Posterior reversible encephalopathy syndrome [PRES (also known as reversible posterior leukoencephalopathy syndrome)] presents with rapid onset of symptoms including headache, seizures, altered consciousness, and visual disturbance (1,2). It is often—but by no means always—associated with acute hypertension (1,2). If promptly recognized and treated, the clinical syndrome usually resolves within a week (2,3), and the changes seen in magnetic resonance imaging (MRI) resolve over days to weeks (2-4).
Chronic kidney disease and acute kidney injury are both commonly present in patients with PRES (4), and PRES is strongly associated with conditions that co-exist in patients with renal disease, such as hypertension, vascular and autoimmune diseases, exposure to immunosuppressive drugs, and organ transplantation. It is therefore important to consider PRES in the differential diagnosis of patients with renal disease and rapidly progressive neurologic symptoms. Posterior reversible encephalopathy syndrome is an increasingly recognized disorder, with a wide clinical spectrum of both symptoms and triggers, and yet it remains poorly understood.
CLINICAL FEATURES
Commonly, PRES evolves over a matter of hours, with the most common presenting symptoms being seizures, disturbed vision, headache, and altered mental state (4) (Figure 1). More than 70% of patients with PRES are hypertensive, though a significant proportion have normal or only mildly raised blood pressure (3-7).
Figure 1.
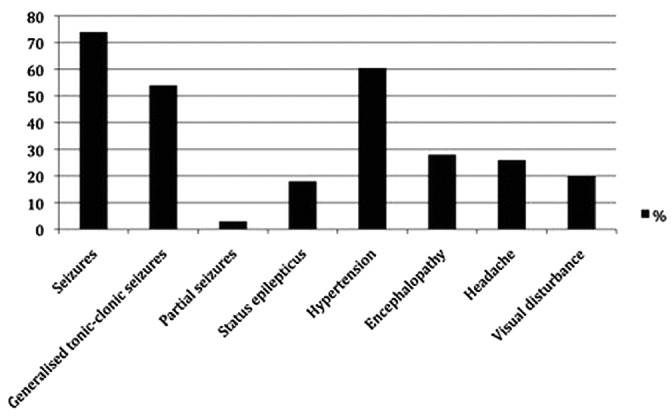
— Frequency of posterior reversible encephalopathy syndrome signs and symptoms seen in a series of 120 cases (4).
The severity of clinical symptoms varies. For example, the visual disturbance can manifest as blurred vision, homonymous hemianopsia, or even cortical blindness (2). Patients may be mildly confused or agitated, but can become comatose (2). Other symptoms less commonly seen include nausea, vomiting, and brainstem deficits (2,3). Seizures and status epilepticus are common, and nonconvulsive status epilepticus may be more frequent than generalized status epilepticus (8). Nonconvulsive status should be suspected in patients with prolonged states of altered consciousness and may be mistaken for postictal confusion. Signs of nonconvulsive seizures include stereotypic movements such as staring, eye blinking, or head turning. Postictal confusion lasts for hours, but PRES and nonconvulsive status can both persist for several days and be mistaken for psychosis, drug intoxication, or psychogenic states (9).
A trigger is usually identifiable—most commonly, acute hypertension—but patients often have other comorbidities that may predispose them to developing PRES. Peak systolic blood pressure is usually between 170 mmHg and 190 mmHg (2,4), but 10% - 30% of patients have normal or only mildly elevated blood pressure (2,4). In PRES, the causes of acute hypertension are commonly acute kidney injury or eclampsia (3,4), but hypertension is also reported in cases of autonomic disturbance, for example Guillain-Barré syndrome (10,11), and after illicit drug use (1). In a large series of cases recording the comorbidities of patients with PRES, more than half the patients had chronic hypertension, and 38% had chronic kidney disease (4). Autoimmune diseases— including thrombotic thrombocytopenic purpura (4) and systemic lupus erythematosus—were present in 45% of the patients, and exposure to immunosuppressive drugs such as cyclosporine, tacrolimus, or chemotherapy agents was present in a similar number, particularly in the context of transplantation (2,4,12).
