Infectious complications remain the most significant cause for morbidity in pediatric patients receiving chronic peritoneal dialysis (PD). Although prophylactic measures have led to improved results in some centers, the frequency of peritonitis in children on PD continues to exceed that seen in adults, and peritonitis remains the most common reason for changing dialysis modality in children (1,2). The serious nature of this infection led to the creation and publication in 2000 of the Consensus Guidelines for the Treatment of Peritonitis in Pediatric Patients Receiving Peritoneal Dialysis (3), under the auspices of the International Society for Peritoneal Dialysis (ISPD). Those largely opinion-based guidelines were composed by an international committee of experts in the field of pediatric dialysis and served as the first such set of recommendations specific to the pediatric PD population. After the publication of those guidelines, the International Pediatric Peritoneal Dialysis Registry (IPPR) was established to support evaluations of the impact of implementing the guidelines on a global basis and to collect data to serve as evidence upon which future guidelines could be based. Data generated from 501 episodes of peritonitis were collected by the IPPR and serve as a foundation for many of the recommendations made in the present publication (4,5).
As with the earlier publication, an international group of experts consisting of pediatric nephrologists, a pediatric dialysis nurse, and a pediatric infectious disease specialist collaborated in the effort. Committee discussions took place face-to-face, during conference calls, and by e-mail.
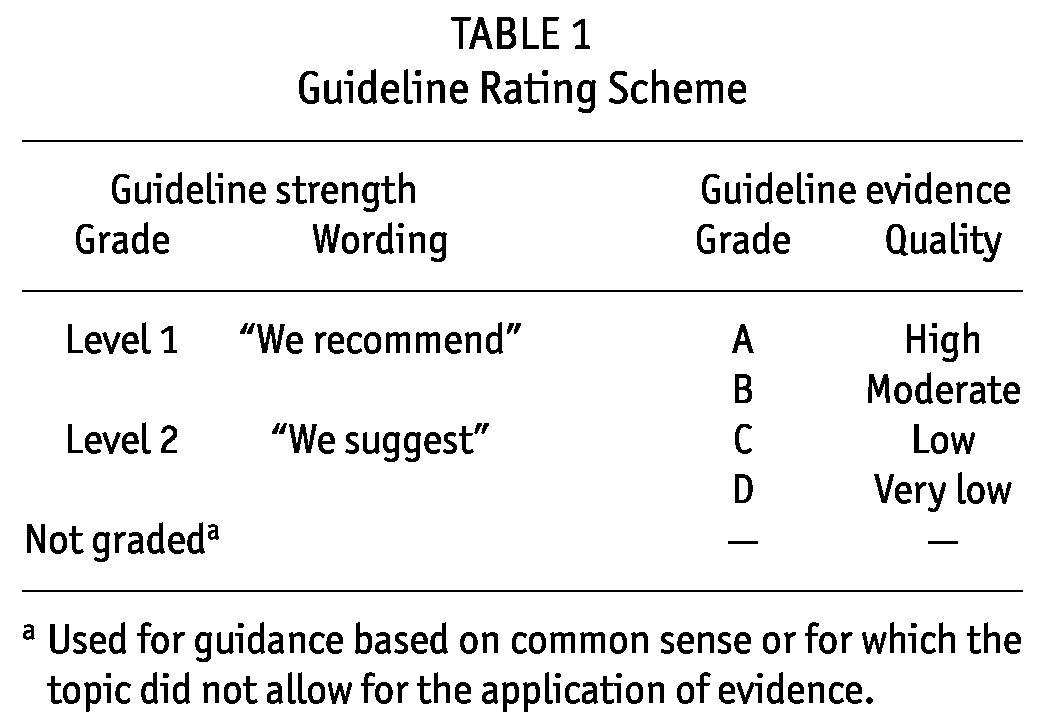
The strength of each guideline statement is graded as Level 1 or 2, or Not Graded, and the quality of the supporting evidence as A, B, C, or D in accordance with the rating scheme used in the KDIGO (Kidney Disease: Improving Global Outcomes) Clinical Practice Guideline for the Care of the Kidney Transplant Recipient (6). Table 1 describes the scheme.
TABLE 1.
Guideline Rating Scheme
Finally, wherever possible, efforts were made to achieve harmonization between the recently published adult treatment recommendations and those designed for children (7). In addition, supporting information (for example, reporting of peritonitis rates, definitions) that is included in the publication pertaining to adults and that is equally applicable to pediatric populations was included in the present publication.
GUIDELINE 1 – TRAINING
1.1 We suggest that PD training be performed by an experienced PD nurse with pediatric training, using a formalized teaching program that has clear objectives and criteria, and that incorporates adult-learning principles (2C).
1.2 We suggest that retraining be provided to all caregivers periodically. We also suggest that re-evaluation of the PD technique be conducted after development of a peritonitis episode (2C).
RATIONALE
Guideline 1.1: Although dialysis training is recognized to be paramount in a successful PD program and in the prevention of PD-related infections, systematic studies looking at the training process itself and at its relationship with patient outcomes are in short supply (8,9). Most of the published studies are adult-based. In the pediatric and adult settings alike, huge variations have been identified, nationally and internationally, in the practices within PD patient training programs—including practices relating to training content, duration, nurse-to-patient ratios, training venue (hospital or home), and trainer experience (8–10). Recent international adult surveys found no relationship between training times and peritonitis rates, but an international pediatric survey did find that peritonitis rates were significantly lower (p < 0.01) in PD programs characterized by longer training times and larger patient numbers (9–12). Further study is required to determine if this difference between the adult and pediatric experiences is related to the recipient of the education—namely, the patients themselves or the parents or caregivers.
The dialysis nurse typically conducts the PD training of patients, but unfortunately, few nurses have any formal preparation in patient education or exposure to adult-learning theory (9,13). The ISPD previously recommended that all new nephrology nurses should receive at least 12 weeks of instruction and experience within a PD unit; included should be 6 – 8 weeks of orientation, with supervision by an experienced PD nurse and observation of procedures, patient education, and clinical care (14). More recently, the ISPD further recommended that new PD trainers be supervised for at least 1 patient training course before they can serve as independent trainers (8). However, a retrospective adult study from Hong Kong surprisingly found that even patients trained by nurses with multiple years of clinical experience had an increased risk for gram-positive peritonitis (15). That finding highlights the fact that having nursing experience and clinical skill does not necessarily equate with teaching expertise. For successful PD teaching, the trainer must be willing and able to incorporate the principles of adult learning into their training program to develop proper training skills. For PD trainers, the need for continued education is also essential to ensure that skills do not become stale and the ability to apply the principles of adult learning are not lost (8).
