Abstract
♦ Background: Peritoneal dialysis (PD) is the preferred available option of renal replacement therapy for a significant number of end-stage kidney disease patients. A major limiting factor to the successful continuation of PD is the long-term viability of the PD catheter (PDC). Bedside percutaneous placement of the PDC is not commonly practiced despite published data encouraging use of this technique. Its advantages include faster recovery and avoidance of general anesthesia.
♦ Methods: We carried out a retrospective analysis of the outcomes of 313 PDC insertions at our center, comparing all percutaneous PDC insertions between July 1998 and April 2010 (group P, n = 151) with all surgical PDC insertions between January 2003 and April 2010 (group S, n = 162).
♦ Results: Compared with group P patients, significantly more group S patients had undergone previous abdominal surgery or PDC insertion (41.8% vs 9.3% and 33.3% vs 3.3% respectively, p = 0.00). More exit-site leaks occurred in group P than in group S (20.5% vs 6.8%, p = 0.002). The overall incidence of peritonitis was higher in group S than in group P (1 episode in 19 catheter-months vs 1 episode in 26 catheter-months, p = 0.017), but the groups showed no significant difference in the peritonitis rate within 1 month of catheter insertion (5% in group P vs 7.4% in group S, p = 0.4) or in poor initial drainage or secondary drainage failure (9.9% vs 11.7%, p = 0.1, and 7.9% vs 12.3%, p = 0.38, for groups P and S respectively).Technical survival at 3 months was significantly better for group P than for group S (86.6% vs 77%, p = 0.037); at 12 months, it was 77.7% and 68.7% respectively (p = 0.126). No life-threatening complications attributable to the insertion of the PDC occurred in either group.
♦ Conclusions: Our analysis demonstrates further encouraging outcomes of percutaneous PDC placement compared with open surgical placement. However, the members of the percutaneous insertion group were primarily a selected subset of patients without prior abdominal surgery or PDC insertion, therefore limiting the comparability of the groups. Studies addressing such confounding factors are required. Local expertise in catheter placement techniques may affect the generalizability of results.
Keywords: Continuous ambulatory peritoneal dialysis, PD catheter, technical survival, percutaneous insertion, surgical insertion
Success in carrying out peritoneal dialysis (PD) requires a functioning catheter and the ability of the patient or carer to master the PD technique. Several methods for peritoneal dialysis catheter (PDC) insertion have evolved, including an open surgical procedure; laparoscopic insertion; and the percutaneous Seldinger, peritoneoscopic, and fluoroscopic placement techniques. The percutaneous Seldinger method is the least invasive technique, and yet it is not commonly practiced. The advantages of percutaneous PDC insertion include avoidance of general anesthesia (GA) and the delays imposed by wait-listing for surgery and for theater time allocation; potentially less post-procedure pain, with faster recovery and ambulation; and efficient use of resources. Recent studies have shown favorable outcomes with percutaneous PDC placement, demonstrating the efficacy and safety of the technique (1,2).
Here, we report the outcomes of 151 PDCs inserted percutaneously between July 1998 and April 2010 (group P) and 162 catheters inserted by conventional surgical technique between January 2003 and April 2010 (group S) in our unit. To our knowledge, this study includes the largest cohort of patients with percutaneously inserted PDCs reported in a comparative analysis of PDC placement techniques.
METHODS
DATA COLLECTION
Information was obtained from our database of patients undergoing PDC placement between July 1998 and April 2010. However, surgical insertions were not recorded in the database before January 2003, and thus, the target study group included all percutaneous PDC insertions during the specified period, but the comparator group comprised PDCs inserted surgically between January 2003 and April 2010. Clinical outcomes and complications of all PDCs were analyzed up to April 2011.
Medical records of patients who had received catheters were reviewed for clinical data, and the hospital’s laboratory database was used to confirm peritonitis episodes. The study was granted ethical approval by the Research Ethics Committee of St James’s Hospital/Adelaide & Meath Hospital, Dublin, incorporating the National Children’s Hospital.
CATHETER INSERTION
Contraindications to percutaneous catheter insertion were open abdominal surgery other than appendectomy or cesarean section, a bleeding tendency, and preexisting hernia requiring simultaneous repair. Otherwise, the chosen method of insertion depended largely on the caring physician’s preference and operator availability.
Percutaneous catheter insertion was carried out as a side-room procedure in the renal ward. A curled two-cuffed Tenckhoff catheter was placed using the Seldinger technique under aseptic conditions by the method described in a previous paper (3). The catheter was inserted under local anesthesia and conscious sedation with oral diazepam 10 mg. Premedication as part of the PDC insertion protocol included intravenous piperacillintazobactam 4.5 g, and DF118 (dihydrocodeine) 60 mg. Percutaneous insertions were performed by a consultant nephrologist and occasionally by senior nephrology trainees under supervision.
Surgical insertion of the same two-cuffed coiled Tenckhoff catheters was carried out under GA in a theater room using the standard open surgical technique (4). The procedure was performed by consultant surgeons or surgical trainees under direct supervision.