The patient reported by Ogawa and colleagues in this issue of Peritoneal Dialysis International had a number of risk factors for PRES, including end-stage renal disease and chronic hypertension. It is likely that the trigger was the acute increase in blood pressure subsequent to poor compliance with peritoneal dialysis and medication. Instances of PRES have been reported in other patients using hemodialysis and peritoneal dialysis (7,12-14).
PATHOPHYSIOLOGY
The cause of PRES remains controversial, but the most popular theory is that severe hypertension causes interruption to brain autoregulation (2,15). Cerebral blood flow is usually regulated by dilatation and constriction of vessels to maintain adequate tissue perfusion (15) and to simultaneously avoid excessive intracerebral hypertension. Breakdown in autoregulation occurs above a mean (two thirds diastolic, plus one third systolic) arterial blood pressure of 150 - 160 mmHg; in chronic hypertension, it occurs at higher pressures (15). Uncontrolled hypertension leads to hyperperfusion and cerebral vessel damage, resulting in interstitial extravasation of proteins and fluids, causing vasogenic edema (15). Irreversible damage is seen at mean arterial pressures above 200 mmHg (15). Conditions commonly co-existing in PRES, such as chronic hypertension and atherosclerosis, are known to reduce the effectiveness of autoregulation (4). However, the autoregulation theory does not explain why blood pressure in PRES does not usually reach the upper limit of autoregulation (1), why PRES occurs in the absence of hypertension (1,2,4), and why the extent of the edema is not directly related to the severity of the blood pressure (15). Furthermore, although this theory suggests brain hyperperfusion, evidence from some positron-emission tomography studies actually demonstrates cerebral hypoperfusion (15).
An alternative theory is that PRES is a result of a systemic inflammatory state causing endothelial dysfunction (15). That postulate is supported by the observation that PRES is usually associated with a systemic inflammatory process such as sepsis, eclampsia, transplantation, and autoimmune disease (15). Angiography in PRES demonstrates reversible focal and diffuse abnormalities (15), which are thought to reflect endothelial dysfunction. When blood pressure is high, the vasoconstriction that occurs during autoregulation could exacerbate such a pre-existing inflammatory endothelial dysfunction, causing hypoxia and subsequent vasogenic edema. A mechanism of that kind would explain why control of hypertension allows for recovery. But the theory does not explain why some cases of PRES seem to occur in the absence of any inflammatory state (4). It is clear, however, that further research is required to understand this potentially devastating but truly treatable condition.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of PRES is broad, and history may be limited. Venous sinus thrombosis (Figure 2) or subdural, intracerebral, or subarachnoid hemorrhage can all present with headache, seizures, reduced consciousness, and focal neurologic signs. Infective encephalitis or meningitis, particularly herpes simplex encephalitis (Figure 3), should be considered, and rapid treatment with intravenous acyclovir and antibiotics may be lifesaving while a diagnosis is still being pursued. It is important to consider the diagnosis of posterior circulation stroke, because its treatment (which may include urgent thrombolysis) and prognosis both differ from those in PRES. Basilar artery thrombosis (Figure 4) can present with progressive neurologic deficits and can result in tetraparesis, coma, or locked-in syndrome.
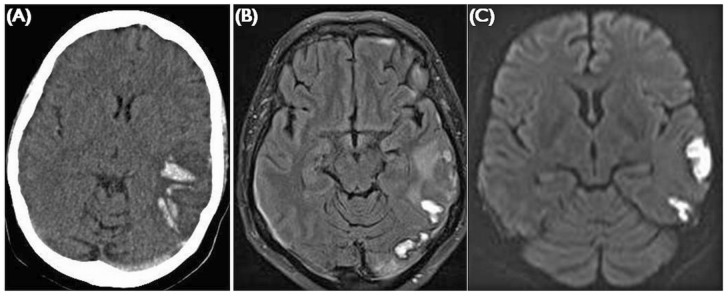
Figure 2.