One of the few studies to examine the impact of a PD training program on patient outcomes consisted of an industry-sponsored program that used a theory-based curriculum (13). The new curriculum was developed by an educational specialist and included clear learning objectives pertaining to cognitive, psychomotor, and affective domains of learning. The curriculum required a significantly longer training time (29 hours compared with the conventional training time of 22.6 hours). Each lesson was repeatedly taught until the trainee met each objective. Compared with patients who received conventional training, patients in the new curriculum group had a lower rate of exit-site infection (ESI: 0.22 vs 0.38 episodes per year, p < 0.004) and a borderline lower peritonitis rate (0.34 vs 0.44 episodes per year, p = 0.099) (13). Unfortunately, the study curriculum has not been released into the public domain for use. Although an evaluation of the curriculum was not part of a randomized study, that experience and the long-term experience of “prolonged” training in Japanese centers suggest that a well-structured curriculum, characterized in part by longer training times, may be associated with improved patient outcomes.
Although an optimal duration of training remains unclear, the pediatric workgroup agrees with the ISPD Nursing Liaison Committee that, for a PD training program to be deemed successful, the trainee must be able to meet (at a minimum) these 3 objectives (8):
Safely perform all required procedures.
Recognize contamination and infection.
List all appropriate responses to contamination and infection.
Because no literature has addressed the impact of teaching more than 1 patient or family simultaneously, the ISPD suggests, and we agree, that PD training should ideally occur on a 1:1 basis. A standardized teaching plan with learning objectives should be used, and all procedures taught should also be provided in written or pictorial form to the learner. It is important that all educational material be written at the fifth or sixth grade (elementary) level (10 – 12 years of age) to ensure that it can be understood by most caregivers (16,17). Resources also need to be available in various languages to accommodate all learning needs. The teaching plan then needs to be individualized to take into account a family’s previous experience and coping mechanisms, and to incorporate any additional barriers to learning such as illness, external stressors, and learning impairments (16).
To ensure that the caregiver or parent is competent to deliver home PD, essential core topics have to be taught within the standardized teaching plan. Table 2 presents a summary of PD training content, with core topics related to infection shown in boldface type. To assess whether the training objectives have been met, competencies or a post-training test are also highly recommended, with the evaluation designed to incorporate both concept and skill testing (13,15).
TABLE 2.
Peritoneal Dialysis (PD) Training Content
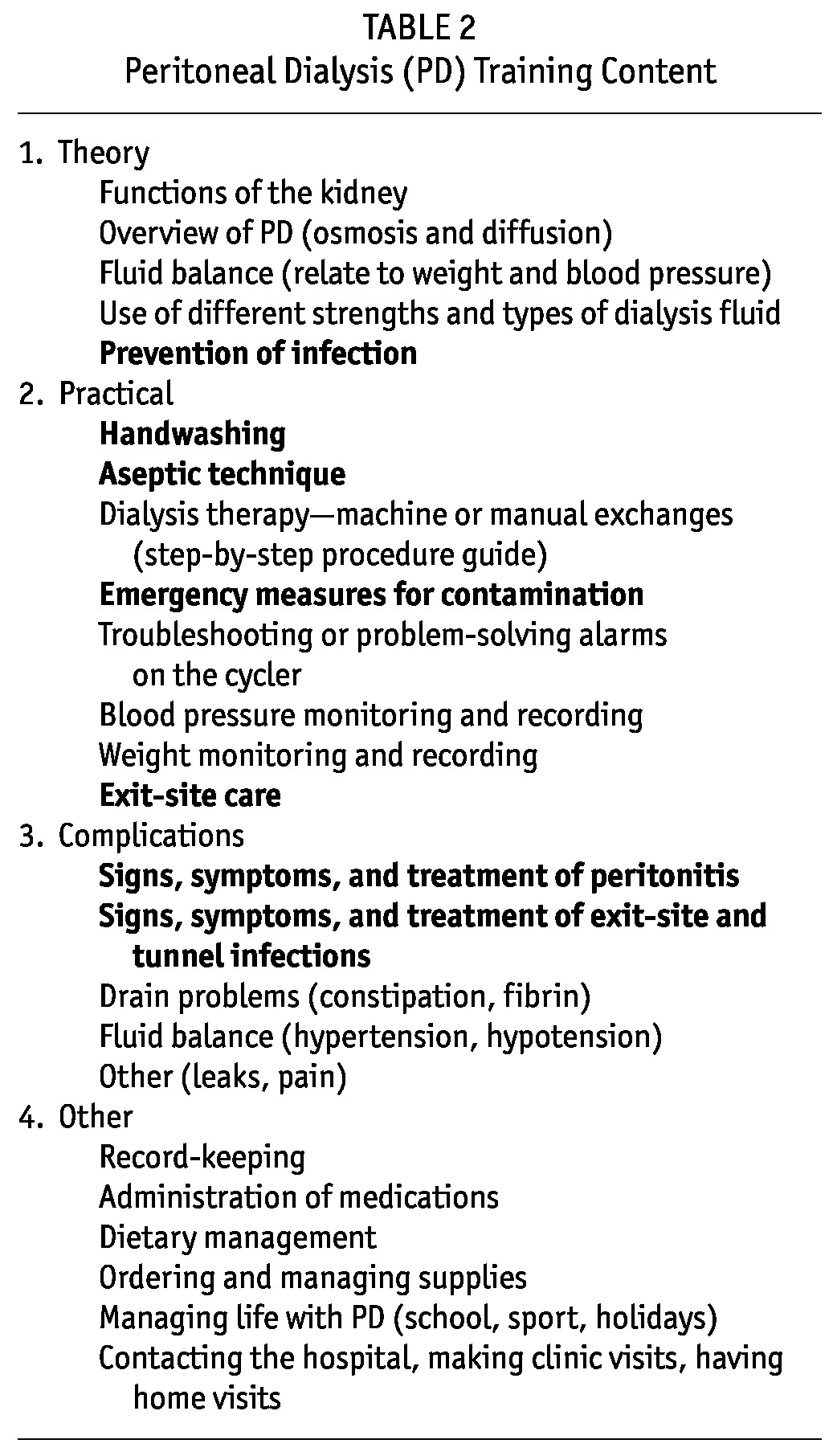
Handwashing is essential to preventing contamination and infection. Caregivers must be taught to thoroughly wash their hands before any care procedures (18). It is then paramount that the hands be dried completely with a clean towel, because hand dampness after handwashing can cause bacterial translocation through touch contamination (19). Caregivers must ensure to avoid contaminating their hands after washing by, for example, turning off the faucet (tap) with a bare hand; a towel should be used for this maneuver, if necessary. Further study on the subject of the optimal duration of handwashing is required. A recent PD literature review and the World Health Organization guidelines have both discussed this subject and have provided recommendations regarding the duration for handwashing and handrubbing (18,20,21)
The recommendation of an antibacterial soap for handwashing has historically been common practice. However, a recent comprehensive literature review by Baxter Healthcare on hand hygiene in PD suggests that, because bacterial resistance has been found with both triclosan- and chlorhexidine-based soaps, plain soap and water can be used for initial washing to remove any grime and transient bacteria present; then, after thorough drying, an alcohol-based liquid or gel should be applied to the hands (20). The use of pictorial handwashing guidelines (for example, those from the World Health Organization guidelines on hand hygiene) can help parents and caregivers learn a systematic, consistent approach to handwashing (21). When used as part of the PD training process, such aids help to ensure that parents and caregivers wash all areas of their hands thoroughly, and the aids can also be used as a component of an assessment tool for monitoring technique.