Between January 2003 and April 2010, 170 PDCs were inserted by the surgical method. We excluded 8 of those catheters: 4 because of insufficient or missing data, and another 4 because they were not used (1 patient switched to hemodialysis for personal reasons, 1 required extensive abdominal surgery for newly diagnosed bladder cancer, 1 experienced recurrence of a large abdominal hernia that precluded PD, and 1 underwent transplantation before catheter use). Of the 162 catheters inserted percutaneously between July 1998 and April 2010, 10 were excluded because of missing data, and 1, because it was never used (the patient died of acute myocardial infarction).
Peritonitis was identified by clinical history and physical findings documented in the medical notes (abdominal pain, fever, nausea, diarrhea, abdominal tenderness), and also by laboratory data (dialysate white cell count exceeding 100/mm3, or a positive effluent culture, or both). Positive swab cultures from patients with suspected exit-site infection were identified from the laboratory database. We defined poor initial drainage as significant drainage failure within 48 hours of commencing PD exchanges, necessitating either catheter manipulation or replacement or discontinuation of PD. We defined secondary drainage failure as catheter blockage or dysfunction occurring more than 48 hours after successful initial drainage and resulting in catheter manipulation or replacement or discontinuation of PD. We differentiated between catheter removal for mechanical or infectious complications of the PDC and non-catheter-related reasons for discontinuation of PD, including death, transplantation, recovery of renal function, and transfer to hemodialysis because of ultrafiltration failure, poor clearance, or patient choice. In addition to catheter replacement and ongoing catheter use, the foregoing clinical endpoints or events that resulted in discontinuation of PD were considered primary outcomes. The analysis looked at individual catheter outcomes rather than patient outcomes. As in similar earlier studies, that approach allowed for representation of patients more than once if they received more than 1 PDC within the study period, thus avoiding bias imposed by deselecting re-insertions during that time. Whenever a PDC catheter was replaced, either surgically or percutaneously, the new catheter was analyzed as a separate event. Primary outcomes were recorded retrospectively at 3 and 12 months after insertion of the PDC.
STATISTICAL ANALYSIS
Mean and standard deviations were calculated for continuous parametric data, and medians and interquartile ranges for nonparametric data. We report frequencies for categorical data. For group comparisons, we used the Student t-test, Mann-Whitney test, or chi-square test (Pearson or Fisher exact test), as appropriate. For rates, we calculated rate ratios with 95% confidence intervals. We used two methods to assess technical survival of PDCs. In an initial analysis, we excluded PDCs that were no longer in use at specified point intervals (3 and 12 months after catheter insertion) because of patient-related factors as opposed to catheter-related complications. Alternatively, Kaplan-Meier curves were generated to represent catheter survival in the two groups for the first 12 months after catheter insertion (censored for PD cessation for reasons other than catheter complications). The statistical analysis was carried out using the SPSS software application (version 15.0: SPSS, Chicago, IL, USA).
RESULTS