— Venous sinus thrombosis. (A) Unenhanced computed tomography shows posterior temporal parenchymal hemorrhage and a hyperdense left transverse sinus. (B) FLAIR (fluid attenuated inversion recovery) imaging shows high signal in the area of hemorrhage, with vasogenic edema in the white matter. (C) Diffusion-weighted imaging can be confounded in areas of hemorrhage, giving a picture of apparent restriction. Findings are usually unilateral and restricted to a venous drainage territory.
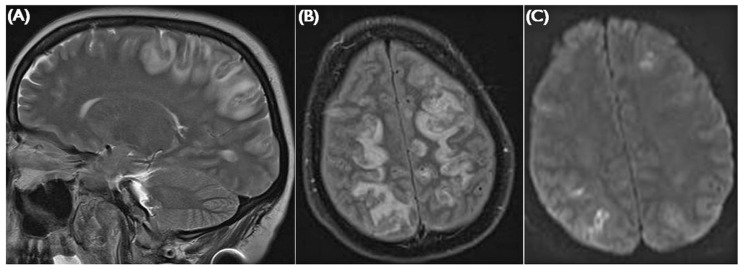
Figure 3.
— Herpes simplex encephalitis. (A) In this case, FLAIR (fluid attenuated inversion recovery) imaging shows high signal in the left insular cortex, posterior temporal lobe, cingulate gyrus, and caudate nucleus. Findings are usually bilateral and asymmetrical, and can mimic an atypical posterior reversible encephalopathy syndrome with frontal distribution. (B) Diffusion weighted changes can also occur.
Figure 4.
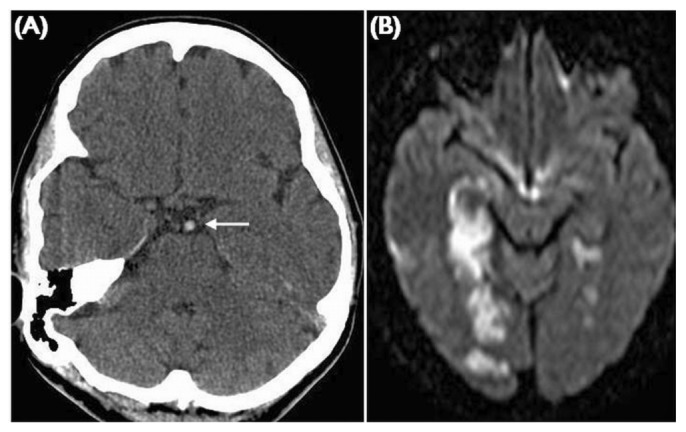
— Basilar artery thrombosis. (A) Unenhanced computed tomography demonstrates a hyperdense basilar artery indicating acute thrombus (white arrow). The diffusion weighted imaging shows bilateral posterior circulation infarcts involving the white and grey matter.
Central nervous system vasculitis can present with symptoms similar to those in PRES, but the MRI findings are usually more diffuse, and many of the clinical features and MRI findings are irreversible. The diagnosis may be difficult because systemic signs of inflammation are often absent (2,15), but rapid treatment is vital to prevent further complications. Autoimmune encephalitis and metabolic encephalopathies such as deranged serum glucose, sodium (including central pontine myelinolysis), uremia, or drug toxicity (for example cyclosporine) can also have similar progressive symptoms.
INVESTIGATIONS
In the acute setting, computed tomography (CT) imaging allows for rapid assessment. It can also exclude major cerebral hemorrhage and space-occupying lesions. Although not 100% sensitive, CT may also demonstrate venous sinus thrombosis (Figure 2) or arterial ischemia or thrombosis (Figure 4). In many situations, including in PRES, CT imaging can be normal and therefore should not provide reassurance (2). Typical MRI findings in PRES (Figure 5) are of bilateral white-matter abnormalities in vascular watershed areas in the posterior regions of both cerebral hemispheres, affecting mostly the occipital and parietal lobes (1,4,16). Atypical features— including hemorrhage, asymmetrical changes (such as in the case described by Ogawa and colleagues), isolated involvement of the frontal lobes, and cortical lesions (1,4,16)—are common. Using standard MRI, it may not be easy to differentiate PRES clinically from other acute vascular diseases, but more specialized sequences may help to identify ischemia, subtle hemorrhage, edema, or inflammation. Diagnosis requires careful selection of the appropriate imaging techniques as well as consideration of the nephrotoxic effects of some contrast media. Venous sinus thrombosis can be rapidly diagnosed by CT or magnetic resonance venography (Figure 2). Magnetic resonance venography has the advantage of not requiring nephrotoxic contrast, but it may not be available in all centers. Angiography can identify vessel thrombosis, dissection, or vasculitis.