Within pediatric programs, it is common practice and advisable to train 2 family members or caregivers (1 of whom can be the patient, if deemed capable) (22). This approach ensures that support is available at home to help meet the daily burden of PD care and may reduce the risk of burnout. The possibility of training extended family members or caregivers as a means of providing parents with regular respite may also be beneficial, but does somewhat mandate regular performance of the procedure by the extended providers to maintain their proficiency. The availability of sufficient training staff to educate the additional caregivers is also mandatory. As children on dialysis mature, the teenagers or young adults should be encouraged to take a more active role in their own care, and additional teaching will be required for them. It is important to ensure that the teaching content and style is based on the patient’s developmental age, not chronological age (16). To date, no prospective studies have been conducted to address the training of adolescents to manage their own PD care needs. In one recent study on adherence, no relationship was observed between the peritonitis rate and the participation of adolescent patients in the provision of PD (23).
Finally, peritonitis has been reported to occur as a result of domestic animals (cats, dogs) biting dialysis tubing. Patients and families should be educated about the importance of excluding animals from the room in which dialysis is being conducted (24,25).
Guideline 1.2: For patients and families participating in the provision of PD, the ISPD Nursing Liaison Committee recommends, and we agree with, retraining both periodically and after infection or after a prolonged interruption in PD. Further study is required to determine exactly when and how retraining should be conducted (8). Home visits are also recommended as part of the continuation of training and education because such visits allow the nurse to assess the patient or caregiver’s PD knowledge and skills in the home setting (8,18,22,26).
An observational multicenter adult study from Italy, through a questionnaire and home visits (the latter now being a required component of dialysis care in the United States), found that, with respect to infection control, 29% of patients required reinforcement of their knowledge and ability to correctly perform PD (26). The authors found that the need for retraining was greatest in patients less than 55 years of age, in those with lower educational degrees, and in those in the early or late phase of their PD therapy (<18 or >36 months). It is important to remember that education and training of the patient and family should involve a continual process of assessment, planning, teaching, and evaluation (16). Given that peritonitis remains the primary reason for PD technique failure in children, root-cause analysis should be applied to each episode of peritonitis in an attempt to establish the causes of the infection and to implement interventions designed to reduce the risk of recurrence (10,18,7). All members of the multidisciplinary PD team should be involved in the root-cause analysis, including the physician, PD nurse, and social worker, with dialysis retraining being provided when deemed necessary (18,27). Retraining may be particularly important after episodes of peritonitis that occur soon (0 – 3 months) after initiation of PD.
LIMITATIONS
Currently, much of the advice surrounding PD training is opinion-based, especially with respect to the pediatric patient.
FURTHER STUDY
Studies are required to further address the methods used to teach parents and caregivers the management of home PD. The content of teaching provided to adolescent patients also requires evaluation. Observational data should be collected to better determine the impact of specific components of training on patient outcomes. Factors to be addressed include length of training time, the setting of the training (hospital or home), the timing and frequency of periodic retraining, the content of the training examination, and the value of retraining after peritonitis episodes.
GUIDELINE 2 – CATHETER TYPE AND PLACEMENT
2.1 We suggest the use of a double-cuff Tenckhoff catheter with a downward or lateral subcutaneous tunnel configuration that is placed by a surgeon or nephrologist experienced in PD catheter placement (2B).
2.2 We recommend that perioperative antibiotic prophylaxis be used within 60 minutes before the incision for PD catheter placement to reduce the incidence of early-onset peritonitis (1A).
RATIONALE
Guideline 2.1: Data from the 2008 North American Pediatric Renal Trials and Collaborative Studies report showed that use of the double-cuff Tenckhoff catheter with a swan-neck tunnel and a downward-directed exit site was associated with a better annualized peritonitis rate and a longer time to a first peritonitis episode when compared with other combinations of catheter characteristics in pediatric PD patients (2). Similar data on the beneficial effects of two cuffs and a downward-directed exit site in adult patients have also been published, although those findings have not been confirmed in prospective randomized trials (28–30).
A clear benefit for a coiled compared with a straight configuration of the intraperitoneal portion of the catheter with respect to the prevention of catheter-related infections has not been demonstrated in either pediatric or adult patients (2,31). However, data collected by the IPPR has revealed that the use of Tenckhoff catheters with a straight ending within the peritoneal cavity is associated with an increased rate of post-peritonitis technique failure, possibly as a result of an inability to completely drain the peritoneal cavity when post-infection adhesions are present (5).
Finally, a single-cuff catheter and a downward-pointing exit site proved to be independent risk factors for relapsing peritonitis in a multivariate analysis conducted on 490 episodes of non-fungal peritonitis (non-FP) reported by the IPPR, and in the same IPPR experience, a single-cuff catheter was associated with a nearly 13 times increased risk for gram-negative peritonitis (32,33). The observed increase in the relapse risk associated with downward-pointing exit sites is not readily explained and is surprising, given that previous studies reported a decreased risk for peritonitis with a downward-pointing configuration for the exit site (32,34,35). The new finding will require further evaluation in future studies.
Proper patient preparation and catheter placement technique play key roles in preventing catheter-related infections. The location of the exit site should be determined in advance of the surgical procedure, and it should be placed away from the belt line, from diapers, and from stomas (gastrostomy, ureterocutaneostomy). In children with a history of recurrent ESI and in those wearing diapers or having fecal incontinence or an ostomy, the use of a swan-neck presternal catheter may be beneficial (36,37). Although no difference in the risk of peritonitis and ESI or tunnel infection (TI) has been demonstrated in comparisons of midline and lateral catheter insertion sites in adult patients, a paramedian fascial incision is usually preferred in infants and children to avoid herniation or dialysate leakage that may predispose to infection-related complications (31,38).