Table 1 presents the baseline characteristics of the two PDC groups. No differences in age, sex, or duration of follow-up were observed between the two groups. Significantly more patients in group S than in group P had undergone previous abdominal surgery or PDC insertion (41.8% vs 9.3% and 33.3% vs 3.3% respectively, p = 0.00). Patients with polycystic kidney disease (PCKD) were more likely to have undergone surgical PDC insertion in view of the theoretic risk of cyst rupture during the blind percutaneous technique. Total accumulated PDC experience was 2567 patient-months in group P and 2717 patient-months in group S.
TABLE 1.
Baseline Characteristics of the Study Groups
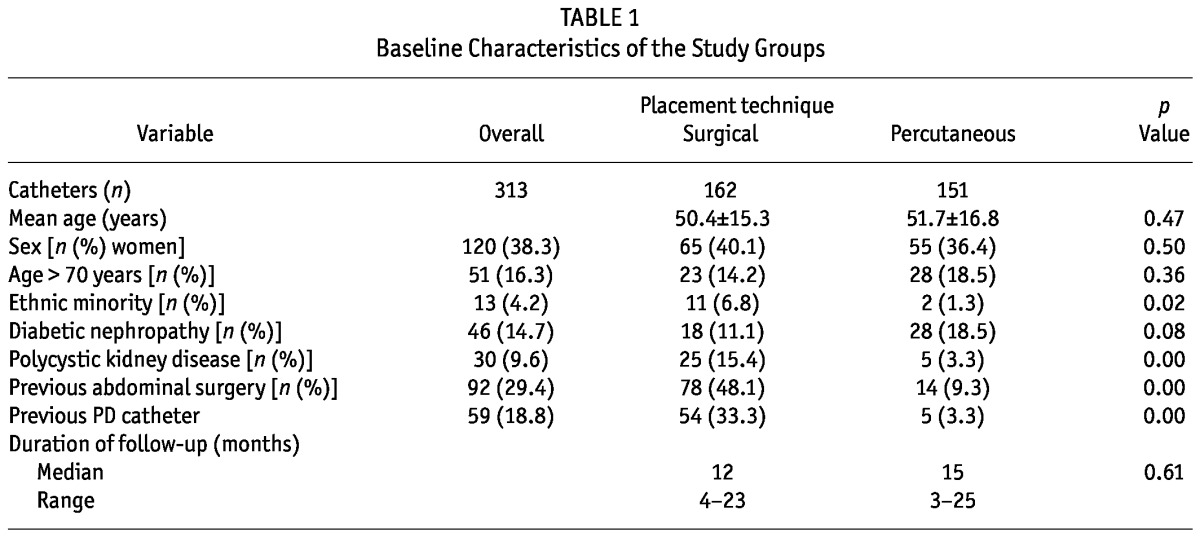
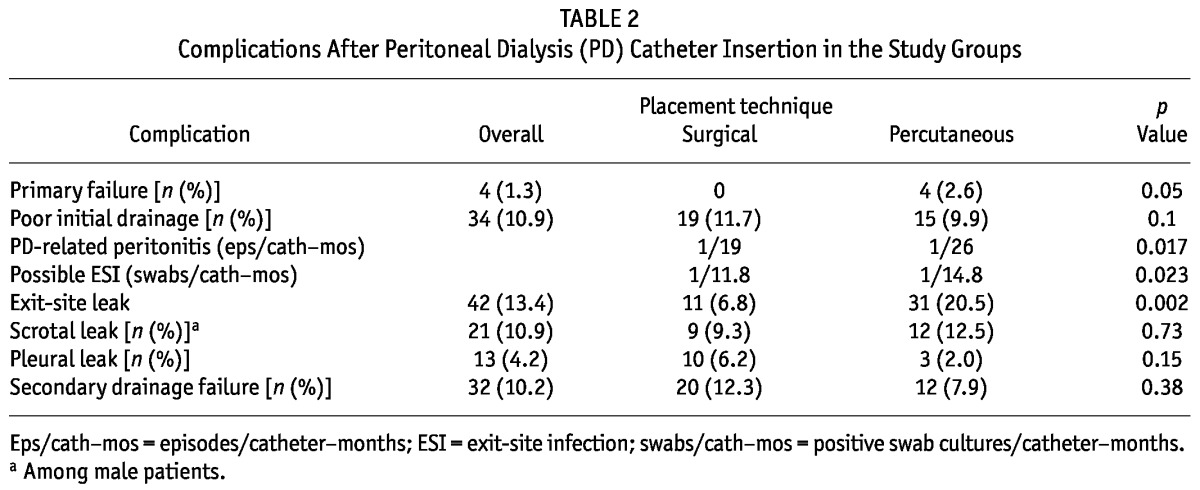
Table 2 shows treatment-related complications after PDC insertion. More exit-site leaks occurred in group P than in group S (20.5% vs 6.8%, p = 0.002), but we observed no statistically significant differences between the groups in poor initial drainage (9.9% vs 11.7%, p = 0.1) or secondary drainage failure (7.9% vs 12.3%, p = 0.38). Peritonitis rates were 3.85 and 5.26 episodes per 100 patient-months for group P and group S respectively (rate ratio: 1.36; 95% confidence interval: 1.06 to 1.76; p = 0.017). No difference was evident in the incidence of early peritonitis (within 1 month of catheter insertion) between the two groups (5% in group P vs 7.4% in group S, p = 0.4). The mean interval between first PDC use and late-onset peritonitis (more than 1 month after catheter insertion) was 15.9 months in group P and 18.3 months in group S. Exit-site leaks almost always occurred early (within 1 month of insertion); the one exception was a leak that occurred 8 weeks after percutaneous PDC insertion. All exit-site leaks settled conservatively after PD was withheld for a few days. Rates of positive exit-site swabs taken for suspected infection were 1 in 14.8 patient-months in group P and 1 in 11.8 patient-months in group S (rate ratio: 1.26; 95% confidence interval: 1.03 to 1.54; p = 0.02).
TABLE 2.
Complications After Peritoneal Dialysis (PD) Catheter Insertion in the Study Groups
The incidence of abdominal-wall hernia after PDC placement was 10% in group P and 15.4% in group S. In both groups, approximately one third of the hernias were inguinal. Most hernias were repaired. Only 1 patient of the 12 in group P who developed abdominal-wall hernia had PCKD; 1 patient had previously undergone nephrectomy. Of the group S patients, 25% of those who developed abdominal wall hernias had PCKD; 40% had undergone abdominal surgery before the PDC insertion.
Of the group P catheters, 10% required laparoscopic manipulation to restore catheter function. At 12 months from the original insertion date, two thirds of the manipulated catheters in group P were in use, and none had been removed because of catheter-related complications. Of the group S catheters, 10.5% required manipulation. At 12 months from the original insertion date, 8 had been removed because of catheter-related complications; 3 had been removed for patient-related indications; and 3 remained in use. During the study period, 10.6% of group P catheters and 13% of group S catheters were replaced. The primary failure rate was 2.6% (4 patients) in group P and 0% (no insertion failures) in group S. Unsuccessful insertions resulted from failure to advance the guidewire.
No major or life-threatening complications resulted from either surgical or percutaneous PDC placement. No significant bleeding occurred after insertion in either group.