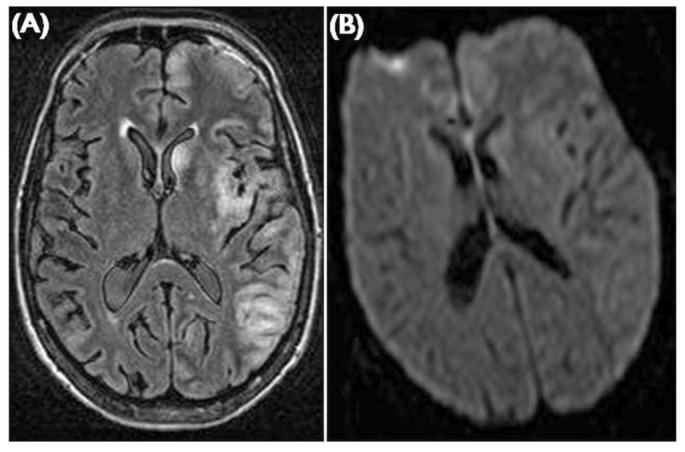
Figure 5.
— Posterior reversible encephalopathy syndrome. (A) Sagittal T2-weighted image shows the distribution of subcortical edema typically involving the posterior frontal, parietal, and occipital lobes. (B) Axial FLAIR (fluid attenuated inversion recovery) imaging eloquently demonstrates the subcortical edema. (C) B1000 images from a diffusion-weighted sequence shows restricted diffusion in the cortex. Findings are usually bilateral and asymmetrical.
Electroencephalography can identify subclinical seizures and can point to other causes of encephalopathy. Lumbar puncture can diagnose infection or subarachnoid hemorrhage, but may be normal early in the illness or after antibiotic treatment.
MANAGEMENT
No clinical trials have evaluated the management of PRES, but rapid withdrawal of the trigger appears to hasten recovery and to avoid complications: for example, aggressive blood pressure management (which may include increased ultrafiltration), withdrawal of the offending drug, or delivery in eclampsia (2). Antiepileptic drugs should be used to treat seizures, and anesthesia and ventilation should be instituted in generalized status epilepticus and to protect the airway in obtunded patients. Corticosteroids should theoretically improve vasogenic edema, but there is no evidence for their use in PRES (2).
PROGNOSIS
The patient described by Ogawa and colleagues required anesthesia because of poorly controlled seizures. Once increased peritoneal dialysis and antihypertensive agents were commenced, the patient’s clinical state improved, and she rapidly recovered. Her clinical course is typical, given that the symptoms of PRES usually resolve with a week of presentation if the triggering condition is treated and if the complications of seizures and coma are prevented. The MRI findings in the case reported by Ogawa and colleagues resolved within 8 days, but radiologic resolution can take weeks despite the more rapid clinical recovery (2-4). Persistent neurologic deficits have been reported, but are rare (2). It is unlikely that patients with uncomplicated PRES such as Ogawa’s patient will require long-term anticonvulsant therapy (2). Recurrent episodes of PRES have been reported (4,7,13), particularly in patients requiring dialysis (7,13), and it will therefore be imperative to closely monitor blood pressure and to avoid further precipitants.
SUMMARY
PRES should be considered in patients who present with seizures, altered consciousness, visual disturbance, or headache, particularly in the context of acute hypertension.
PRES has been associated with chronic and acute kidney disease, solid organ transplantation, and use of immunosuppressive drugs.
Typical MRI findings include reversible, symmetrical, posterior subcortical vasogenic edema.