Preoperative bowel preparation and showering or bathing with an antiseptic soap may help to reduce the risk of postoperative infections. As an alternative to standard surgical insertion of the catheter, a laparoscopic PD catheter placement technique has been adopted by some pediatric centers, with the advantage of a less-invasive procedure and a smaller-diameter peritoneal perforation, resulting in elastic sealing of the insertion site (39). However, in three trials conducted in adult patients, no significant difference in the risk of peritonitis has been shown when a laparoscopic approach to insertion of a PD catheter has been compared with a surgical approach (31). Similarly, retrospective single-center pediatric trials have not shown any difference in the infection rate between these two catheter placement techniques (40,41).
Regardless of the insertion technique, the outer cuff should be situated approximately 2 cm from the exit site to decrease the likelihood of cuff extrusion, a complication associated with an increased risk for ESI.
Once the catheter is inserted, sutures should not be placed at the exit site, because sutures increase the possibility of bacterial colonization. Fibroblast ingrowth of the Dacron cuff is sufficient to anchor the catheter, obviating the need for suture material (42,27). The exit site should be round and small enough to allow for a snug fit of the catheter within the surrounding skin. The catheter should be securely anchored close to the exit site to minimize movement and the potential for traction injury, which represents a risk factor for ESI. Commercially available catheter immobilization devices can be used, but tape or a dressing is typically adequate. The method of immobilization should be individualized to the patient’s needs.
In a prospective, open-label randomized study performed in a single pediatric center, the application of fibrin glue to the peritoneal cuff suture prevented early dialysate leakage (43). The fibrin glue technique may be considered in cases in which dialysis will be initiated shortly after catheter implantation. However, the application of fibrin glue was not associated with differences in the ESI or peritonitis rates during the initial 60 days after catheter implantation (43).
Guideline 2.2: Administration of an antibiotic just before peritoneal catheter placement has been shown to lower the incidence of early infectious complications such as wound infection and peritonitis in adult and pediatric PD populations. In pediatric chronic PD patients, Sardegna et al. (44) conducted a retrospective study that showed a benefit associated with the use of prophylactic antibiotics. In that study, peritonitis was found to be less common in patients receiving prophylaxis with cephalosporins, vancomycin, ampicillin, or nafcillin–gentamicin than in patients receiving no prophylaxis. In a systematic review published in 2004 (45), an analysis of randomized prospective studies encompassing a combined 335 adult patients showed that, compared with no treatment or with placebo, the use of perioperative intravenous antibiotics significantly reduced the risk of peritonitis within 1 month of surgery [relative risk (RR): 0.35; 95% confidence interval (CI): 0.15 to 0.80]. Of the prospective studies analyzed, three with short follow-up periods of less than 4 weeks (46–48) showed a significant reduction in the incidence of peritonitis. In the large prospective study conducted by Gadallah et al., 221 patients undergoing PD catheter placement were randomly assigned to intravenous vancomycin (1 g given 12 hours before the procedure, n = 86), intravenous cefazolin (1 g given 3 hours before placement, n = 85), or no antibiotics (n = 83) (49). At 2 weeks, the incidence of peritonitis was significantly lower in the patients receiving antibiotics, particularly vancomycin (1% for vancomycin, 7% for cefazolin, and 12% for no treatment, p = 0.02). Single-dose vancomycin was superior to single-dose cefazolin; however, peritonitis episodes were documented only for the first 14 days post catheter implantation. The possibility that vancomycin was most effective because of its long half-life was not investigated.
Given the emergence of vancomycin-resistant organisms, the routine use of vancomycin for prophylaxis before catheter insertion is not recommended (50). Atta et al. reported the incidence of vancomycin-resistant enterococci (VRE) colonization among adult outpatient hemodialysis (HD) and PD patients as 17.8%. Of the patients not receiving vancomycin, none became colonized with VRE, but 26% of the patients receiving vancomycin became colonized (51). Vancomycin-resistant enterococci have also been isolated in pediatric HD and PD patents (52). Although peritonitis with VRE is uncommon in stable patients receiving continuous ambulatory PD (CAPD), when it occurs, it has characteristically been associated with recent hospitalization and the use of antibiotics, mainly vancomycin, or with nosocomial infection (53–55). Surgical prophylaxis and routine prophylaxis for patients on chronic PD should therefore be acknowledged as situations in which vancomycin use is to be discouraged (50).
A 2002 review written in collaboration with major national societies recommends the administration of a first-generation cephalosporin, given intravenously 1 hour before PD catheter insertion (56). In contrast, the 2005 ISPD guidelines state that each program should consider giving vancomycin, with a view to the benefit–risk ratio with that drug (57). Another 2005 updated review of contemporary developments in peritoneal catheters and exit-site practices favored a single dose of a first- or second-generation cephalosporin and did not recommend routine prophylaxis with vancomycin because of the risk of VRE emergence (58). Similarly, the 2005 European Best Practice Guidelines recommend a first-generation cephalosporin such as cefazolin 1 g, either orally 1 – 2 hours before or parenterally 30 minutes before the procedure. Vancomycin is suggested as an alternative (59).
The choice of the specific antibiotic to be used for perioperative prophylaxis should also take center-specific susceptibility patterns and public health concerns into consideration.
LIMITATIONS
Given that the recommendation in favor of a downward-pointing configuration for the exit site in children is largely derived from multicenter observational studies, center effects cannot be excluded.
The evidence for the efficacy of perioperative antibiotic prophylaxis is limited to a few prospective studies in adult patients.
RESEARCH RECOMMENDATIONS
A prospective multicenter study evaluating standardized approaches to exit-site and tunnel configurations and the associated infection rates should be performed in children across the pediatric age range.
The emergence of resistant bacterial strains should be followed prospectively in centers worldwide, with attention to the use (or lack thereof) of prophylactic antibiotic protocols.
GUIDELINE 3 – EARLY EXIT-SITE CARE
3.1 We recommend once-weekly sterile dressing changes to the exit site, performed by experienced health personnel according to a standardized protocol, until the exit site is well healed (2B).
3.2 We recommend catheter immobilization to prevent trauma to the exit-site and to optimize early healing (1B).
RATIONALE
Guideline 3.1: The aims of early PD exit-site care after implantation are
to prevent bacterial colonization during the healing phase,
to minimize multiplication of bacteria, and
to prevent local trauma through catheter immobilization at the exit site (60).
Unfortunately, no pediatric studies and only limited adult studies have addressed this period of catheter care. Thus, the recommendations made, including those by the ISPD in 1998 and 2005, incorporate broad general principles of early exit-site care and are primarily based on the work carried out by Twardowski and Prowant (61). Table 3 summarizes the details that follow.
TABLE 3.
Cleaning Guidelines for the Healing Peritoneal Dialysis (PD) Exit Site

After catheter implantation, dressing changes should be avoided during the first postoperative week. They should then be performed only once weekly, using sterile technique until the site is healed as characterized by the description “when the skin around the exit site looks normal without gaping” (18,61). The weekly sterile dressing changes should continue until that state is achieved—a minimum of 2 – 3 weeks, although healing can take up to 6 weeks.