Table 3 shows the causes of dropout to hemodialysis. All group P patients whose PD was interrupted because of unresolved drainage failure resumed PD with a new catheter; none transferred to hemodialysis because of catheter dysfunction.
TABLE 3.
Reasons for Transfer to Hemodialysis in the Study Groups
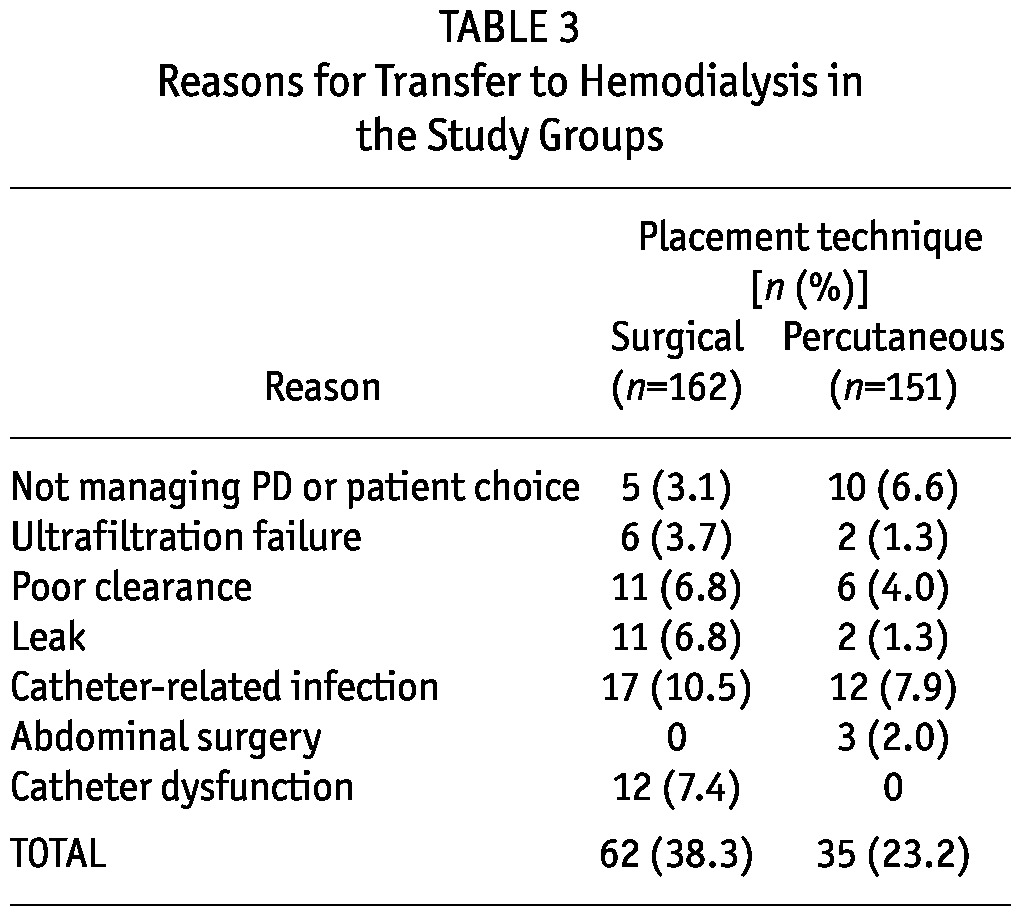
Table 4 shows the primary catheter outcomes at the 3- and 12-month time points. Significantly more patients in group S switched to hemodialysis because of catheter-related complications (p = 0.00 at 3 and 12 months). Catheter technical survival was significantly better for group P than for group S at 3 months (86.6% vs 77%, p = 0.037); at 12 months, the rates were nonsignificantly different (77.7% vs 68.7%, p = 0.126, Table 5). Figure 1 shows Kaplan-Meier survival plots for the two groups.
TABLE 4.
Primary Outcomes of Peritoneal Dialysis Catheter Insertions in the Study Groups at 3 and 12 Months
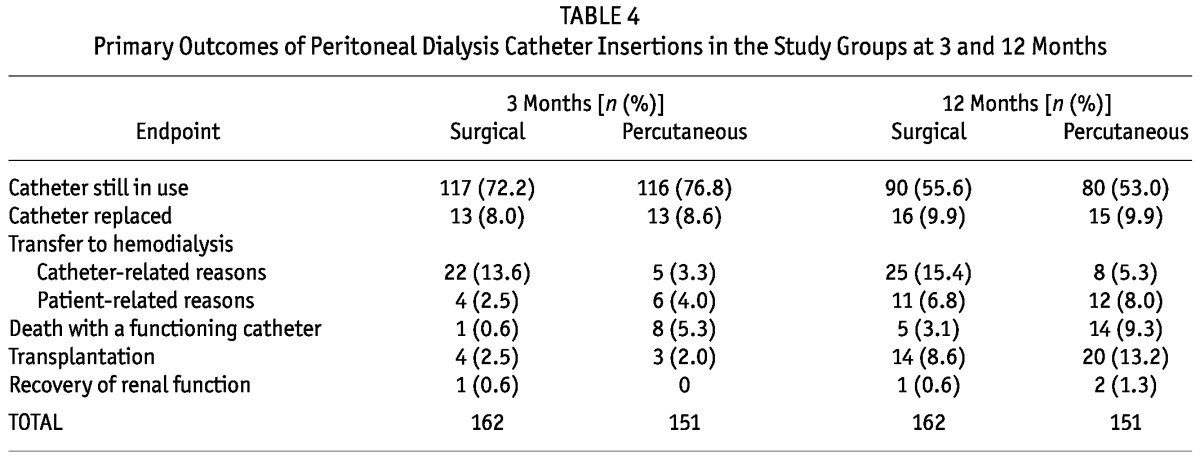
TABLE 5.
