-intestine tissue from a representative patient with encapsulating peritoneal sclerosis. (A) Staining for CD31 (brown). 400× magnification. (B) Staining for CD34 (red) and podoplanin (blue). The vessels show merged staining for CD34 and podoplanin. 400× magnification. (C) Staining for CD34 (brown). 400× magnification. (D) Staining for podoplanin (brown). 400× magnification.
ANGIOGENESIS AND LYMPHANGIOGENESIS IN PARIETAL COMPARED WITH VISCERAL TISSUES IN PATIENTS WITH EPS
Of the 18 patients with EPS, samples from 7 used to investigate differences between the parietal and visceral peritoneum. As Table 3 shows, the parietal and visceral peritoneal tissues showed no significant difference with respect to CD34-positive capillaries. By contrast, podoplanin-positive capillaries were significantly more numerous in visceral peritoneal tissue than in parietal peritoneal tissue (p < 0.05).
TABLE 3.
CD34- and Podoplanin-Positive Capillaries in Parietal and Visceral Peritoneal Tissue from Patients with Encapsulating Peritoneal Sclerosis

IMMUNOGOLD ELECTRON MICROSCOPY ANALYSIS IN PATIENTS WITH EPS
The immunoelectron-microscopy analysis revealed much gold-labeled podoplanin expression around the endothelial cells. Those cells lacked basal lamina and had anchoring filaments, which are a characteristic feature of lymphatic vessels [Figure 7(A)]. By contrast, in the degenerated layer, much gold-labeled podoplanin expression was confirmed in fibroblast-like cells but not in endothelial cells [Figure 7(B)].
Figure 7.
— Immunogold electron microscopy analysis of podoplanin in small-intestine tissue from a representative patient with encapsulating peritoneal sclerosis. (A) The lumen of the compact zone (asterisk) shows a thin-walled single layer with no pericytes or smooth-muscle cells that have anchoring filaments (white arrowhead). (B) Fibroblast-like cell of the degenerated layer surrounded by numerous collagen fibers. Immunogold-labeled podoplanin is seen (black arrowhead). From Ishiyama et al. (20).
CORRELATION BETWEEN ANGIOGENIC RESPONSE AND CLINICAL DATA IN EPS
We used Spearman rank correlations and the Mann-Whitney U-test to investigate correlations between the angiogenic response (angiogenesis and lymphangiogenesis) and clinical parameters in the patients with EPS (sex, age, duration of continuous ambulatory PD, dialysate-to-plasma ratio of creatinine, UFF, and history of peritonitis; Table 4). The clinical parameters showed no significant correlation with the angiogenic response.
TABLE 4.
Angiogenic Response and Clinical Data in Patients with Encapsulating Peritoneal Sclerosis

DISCUSSION
One of the main new findings of the present study is that podoplanin-positive vessels are more common in visceral peritoneum than parietal peritoneum. In control visceral peritoneal tissue, lymphatic vessels were found only around arterioles in the longitudinal layer of muscle, as shown in Figure 1. In EPS, however, there is dramatically increased angiogenesis and lymphangiogenesis at a distinct fiber thickness in the compact zone, as shown in Figure 2. Sherif et al. (9) reported a morphology analysis of the parietal peritoneum that used the Masson-Noguchi method in patients with EPS; they found that microvessels increased by an average of 37.2 ± 21.9/mm2. We found a total number of CD34- and podoplanin-positive lumens nearly equal to their overall total (41.9 ± 16.9/mm2 plus 2.8 ± 3.4/mm2). Therefore, the reported morphology analysis of parietal peritoneum angiogenesis found, predominantly, CD34-positive lumens. Conversely, in visceral peritoneum (compared with parietal peritoneum), lymphatic vessels were dramatically increased (37.8 ± 22.9/mm2 vs 2.8 ± 3.4/mm2), but CD34-positive lumens showed no statistical difference (Table 3). With respect to lymphangiogenesis, lymphatic vessels develop under several conditions, including wound healing from inflammation (21), tumor metastasis (22), granulation tissue in myocardial infarction (17), and transplanted kidneys with inflammatory infiltrates (11). Notably, a diabetic state, with advanced glycation end-products and glucose degradation products resulting from high glucose, could be related to the increased growth of lymphatic vessels in diabetic nephropathy (23). The diabetic state closely mimics PD therapy because of high glucose use (6). In this context, one possible mechanism for preferential lymphangiogenesis in the visceral peritoneum is the effect of anatomy and gravity, which expose the visceral peritoneum to PD fluid, because the patient is mostly in a supine position (while sleeping and during PD fluid exchanges). Unfortunately, we could not determine whether this unique lymphangiogenesis response is specific to the visceral peritoneum in patients with EPS or is the usual response with long-term exposure to PD fluid, because our controls were autopsy subjects who did not have simple sclerosis or pre-EPS. Interestingly, our additional data indicate that CD34+Ki-67 was seen in only 4.6% of angiogenesis sites and podoplanin+Ki-67 was seen in only 0.8% of lymphangiogenesis sites. Because steroid therapy is used to reduce inflammatory activity before surgery, conditions other than inflammation might induce lymphangiogenesis in patients with EPS. Further analysis is needed to determine the specificity of this phenomenon.