It is generally accepted that the foregoing dressing changes should be performed by specially trained staff (62). Less-frequent dressing changes are advocated during this period because each change requires manipulation of the catheter, which can increase the risk of trauma to the exit site. With each dressing change, the exit site could also become contaminated with bacteria, even if aseptic technique is followed (63). Dressing changes should be performed more frequently only if excessive drainage is noted at the exit site or if excessive sweating causes wetness at the exit site (61). In pediatrics, soiling of the dressing as a result of the catheter being positioned near the diaper region would also necessitate a dressing change.
If the healing process is felt not to be progressing normally (as reflected by deterioration or signs of infection), a culture should be taken from the exit site, because bacterial colonization is already likely to be present, and more frequent cleaning will be required (61). Antibiotic treatment may also be necessary.
To reduce the number of bacteria and to remove debris during each dressing change, the exit site should be cleaned with a nonirritating, nontoxic agent. Strong agents such as hydrogen peroxide and povidone iodine should be avoided because they are cytotoxic and can be damaging to granulation tissue in the sinus tract (59,60). Although no consensus has been reached about a specific sterile cleansing agent to use and further controlled study is required, chlorhexidine, normal saline, and the nonionic surfactant agent poloxamer 188 (Shur-Clens: ConvaTec Professional Services, Skillman, NJ, USA) have all been suggested as suitable options.
Application of a topical antibiotic cream or ointment at the time of the weekly sterile dressing change has also been recommended (18). However, no data are currently available on the duration of action of these topical agents, making it unclear whether weekly application is truly beneficial during the immediate post-insertion period (compared with use of such agents as a component of chronic exit-site care).
Because of the large amount of drainage that can occur during the post-implantation period, several layers of sterile gauze dressing should be applied over the thoroughly dried exit site to wick away any drainage and to keep the site dry. Use of semipermeable and occlusive dressings directly onto the wound should be avoided because of the resultant pooling at the exit site of any drainage, which provides a good medium for bacterial growth (60).
During this early healing phase, submerging the catheter and exit site in water has to be avoided, and so bathing and showering is not advised. This recommendation is meant to prevent colonization with waterborne organisms and skin maceration (60).
Guideline 3.2: The dialysis catheter has to be secured with an adhesive to anchor it and to prevent torquing movement (61). Commercially available catheter immobilization devices can be used, but tape or a dressing is typically adequate. The method of immobilization should be individualized to the patient’s needs. Sutures should not be placed at the exit site because the suture may act as a nidus for bacterial infection. Fibroblast ingrowth of the Dacron cuffs obviates the need for suture material (42,27).
LIMITATIONS
The work by Twardowski and Prowant in the early to mid-1990s continues to be the foundation for all current early exit-site care guidelines. Evidence on the topic of early exit-site care is limited, especially evidence specific to the pediatric setting.
RESEARCH RECOMMENDATIONS
Randomized controlled trials (RCTs) to look at early exit-site care in the pediatric setting are required. Factors to be addressed include the frequency of dressing changes, the choice of cleansing solution, and whether any benefit accrues to once-weekly application of topical antibiotic ointments or creams during the healing phase.
GUIDELINE 4 – CHRONIC EXIT-SITE CARE
4.1 We recommend cleansing the exit site with a sterile antiseptic solution and sterile gauze (1C).
4.2 Each program should evaluate the type, frequency, and resistance patterns of organisms causing ESIs and institute a center-specific protocol to diminish such risk (not graded).
4.3 We suggest that a topical antibiotic be applied to the peritoneal catheter exit site as a component of chronic exit-site care (2B).
RATIONALE
Guideline 4.1: The ultimate goal of exit-site care is to keep the exit site clean, dry, scab-free, crust-free, painless, and noninflamed. Immobilization of the catheter and protection from trauma is essential (60). Excellent hand hygiene is also vitally important before any examination of the exit site by the patient, caregivers, and health care professionals. Handwashing, followed by thorough drying, before changes of dressings and dialysate are essential for preventing PD-associated infections (18). Accordingly, those aspects of care should be a component of patient training in all PD centers (see Guideline 1 – Training).
The role and efficacy of topical disinfectants (povidone iodine, chlorhexidine, hydrogen peroxide, sodium hypochlorite, octenidine, etc.) for chronic exit-site care remain unclear. In an early RCT in adults (64), local application of povidone iodine solution at the exit site was compared with local treatment using water and non-disinfectant soap and was found to significantly reduce the rate of ESIs. Retrospective pediatric data showed that the use of chlorhexidine (compared with povidone iodine) was associated with a significant decline in the frequency of ESIs (65). A recent pediatric survey from Japan found that neither peritonitis nor ESI or TI were prevented with the use of topical povidone iodine (66). Additionally, the European Best Practice Guidelines for PD emphasize that, because of epithelial toxicity, povidone iodine preparations and hydrogen peroxide should be avoided, especially during the early healing phase immediately after catheter implantation (59).
Amuchina (Aziende Chimiche Riunite Angelini Francesco, Casella Genova, Italy) is another agent that is used for exit-site care. The ESI rates with Amuchina 10% (electrolytically produced sodium hypochlorite solution) and Amuchina 5% are similar to or lower than those seen with povidone iodine or chlorhexidine in adults. A recent RCT in children compared pH-neutral soap with Amuchina 10% solution and showed a favorable effect for Amuchina in preventing ESIs (67,68). Another recent retrospective study in 83 children demonstrated similar results, in that the combination of mupirocin and sodium hypochlorite for daily exit-site care was very effective and superior to mupirocin alone as a means of reducing PD catheter–associated infections and of prolonging catheter survival (69).
The IPPR has also generated pediatric-specific data on the topic of chronic exit-site care, with clear differences in practice patterns observed around the globe, highlighting the absence of a standard (5). Chronic exit-site care is conducted daily in 93% of centers in America and Asia, in 64% of centers in eastern Europe and Turkey, but in only 8% of western European centers. Large regional differences also exist with respect to the choice of an exit-site cleansing agent. Soap or sodium hypochlorite are the primary agents in North America, and povidone iodine is often used in Turkish and some European centers, but rarely in Asian or US centers. Many European sites use the quaternary ammonium compound beta-octenidine.
Data from the IPPR also suggest that the global variation in gram-negative peritonitis may well be related to chronic exit-site care and mupirocin use. Compared with centers in western Europe, US centers had an incidence of Pseudomonas peritonitis that was higher by a factor of 8 and that was associated with exit-site care practices characterized by daily washing with nonsterile cleansing agents and application of mupirocin (5).