Peritoneal Dialysis Catheter Survivala in the Study Groups
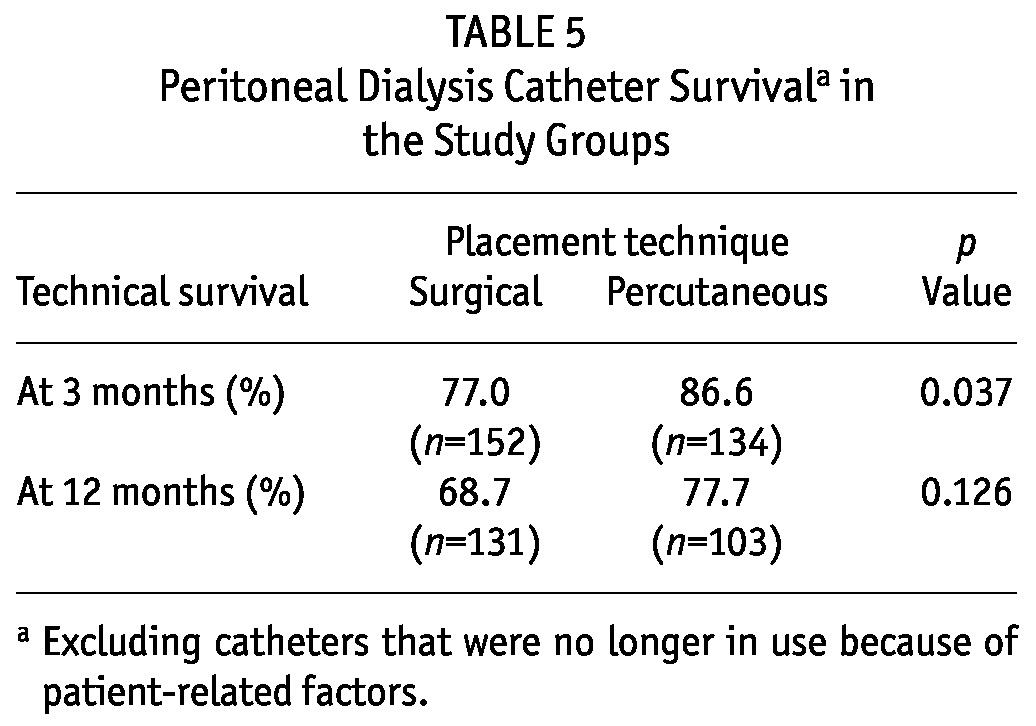
Figure 1.
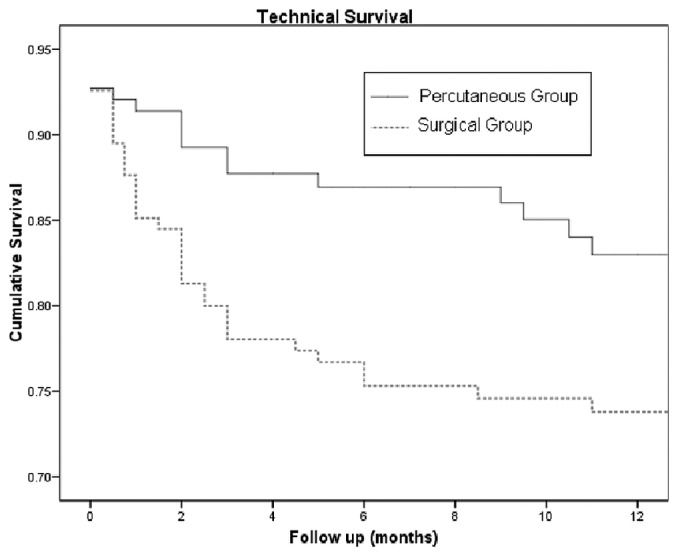
— Kaplan-Meier survival plots for the percutaneously and surgically placed catheters.
More patients in group P than in group S died with a functioning catheter at 3 and 12 months after catheter insertion (p = 0.02). Of the group P catheters, most (96.7%) were first catheters, because re-insertion was usually done surgically. Of the group S catheters, two thirds were first catheters, and one third were reinsertions. Of the surgical reinsertions, 30% occurred in patients whose first catheter had been inserted percutaneously during the study period; those patients were therefore represented in both study groups. The others either underwent catheter insertion before the study period started or were represented more than once within group S—that is, they underwent more than 1 surgical PDC insertion during the study period.