Another main finding, which Braun et al. (13) have already reported, was that the podoplanin-positive fibroblast-like cells were predominantly seen in the degenerated layer. These fibroblast-like cells were positive not only for podoplanin, but also for smooth-muscle actin. Braun et al. (13) suggested that these cells are an intermediate phenotype between myofibroblasts and differentiated mesothelial cells. As discussed elsewhere, transforming growth factor β (TGF-β) is considered the master molecule in peritoneal injury during PD (3,4). A recent report showed that TGF-β can induce podoplanin expression in human fibrosarcoma cells (24). Another demonstrated that podoplanin promoted the epithelial-to-mesenchymal transition (EMT) by binding to ezrin, radixin, and moesin (25). In that context, our data suggest an additional mechanism for EMT: TGF-β enhanced podoplanin expression and promoted the EMT reaction of mesothelial cells to myofibroblasts.
From a clinical perspective, most patients with EPS (8 of 10, 80%) were classified as having UFF, another factor contributing to PD discontinuance. Our findings may partly explain type 3 UFF, because one of the main functions of the lymphatic vessels is to return protein-rich fluid, macromolecules, and cells such as extravasated leukocytes and activated antigen-presenting cells back to the circulating blood (23,26). Unfortunately, our data show no correlation between the lymphatic vessels and UFF. Moreover, we could not find a correlation between lymphangiogenesis and any clinical parameter. One possible reason is that all our data came from patients with EPS, which is an extremely advanced peritoneal injury. Data from patients with simple sclerosis and pre-EPS should be examined to clarify the significance of lymphangiogenesis in patients with EPS. Whether UFF can be explained by the increased lymphatic vessels in patients with EPS is an issue for future investigation.
CONCLUSIONS
The present study demonstrated that lymphatic vessels are increased in the visceral peritoneum of patients with EPS.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
Acknowledgments
We thank Mrs. Miyako Ishiyama (Kanagawa Rehabilitation Hospital) for skillful technical assistance with electron microscopy. We thank Mrs. Sachiko Ishii (Kashiwa Hospital, The Jikei University School of Medicine) for skillful technical assistance with immunohistochemistry.
REFERENCES
- 1. Kawaguchi Y, Hasegawa T, Nakayama M, Kubo H, Shigematu T. Issues affecting the longevity of the continuous peritoneal dialysis therapy. Kidney Int Suppl 1997; 62:S105–7 [PubMed] [Google Scholar]
- 2. Honda K, Oda H. Pathology of encapsulating peritoneal sclerosis. Perit Dial Int 2005; 25(Suppl 4):S19–29 [PubMed] [Google Scholar]
- 3. Selgas R, Bajo A, Jiménez-Heffernan JA, Sánchez-Tomero JA, Del Peso G, Aguilera A, et al. Epithelial-to-mesenchymal transition of the mesothelial cell—its role in the response of the peritoneum to dialysis. Nephrol Dial Transplant 2006; 21(Suppl 2):ii2–7 [DOI] [PubMed] [Google Scholar]
- 4. Aroeira LS, Aguilera A, Sánchez-Tomero JA, Bajo MA, del Peso G, Jiménez-Heffernan JA, et al. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol 2007; 18:2004–13 [DOI] [PubMed] [Google Scholar]
- 5. Ha H, Cha MK, Choi HN, Lee HB. Effects of peritoneal dialysis solutions on the secretion of growth factors and extracellular matrix proteins by human peritoneal mesothelial cells. Perit Dial Int 2002; 22:171–7 [PubMed] [Google Scholar]
- 6. Boulanger E, Grossin N, Wautier MP, Taamma R, Wautier JL. Mesothelial RAGE activation by AGEs enhances VEGF release and potentiates capillary tube formation. Kidney Int 2007; 71:126–33 [DOI] [PubMed] [Google Scholar]