Finally, Italian pediatric PD registry data have shown that there is no difference in catheter survival with the use of either povidone iodine or hydrogen peroxide as the antiseptic solution, and with exit-site cleansing on a daily or alternate-day schedule (38). In light of the available data, we recommend exit-site cleansing with sterile gauze and sterile antiseptic solutions (preferably chlorhexidine, sodium hypochlorite, or octenidine) conducted by a well-trained caregiver. The optimal frequency of exit-site care—for example, daily compared with alternate-day or less frequently—has not yet been determined.
Guideline 4.2: A review of every episode of both peritonitis and ESI to determine the root cause of the event should be routine in PD programs (18). A common mistake made in trials of infectious complications in PD is to omit to provide or analyze the infection rates for individual organisms, but to give the organisms as percentages of the total, which may be misleading. If the incidence of a specific organism is reduced, the proportion of ESIs caused by other agents may increase, without a change in absolute numbers. A way to overcome this limitation is, as proposed by Piraino et al., to report results as incidence rates—that is, the number of infections by a specific organism divided by time at risk (70).
We therefore suggest that each center examine the susceptibilities of the bacteria causing infections and make a decision about antibiotic prophylaxis. If a center has a very low ESI incidence rate, there may be no need to use any prophylaxis for reducing catheter-related infections. The routine application of an antibacterial ointment or cream such as mupirocin or gentamicin to the catheter exit site is, however, a strategy that has been studied and found to be associated with a reduction in the rate of catheter-related infections (71,72). (It should be noted that antibiotic ointments containing polyethylene glycol base should not be applied to the exit site when the catheter is made of polyurethane because of the associated risk of catheter rupture.) Other topical agents that have been studied include Medihoney [Comvita New Zealand, Te Puke, New Zealand (commercially available medical honey with antimicrobial action)] and Polysporin Triple (Johnson and Johnson, Markham, Ontario, Canada) compound (73,74). The use of gentamicin might be preferred over the use of mupirocin in centers that have experienced an increased frequency of ESIs secondary to Pseudomonas species rather than to Staphylococcus aureus.
Guideline 4.3: Exit-site colonization or infection with Pseudomonas aeruginosa and nasal or exit-site carriage of S. aureus are widely accepted as risk factors for peritonitis and ESIs in adults and children undergoing chronic PD and as possible targets of prophylactic antibiotic therapy (75–79). However, as indicated in the 2010 update of the adult PD-related infections recommendations, the benefit of screening for S. aureus carriage, either after a staphylococcal peritonitis episode or routinely in the PD program, needs to be clarified (7). Approximately one half of PD patients have been found to be S. aureus nasal carriers, but the catheter exit site (rather than the nose and the nails) has also been shown to possibly be the most frequent site for colonization with S. aureus strains identical to those causing peritonitis (80,81). Screening for exit-site rather than nasal colonization may therefore be more advisable, although this practice is not routinely recommended at the present time.
Mupirocin is a topically active antibacterial agent with demonstrated benefit in eradicating colonization with S. aureus (82). Since the early 1990s, numerous studies have evaluated the efficacy of prophylactic intranasal or topical mupirocin application at the catheter exit site in the chronic PD population (83–91). Despite some conflicting reports, one of which is a pediatric study from Japan, most studies demonstrated that the prophylactic use of mupirocin either intranasally or at the exit site reduces the incidence of both ESI and peritonitis caused by S. aureus (45,84–88,91–95). The recommended frequency and route of usage is quite variable, as evidenced by the fact that daily application of exit-site mupirocin in all patients, application 3 times daily intranasally for 7 days for each positive nose culture, or application once monthly intranasally in nasal carriers have all proven to be effective options (84–88,90).
On the other hand, a recent evidence-based review, including renal and nonrenal patients, does not support the routine use of prophylactic intranasal mupirocin in patients with the goal of reducing the rate of staphylococcal infection, despite the efficacy of mupirocin in reducing nasal carriage (96). The authors were concerned about the possibility of micro-organism replacement, in which S. aureus colonization and infection are reduced, only to allow infection with a different—potentially more virulent—organism.
Based on all the available data, application of prophylactic mupirocin to the exit site with every dressing change has been recommended in many centers as the current method of choice for preventing PD catheter infections caused by S. aureus. Furthermore, topical use of the antibiotic at the exit site after healing is preferable, because it precludes the need for repeated nasal swabs and repeated courses of intranasal treatment, with consequent higher compliance, lower cost, and wider efficacy (59,97). However, concern has been raised about the development of resistance to mupirocin and the possible development of infections secondary to organisms other than S. aureus when mupirocin is used on a frequent basis (98,99). In contrast, a study that examined mupirocin resistance over a 7-year period reported no increased prevalence of mupirocin resistance (2.7% of the patients) over the period of observation (100). But a parallel increase in the incidence of infections secondary to gram-positive micro-organisms other than S. aureus and to gram-negative bacteria has been observed in association with the decreasing rate of S. aureus infections associated with mupirocin prophylaxis (84,85). P. aeruginosa is now the most common cause of combined catheter-related infection and catheter-related peritonitis, partly because of a sharp decrease in S. aureus–related infections subsequent to the introduction of mupirocin prophylaxis (90,92). In fact, IPPR data show that prophylactic treatment with mupirocin at the catheter exit site increased the risk of peritonitis from Pseudomonas species, a finding that also raises concerns about the current concept of topical prophylaxis with mupirocin.
In turn, gentamicin applied daily to the exit site appears to be a promising option (71). Gentamicin is active against S. aureus and P. aeruginosa because it inhibits normal bacterial protein synthesis. In a randomized double-blind multicenter trial in adults, a simple regimen involving daily application of gentamicin (compared with mupirocin) cream to the exit site resulted in a 57% reduction in catheter ESIs and a 35% reduction in peritonitis episodes (72). Additionally, gentamicin cream was highly effective in reducing P. aeruginosa ESIs and has been associated with few side effects (such as easily treatable fungal ESIs); gentamicin was also as effective as mupirocin in preventing S. aureus ESIs (92). Of interest, however, are the findings of a recent retrospective chart review, which showed a trend toward higher peritonitis rates in a gentamicin group (compared with a mupirocin group), largely as a result of gram-positive bacteria (101). Furthermore, resistance to gentamicin may be clinically more problematic than resistance to mupirocin, given that gentamicin is a cornerstone of treatment in some centers for gram-negative peritonitis in patients receiving chronic PD.
Yet another alternative agent is Polysporin Triple compound ointment (bacitracin 500 U/g, gramicidin 0.25 mg/g, and polymyxin B 10 000 U/g, MP3), which is active against coagulase-negative (CNS) and -positive Staphylococcus and against some gram-negative bacteria. This agent has been shown to be effective in preventing HD catheter–related infections (102). Like gentamicin, Polysporin Triple compound has the advantages of low cost, high tolerability, and low resistance. Results of a recent Canadian multicenter trial in adults to evaluate the effectiveness in routine PD care of Polysporin Triple compound compared with mupirocin at the catheter exit site revealed equivalent efficacy in preventing catheter-related infections (103). However, there was an unacceptably high rate of FP with the Polysporin Triple (7 vs 0, p = 0.01). The use of Polysporin Triple compound cannot, therefore, be advocated.