DISCUSSION
Access to timely insertion of a PDC is a crucial factor for improving PD utilization and avoiding urgent hemodialysis (5-7). Since the development of the Tenckhoff catheter in the mid-1960s, and after the introduction of continuous ambulatory PD (CAPD) in the 1970s, PDC placement techniques have continually been advanced, with myriad experiences being reported by various centers (1,2,8-31). The diversity of those experiences reflects the importance of operator skills and local facilities in determining practices and outcomes of PDC insertion. Evidence as to which of the insertion methods produces the best clinical outcomes is still lacking. However, it is generally accepted that open surgical or laparoscopic techniques are recommended for patients in whom abdominal adhesions are suspected or for times when a blind technique is considered less safe—as in very obese patients and those with a bleeding disorder (32,33). Surgical insertion of PDCs by nephrologists has also been described (31).
Recently, the interest in percutaneous PDC placement has been growing (1,2). Although this technique has traditionally been reserved for patients unfit for GA (3), it is now increasingly being adopted as a front-line approach in younger and healthier patients. As a result, outcomes comparisons of percutaneous insertions with conventional surgical insertions in broadly similar groups of patients have become possible. Our results emphasize this trend toward avoiding unnecessary GA and the constraints arising from the need to coordinate theater time and surgeon availability. The simplicity and relative inexpensiveness of the percutaneous technique may be of particular interest in the setting of limited health care resources, addressing not only accessibility in developing countries, but also resource allocation and cost-effectiveness in the developed world. The percutaneous PDC insertion technique maintains its advantageous safety profile and applicability in patients who are not candidates for GA or invasive surgery. On the other hand, surgical and laparoscopic techniques retain their utility, particularly for insertions considered high-risk because of suspected abdominal adhesions or complex underlying anatomy.
Because percutaneous placement is a blind technique, the major and potentially life-threatening complication associated with it is visceral injury. The risk is increased in patients who have undergone previous surgery or experienced severe peritonitis. This particular complication was not observed in our study group. We observed a higher rate of death with a functioning catheter in group P, but those deaths resulted mostly from acute illnesses and underlying disease processes unrelated to PD. One exception was a fungal peritonitis, which may have contributed to death in an elderly patient who had severe Clostridium difficile colitis. Of the group P patients, 3 underwent autopsy to confirm the cause of death.
As in previous studies comparing PDC insertion techniques, our study has several limitations, some universal and yet unique to a group of subjects dependent on renal replacement therapy. The rates of drop-out to an alternative modality of renal replacement are unpredictable and can be independent of patient compliance or the success of catheter insertion. Exclusion or censoring of drop-out attributable to non-catheter-related indications (as in this study) allows for an estimation of survival probability, but can impart undetected selection bias. Premature removal of a PDC because of PD-related complications such as primary failure or poor initial drainage results in unmeasured confounding in the analysis of secondary drainage failure or leakage. Technical survival of PDCs as defined earlier in this paper is affected by patient willingness to continue PD and by the physician’s advice in the face of treatment-related complications. In addition, there is likely to be a significant effect of operator bias on the reported outcomes and complications.
Although the baseline characteristics of our two study groups were broadly similar, there were significant differences in the percentages of patients who had previously undergone abdominal surgery and PDC insertion. Those differences could be relevant in the assessment of outcomes, because adhesions secondary to earlier surgery or peritonitis may contribute to PDC dysfunction. We therefore carried out a subgroup analysis, which showed that, in a cohort of patients with no prior abdominal surgery, technical survival after percutaneous PDC insertion still compared favorably with that after surgical PDC insertion. The results of that analysis will be reported in a separate paper (Medani S, Hussein W, Shantier M, Wall C, Mellotte G. Comparison of percutaneous and open surgical techniques for first-time peritoneal dialysis catheter placement in the unbreached peritoneum. Presented at the Irish Nephrology Society Annual Meeting, 13 May 2011, Dublin).
In the present retrospective comparative analysis, we excluded catheter insertions for patients whose medical notes were incomplete. However, of 333 PDC insertions, only 14 (4.2%) were excluded because of missing data.
The time to catheter use post-insertion was not standardized. We believe that the latter factor might have contributed to the difference seen in the rates of pericatheter leakage between the groups. The average interval between catheter placement and use was 5.5 days in group P and 8 days in group S.
Clinical data on exit-site infections were not consistently recorded. Swabs were usually sent when pericatheter erythema or a purulent discharge was detected. We report the rates of positive exit-site cultures. Given the retrospective nature of the analysis, those rates may not accurately reflect acute exit-site infection rates, because routine re-swabbing in patients previously treated for an exit-site infection or in colonized patients without active infection may be included.
Length of hospital stay after PDC insertion varied considerably from patient to patient in both groups, depending on factors such as comorbidities necessitating inpatient treatment, initiation of pre-transplantation work-up, and social and community support arrangements when needed. Significantly more patients in group S had PCKD, but whether that difference is relevant to the study outcomes is unknown. Small retrospective studies have shown no differences in technical survival and peritonitis rates between PCKD patients receiving PD and matched nondiabetic control subjects (34,35).