- 7. Schilte MN, Celie JW, Wee PM, Beelen RH, van den Born J. Factors contributing to peritoneal tissue remodeling in peritoneal dialysis. Perit Dial Int 2009; 29:605–17 [PubMed] [Google Scholar]
- 8. Io H, Hamada C, Ro Y, Ito Y, Hirahara I, Tomino Y. Morphologic changes of peritoneum and expression of VEGF in encapsulated peritoneal sclerosis rat models. Kidney Int 2004; 65:1927–36 [DOI] [PubMed] [Google Scholar]
- 9. Sherif AM, Yoshida H, Maruyama Y, Yamamoto H, Yokoyama K, Hosoya T, et al. Comparison between the pathology of encapsulating sclerosis and simple sclerosis of the peritoneal membrane in chronic peritoneal dialysis. Ther Apher Dial 2008; 12:33–41 [DOI] [PubMed] [Google Scholar]
- 10. Numata M, Nakayama M, Nimura S, Kawakami M, Lindholm B, Kawaguchi Y. Association between an increased surface area of peritoneal microvessels and a high peritoneal solute transport rate. Perit Dial Int 2003; 23:116–22 [PubMed] [Google Scholar]
- 11. Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol 2004; 15:603–12 [DOI] [PubMed] [Google Scholar]
- 12. Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A 2002; 99:16069–74 [Erratum in: Proc Natl Acad Sci U S A 2003; 100:4970] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braun N, Alscher DM, Fritz P, Edenhofer I, Kimmel M, Gaspert A, et al. Podoplanin-positive cells are a hallmark of encapsulating peritoneal sclerosis. Nephrol Dial Transplant 2011; 26:1033–41 [DOI] [PubMed] [Google Scholar]
- 14. Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int 2000; 20(Suppl 4):S43–55. [PubMed] [Google Scholar]
- 15. Nakamoto H. Encapsulating peritoneal sclerosis—a clinician’s approach to diagnosis and medical treatment. Perit Dial Int 2005; 25(Suppl 4):S30–8 [PubMed] [Google Scholar]
- 16. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. on behalf of the Peritoneal Biopsy Study Group. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 2002; 13:470–9 [DOI] [PubMed] [Google Scholar]
- 17. Ishikawa Y, Akishima-Fukasawa Y, Ito K, Akasaka Y, Tanaka M, Shimokawa R, et al. Lymphangiogenesis in myocardial remodelling after infarction. Histopathology 2007; 51:345–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamamoto I, Horita S, Takahashi T, Kobayashi A, Toki D, Tanabe K, et al. Caveolin-1 expression is a distinct feature of chronic rejection-induced transplant capillaropathy. Am J Transplant 2008; 8:2627–35 [DOI] [PubMed] [Google Scholar]
- 19. Sawada H, Esaki M. Use of nanogold followed by silver enhancement and gold toning for preembedding immunolocalization in osmium-fixed, Epon-embedded tissues. J Electron Microsc (Tokyo) 1994; 43:361–6 [PubMed] [Google Scholar]
- 20. Ishiyama M, Yaginuma T, Yagishita S. Preembedding immunoelectron microscopy using nanogold method from paraffin block [Japanese]. J Electron Microsc Tech Med Biol 2008; 22(2):1–7 [Google Scholar]
- 21. Paavonen K, Puolakkainen P, Jussila L, Jahkola T, Alitalo K. Vascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healing. Am J Pathol 2000; 156:1499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 2002; 161:947–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakamoto I, Ito Y, Mizuno M, Suzuki Y, Sawai A, Tanaka A, et al. Lymphatic vessels develop during tubulointerstitial fibrosis. Kidney Int 2009; 75:828–38 [DOI] [PubMed] [Google Scholar]
- 24. Suzuki H, Kato Y, Kaneko MK, Okita Y, Narimatsu H, Kato M. Induction of podoplanin by transforming growth factor-beta in human fibrosarcoma. FEBS Lett 2008; 582:341–5 [DOI] [PubMed] [Google Scholar]
- 25. Martín-Villar E, Megías D, Castel S, Yurrita MM, Vilaró S, Quintanilla M. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J Cell Sci 2006; 119:4541–53 [DOI] [PubMed] [Google Scholar]
- 26. Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature 2005; 438:946–53 [DOI] [PubMed] [Google Scholar]