Finally, Medihoney is now being suggested as an alternative agent that can effectively prevent catheter-associated infections and minimize antimicrobial resistance and toxicity. Honey has been shown to exert antimicrobial action against a broad spectrum of fungi and bacteria, including methicillin-resistant S. aureus (MRSA), multidrug-resistant gram-negative organisms, and VRE (104,105). A recent randomized controlled trial in HD patients demonstrated that 3-times-weekly application of standardized antimicrobial honey to the HD catheter exit site was safe, inexpensive, and effective, and that it resulted in a rate of catheter-associated infections comparable to that obtained with topical mupirocin prophylaxis (106). Therefore, a multicenter RCT in both adult and pediatric patients in Australia and New Zealand has been designed to determine whether daily Medihoney (compared with standard topical mupirocin prophylaxis) in nasal staphylococcal carriers reduces the risk of catheter-associated infections in PD patients. The results will probably be available in 2012 (73).
LIMITATIONS
No well-designed prospective RCTs on chronic catheter exit-site care practice in pediatric or adult PD patients are available.
RESEARCH RECOMMENDATIONS
A multicenter protocol should be designed to compare daily with 3-times-weekly exit-site care, in terms of the development of catheter-related infections.
The effectiveness of chlorhexidine, sodium hypochlorite, and beta-octenidine in preventing catheter-related infections should be compared in a randomized prospective trial.
A RCT should be performed in children to compare the effectiveness of gentamicin and mupirocin in preventing organism-specific and all-cause catheter-related infections.
GUIDELINE 5 – CONNECTOLOGY
5.1 We recommend using double-bag and Y-set disconnect systems with “flush before fill” for patients receiving continuous ambulatory PD (1A).
5.2 We suggest the use of assist devices for spiking PD solution bags (2B).
RATIONALE
Guideline 5.1: Unequivocal evidence indicates that spiking bags of dialysis fluid predisposes to peritonitis by touch contamination. Of all the connectology-related interventions designed to prevent peritonitis in PD, only the disconnect (twin-bag and Y-set) systems (compared with conventional spike connect systems) have proved to be effective in that respect (31,107). A systematic review of RCTs (108–115) revealed that use of the Y-set (compared with the standard spike system) was associated with a significantly lower risk of peritonitis (seven trials, 485 patients—RR: 0.64; 95% CI: 0.53 to 0.77) and peritonitis rate (eight trials, 7417 patient–months—RR: 0.49; 95% CI: 0.40 to 0.61). No difference was observed in the risk of ESI or TI (three trials, 226 patients—RR: 1.00; 95% CI: 0.70 to 1.43) or the rate of infection (two trials, 2841 patients—RR: 1.24; 95% CI: 0.91 to 1.69) (108,111–113,115). In addition, no difference in the catheter removal or replacement rate was observed (two trials, 126 patients—RR: 0.80; 95% CI: 0.40 to 1.63) (31).
The elimination of one extra connection procedure with the use of twin-bag systems further reduces the risk of peritonitis beyond that achieved by Y-connection systems (116). One of the largest reviews of RCTs (31) found that twin-bag systems were associated with a trend toward fewer patients experiencing peritonitis (p = 0.05). In addition, an earlier systematic review (107) reported a significantly lower risk of peritonitis episodes with double-bag systems compared with Y-systems (odds ratio: 0.44; 95% CI: 0.27 to 0.71). Several twin-bag systems are commercially available and each has minor operating differences. These minor variations in connectology can potentially translate into marked differences in peritonitis rates (117,118).
The “flush before fill” technique (flushing the drain tubing with dialysate before filling the abdomen), which is inherent in both the double-bag and Y-set systems for CAPD, has been shown to be a key factor in potentially lowering the risk of peritonitis from contamination (119–121). Most patients using automated PD (APD) undergo an automatic “flush before fill” because current APD cyclers begin treatment with an “initial drain” mode by default, and that approach, too, has been associated with a lower risk for peritonitis.
Guideline 5.2: Manual spiking of dialysate bags has become obsolete, having been replaced by Luer-lock connection technology in most cases. If manual spiking cannot be avoided because of a lack of availability of Luer-lock, double-bag, or Y-connection systems, the use of assist devices should be considered. The UV Flash Compact [Baxter Healthcare Corporation, Deerfield, IL, USA (germicidal exchange device)] has been shown to be useful for patients with a high peritonitis burden from gram-positive organisms (122).
LIMITATIONS
Although there is good evidence for the adverse impact of spiking and the benefit of double-bag or Y-set and flush-before-fill with respect to peritonitis risk, no studies have directly compared the efficacy of various brands of CAPD and cycler systems in preventing peritonitis in adults or children.
RESEARCH RECOMMENDATIONS
There is a need for prospective trials comparing various brands of double-bag systems and assist devices for their ease of use, safety, and efficacy in reducing the risk of peritonitis.
GUIDELINE 6 – ADJUNCTIVE PROPHYLACTIC ANTIBIOTIC THERAPY
6.1 We suggest that the use of oral nystatin or fluconazole be considered at the time of antibiotic administration to PD patients to reduce the risk of fungal peritonitis (2B).
6.2 We suggest prophylactic antibiotic administration after accidental intraluminal contamination to lower the risk of peritonitis (2B).
6.3 We suggest prophylactic antibiotic administration before invasive dental procedures to lower the risk of peritonitis (2D).
6.4 We suggest prophylactic antibiotic administration before procedures involving the gastrointestinal or genitourinary tract and associated with a high risk of bacteremia to lower the risk of peritonitis (2D).