Lastly, the percutaneous insertions included in the study date back to 1998, but the surgical insertions were those logged from 2003, after commencement of dual recording of insertions. For future prospective studies, a carefully designed computerized database system could provide the means for better monitoring of outcomes, thereby addressing some of the foregoing limitations.
CONCLUSIONS
Percutaneous PDC placement compares favorably with the open surgical technique in terms of technical survival and overall complication rates. The interplay of patient factors, selection bias, and physician preferences may affect measured outcomes, and studies eliminating those confounders are warranted. Local facilities and operator skills should continue to dictate practices in PDC insertion until randomized and, ideally, multicenter prospective studies are conducted in suitable patients to establish whether any particular technique is superior. The percutaneous Seldinger method offers the advantage of ease of insertion with cost-effectiveness and can be performed as a day procedure.
We recommend the implementation of formal training of nephrology trainees in this bedside insertion technique, particularly in health care systems with limited resources.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1. Henderson S, Brown E, Levy J. Safety and efficacy of percutaneous insertion of peritoneal dialysis catheters under sedation and local anaesthetic. Nephrol Dial Transplant 2009; 24:3499–509 [DOI] [PubMed] [Google Scholar]
- 2. Ozener C, Bihorac A, Akoglu E. Technical survival of CAPD catheters: comparison between percutaneous and conventional surgical placement techniques. Nephrol Dial Transplant 2001; 16:1893–9 [DOI] [PubMed] [Google Scholar]
- 3. Mellotte GJ, Ho CA, Morgan SH, Bending MR, Eisinger AJ. Peritoneal dialysis catheters: a comparison between percutaneous and conventional surgical placement techniques. Nephrol Dial Transplant 1993; 8:626–30 [PubMed] [Google Scholar]
- 4. Peppelenbosch A, van Kuijk WHM, Bouvy ND, van der Sande FM, Tordoir JHM. Peritoneal dialysis catheter placement technique and complications. Clin Kidney J 2008; 1(Suppl 4):iv23–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goh BL, Ganeshadeva YM, Chew SE, Dalimi MS. Does peritoneal dialysis catheter insertion by interventional nephrologists enhance peritoneal dialysis penetration? Semin Dial 2008; 21:561–6 [DOI] [PubMed] [Google Scholar]
- 6. Asif A, Pflederer TA, Vieira CF, Diego J, Roth D, Agarwal A. Does catheter insertion by nephrologists improve peritoneal dialysis utilization? A multicenter analysis. Semin Dial 2005; 18:157–60 [DOI] [PubMed] [Google Scholar]
- 7. Asif A, Byers P, Gadalean F, Roth D. Peritoneal dialysis underutilization: the impact of an interventional nephrology peritoneal dialysis access program. Semin Dial 2003; 16:266–71 [DOI] [PubMed] [Google Scholar]
- 8. Allon M, Soucie JM, Macon EJ. Complications with permanent peritoneal dialysis catheters: experience with 154 percutaneously placed catheters. Nephron 1988; 48:8–11 [DOI] [PubMed] [Google Scholar]
- 9. Adamson AS, Kelleher JP, Snell ME, Hulme B. Endoscopic placement of CAPD catheters: a review of one hundred procedures. Nephrol Dial Transplant 1992; 7:855–7 [PubMed] [Google Scholar]
- 10. Weber J, Mettang T, Hübel E, Kiefer T, Kuhlmann U. Survival of 138 surgically placed straight double-cuff Tenckhoff catheters in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 1993; 13:224–7 [PubMed] [Google Scholar]
- 11. Eklund B, Groop PH, Halme L, Honkanen E, Kala AR. Peritoneal dialysis access: a comparison of peritoneoscopic and surgical insertion techniques. Scand J Urol Nephrol 1998; 32:405–8 [DOI] [PubMed] [Google Scholar]
- 12. Euthimiadou A, Thodis E, Passadakis P, Tsalikis D, Kaisas G, Vargemezis V. Nonsurgical implantation of Tenckhoff peritoneal catheters in patients on continuous ambulatory peritoneal dialysis. Adv Perit Dial 1999; 15:101–4 [PubMed] [Google Scholar]
- 13. Wright MJ, Bel’eed K, Johnson BF, Eadington DW, Sellars L, Farr MJ. Randomized prospective comparison of laparoscopic and open peritoneal dialysis catheter insertion. Perit Dial Int 1999; 19:372–5 [PubMed] [Google Scholar]
- 14. Gadallah MF, Pervez A, el-Shahawy MA, Sorrells D, Zibari G, McDonald J, et al. Peritoneoscopic versus surgical placement of Tenckhoff catheters; a prospective randomized study on outcome. Am J Kidney Dis 1999; 33:118–22 [DOI] [PubMed] [Google Scholar]