If recognized and treated promptly, the rapid-onset symptoms and radiologic features usually fully resolve within days to weeks.
-
Triggers and associated conditions:
○ Acute hypertension
○ Acute kidney injury
○ Eclampsia
○ Sepsis and multi-organ failure
○ Autoimmune disease
○ Immunosuppressive drugs (for example, tacrolimus, cyclosporine, chemotherapy)
○ Illicit drugs (for example, cocaine)
○ Organ transplantation
○ Chronic hypertension
○ Chronic kidney disease
-
Differential diagnosis:
-
○ Vascular
- Cerebral venous sinus thrombosis
- Intracranial hemorrhage
- Posterior circulation stroke
- Primary central nervous system vasculitis
-
○ Non-vascular
- Infective encephalitis
- Autoimmune encephalitis
- Metabolic/toxic encephalopathy
-
DISCLOSURES
The authors have no relevant financial disclosures.
REFERENCES
- 1. McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol 2007; 189:904–12 [DOI] [PubMed] [Google Scholar]
- 2. Roth C, Ferbert A. The posterior reversible encephalopathy syndrome: what’s certain, what’s new? Pract Neurol 2011; 11:136–44 [DOI] [PubMed] [Google Scholar]
- 3. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med 1996; 334:494–500 [DOI] [PubMed] [Google Scholar]
- 4. Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc 2010; 85:427–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gokce M, Dogan E, Nacitarhan S, Demirpolat G. Posterior reversible encephalopathy syndrome caused by hypertensive encephalopathy and acute uremia. Neurocrit Care 2006; 4:133–6 [DOI] [PubMed] [Google Scholar]
- 6. Onder AM, Lopez R, Teomete U, Francoeur D, Bhatia R, Knowbi O, et al. Posterior reversible encephalopathy syndrome in the pediatric renal population. Pediatr Nephrol 2007; 22:1921–9 [DOI] [PubMed] [Google Scholar]
- 7. Ergün T, Lakadamyali H, Yilmaz A. Recurrent posterior reversible encephalopathy syndrome in a hypertensive patient with end-stage renal disease. Diagn Interv Radiol 2008; 14:182–5 [PubMed] [Google Scholar]
- 8. Kozak OS, Wijdicks EF, Manno EM, Miley JT, Rabinstein AA. Status epilepticus as initial manifestation of posterior reversible encephalopathy syndrome. Neurology 2007; 69:894–7 [DOI] [PubMed] [Google Scholar]
- 9. Kaplan PW. No, some types of nonconvulsive status epilepticus cause little permanent neurologic sequelae (or: “the cure may be worse than the disease”). Neurophysiol Clin 2000; 30:377–82 [DOI] [PubMed] [Google Scholar]
- 10. Delalande S, De Sèze J, Hurtevent JP, Stojkovic T, Hurtevent JF, Vermersch P. Cortical blindness associated with Guillain-Barré syndrome: a complication of dysautonomia? [French]. Rev Neurol (Paris) 2005; 161:465–7 [DOI] [PubMed] [Google Scholar]
- 11. Bavikatte G, Gaber T, Eshiett MU. Posterior reversible encephalopathy syndrome as a complication of Guillain-Barré syndrome. J Clin Neurosci 2010; 17:924–6 [DOI] [PubMed] [Google Scholar]
- 12. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol 2008; 65:205–10 [DOI] [PubMed] [Google Scholar]
- 13. Girişgen I, Tosun A, Sönmez F, Ozsunar Y. Recurrent and atypical posterior reversible encephalopathy syndrome in a child with peritoneal dialysis. Turk J Pediatr 2010; 52:416–19 [PubMed] [Google Scholar]
- 14. Jorge S, Lopes JA, De Almeida E, Martins Prata M. Posterior reversible encephalopathy syndrome (PRES) and chronic kidney disease [Spanish]. Nefrologia 2007; 27:650 [PubMed] [Google Scholar]
- 15. Bartynski W. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 2008; 29:1043–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartynski W. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol 2008; 29:1036–42 [DOI] [PMC free article] [PubMed] [Google Scholar]