- 15. Tsimoyiannis EC, Siakas P, Glantzounis G, Toli C, Sferopoulos G, Pappas M, et al. Laparoscopic placement of the Tenckhoff catheter for peritoneal dialysis. Surg Laparosc Endosc Percutan Tech 2000; 10:218–21 [PubMed] [Google Scholar]
- 16. Savader SJ, Geschwind JF, Lund GB, Scheel PJ. Percutaneous radiologic placement of peritoneal dialysis catheters: long-term results. J Vasc Interv Radiol 2000; 11:965–70 [DOI] [PubMed] [Google Scholar]
- 17. Roueff S, Pagniez D, Moranne O, Roumilhac D, Talaszka A, Le Monies De Sagazan H, et al. Simplified percutaneous placement of peritoneal dialysis catheters: comparison with surgical placement. Perit Dial Int 2002; 22:267–9 [PubMed] [Google Scholar]
- 18. Lu CT, Watson DI, Elias TJ, Faull RJ, Clarkson AR, Bannister KM. Laparoscopic placement of peritoneal dialysis catheters: 7 years experience. ANZ J Surg 2003; 73:109–11 [DOI] [PubMed] [Google Scholar]
- 19. Oğünç G, Tuncer M, Oğünç D, Yardimsever M, Ersoy F. Laparoscopic omental fixation technique vs open surgical placement of peritoneal dialysis catheters. Surg Endosc 2003; 17:1749–55 [DOI] [PubMed] [Google Scholar]
- 20. Kelly J, McNamara K, May S. Peritoneoscopic peritoneal dialysis catheter insertion. Nephrology (Carlton) 2003; 8:315–17 [DOI] [PubMed] [Google Scholar]
- 21. Dequidt C, Vijt D, Veys N, Van Biesen W. Bed-side blind insertion of peritoneal dialysis catheters. EDTNA ERCA J 2003; 29:137–9 [DOI] [PubMed] [Google Scholar]
- 22. Zaman F, Pervez A, Atray NK, Murphy S, Work J, Abreo KD. Fluoroscopy-assisted placement of peritoneal dialysis catheters by nephrologists. Semin Dial 2005; 18:247–51 [DOI] [PubMed] [Google Scholar]
- 23. Ogunc G. Minilaparoscopic extraperitoneal tunneling with omentopexy: a new technique for CAPD catheter placement. Perit Dial Int 2005; 25:551–5 [PubMed] [Google Scholar]
- 24. Soontrapornchai P, Simapatanapong T. Comparison of open and laparoscopic secure placement of peritoneal dialysis catheters. Surg Endosc 2005; 19:137–9 [DOI] [PubMed] [Google Scholar]
- 25. Schmidt SC, Pohle C, Langrehr JM, Schumacher G, Jacob D, Neuhaus P. Laparoscopic-assisted placement of peritoneal dialysis catheters: implantation techniques and results. J Laparoendosc Adv Surg Tech A 2007; 17:596–9 [DOI] [PubMed] [Google Scholar]
- 26. Maya ID. Ultrasound/fluoroscopy-assisted placement of peritoneal dialysis catheters. Semin Dial 2007; 20:611–15 [DOI] [PubMed] [Google Scholar]
- 27. Moon JY, Song S, Jung KH, Park M, Lee SH, Ihm CG, et al. Fluoroscopically guided peritoneal dialysis catheter placement: long-term results from a single center. Perit Dial Int 2008; 28:163–9 [PubMed] [Google Scholar]
- 28. Maio R, Figueiredo N, Costa P. Laparoscopic placement of Tenckhoff catheters for peritoneal dialysis: a safe, effective, and reproducible procedure. Perit Dial Int 2008; 28:170–3 [PubMed] [Google Scholar]
- 29. Crabtree JH, Burchette RJ. Effective use of laparoscopy for long-term peritoneal dialysis access. Am J Surg 2009; 198:135–41 [DOI] [PubMed] [Google Scholar]
- 30. Yang PJ, Lee CY, Yeh CC, Nien HC, Tsai TJ, Tsai MK. Minilaparotomy implantation of peritoneal dialysis catheters: outcome and rescue. Perit Dial Int 2010; 30:513–18 [DOI] [PubMed] [Google Scholar]
- 31. Chow KM, Szeto CC, Leung CB, Kwan BC, Pang WF, Li PK. Tenckhoff catheter insertion by nephrologists: open dissection technique. Perit Dial Int 2010; 30:524–7 [DOI] [PubMed] [Google Scholar]
- 32. Figueiredo A, Goh BL, Jenkins S, Johnson DW, Mactier R, Ramalakshmi S, et al. on behalf of the International Society for Peritoneal Dialysis. Clinical practice guidelines for peritoneal access. Perit Dial Int 2010; 30:424–9 [DOI] [PubMed] [Google Scholar]
- 33. Crabtree JH. Fluoroscopic placement of peritoneal dialysis catheters: a harvest of the low hanging fruits. Perit Dial Int 2008; 28:134–7 [PubMed] [Google Scholar]
- 34. Kumar S, Fan SL, Raftery MJ, Yaqoob MM. Long term outcome of patients with autosomal dominant polycystic kidney disease receiving peritoneal dialysis. Kidney Int 2008; 74:946–51 [DOI] [PubMed] [Google Scholar]
- 35. Li L, Szeto CC, Kwan BC, Chow KM, Leung CB, Li PKT. Peritoneal dialysis as the first-line renal replacement therapy in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis 2011; 57:903–7 [DOI] [PubMed] [Google Scholar]