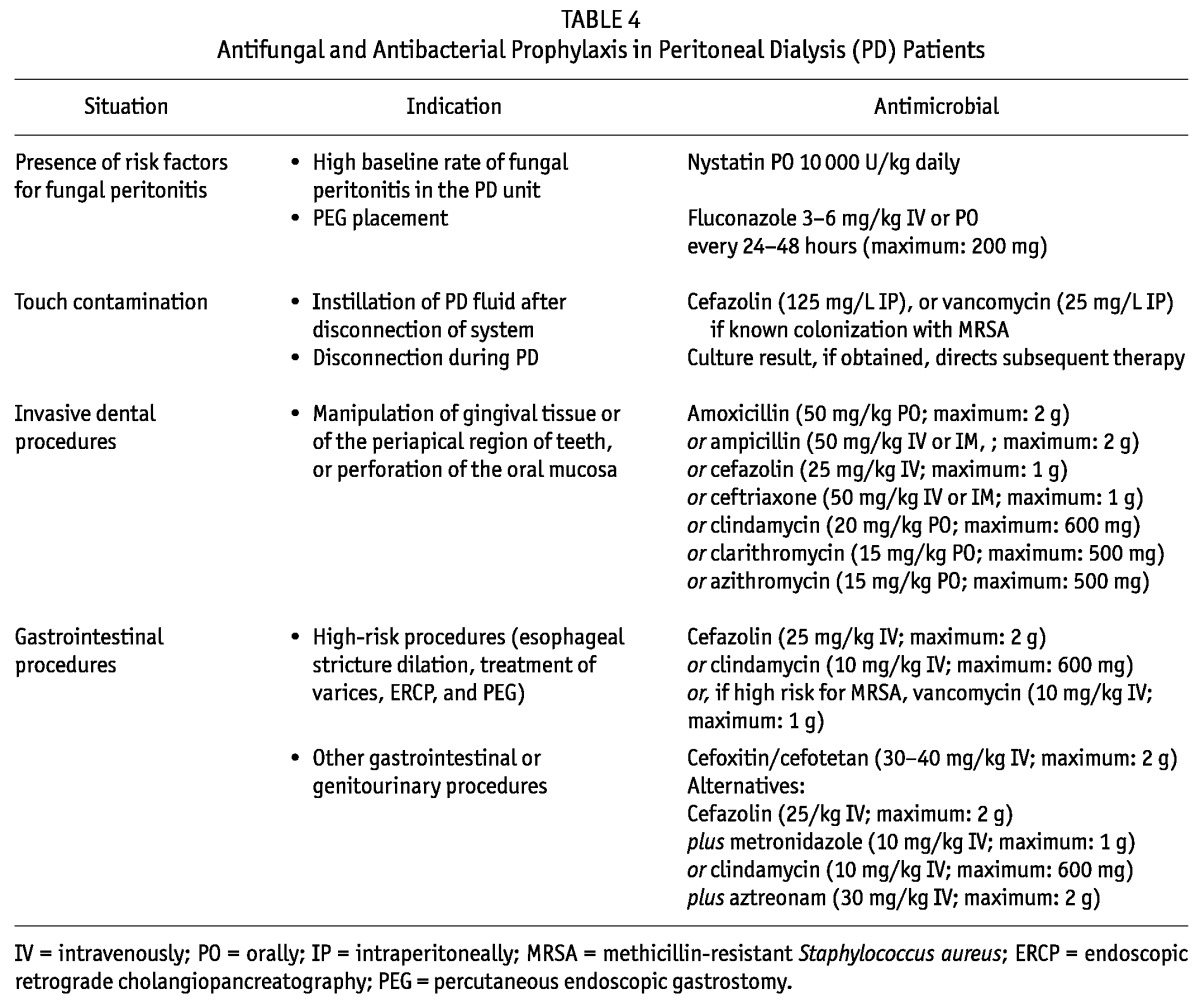
See Table 4 and the guidelines related to prophylactic antibiotic use for catheter placement (guideline 2.2), chronic exit-site care (guideline 4.3), and gastrostomy placement (guideline 7.4).
TABLE 4.
Antifungal and Antibacterial Prophylaxis in Peritoneal Dialysis (PD) Patients
RATIONALE
Guideline 6.1: Fungal peritonitis is uncommon in PD patients, but when it occurs, it is commonly associated with catheter removal, transfer to HD, and death (123,124). The reported prevalence of FP was 2% of all peritonitis episodes in data collected by the IPPR and 2.9% in a pediatric Dutch study (125,4). Recent data in adults reveal rates of 1.5% – 5.8% (126).
Observational studies suggest that frequent peritonitis, particularly episodes with gram-negative organisms, recent antibiotic therapy, and immunosuppression, can all be risk factors for FP in adults and in children (125,127–130). Warady et al. (130) found that 56% of children with FP had received antibiotics in the preceding month, half of them for bacterial peritonitis. In the Dutch study (125), 78% of children had received antibiotic treatment in the previous month, 86% of them for bacterial peritonitis. In both pediatric studies, the overall peritonitis rate was higher in patients experiencing FP than in the PD group in general.
De novo FP episodes—that is, peritonitis episodes caused by a fungus, with no preceding episode of bacterial peritonitis—were reported to occur in only 2.9% of adult patients and 1.3% – 1.6% of patients in a pediatric series (125,130,131). Antibiotic use within the preceding 3 months was noted in 94% of the patients who developed a FP preceded by a bacterial peritonitis; such use was seen in only 61% of patients who developed de novo FP (124).
A number of studies, only two of which are RCTs, have examined the use of FP prophylaxis with either oral nystatin or fluconazole given during the course of antibiotic therapy (131–139). The premise for such therapy is the eradication of the normal flora and the overgrowth of yeast in the digestive tract associated with antibiotic therapy. The first RCT, performed by Lo and coworkers (132), involved PD patients who received antibiotics for any reason. That 2-year study included 199 patients in the intervention arm and 198 patients in the control arm. Oral nystatin 4 times daily (500 000 U) was given to the intervention group throughout the entire course of their antibiotic therapy. Compared with the control group, the nystatin group showed a reduction only in the rate of Candida peritonitis (1.9/100 vs. 6.4/100, p < 0.05). However, not all FP episodes were preceded by antibiotics, and no statistically significant difference was found between the groups with respect to the risk for antibiotic-related Candida peritonitis. The lack of data with respect to nystatin prophylaxis has prompted one author to recommend limiting the use of that agent to centers experiencing a high rate of FP secondary to antibiotic treatment of bacterial peritonitis (140).
In the second RCT (139), patients in the intervention group were given oral fluconazole, 200 mg every other day, during the course of antibiotic therapy for catheter-related infections and were prospectively monitored for 30 – 150 days for the occurrence of FP. A total of 420 bacterial peritonitis and 52 ESI or TI episodes were randomized to either the intervention or the control arm. Compared with the control group, the intervention group experienced a significant reduction in FP (3 vs 15 episodes, p = 0.005). The fact that only 4 of 10 Candida infections tested proved to be susceptible to fluconazole prompted concern that that agent’s therapeutic usefulness may be limited in the future.
A historically controlled pediatric study conducted by Robitaille et al. (134) showed that antibiotic-associated FP episodes were prevented in all patients receiving daily oral nystatin (10 000 U/kg) or ketoconazole (10 mg/kg) compared with those receiving no prophylaxis. The same study also demonstrated that patients with a gastrojejunostomy were more prone to develop FP when treated with antibiotics.
Similarly, several historically controlled adult studies showed a significant benefit with oral antifungal prophylaxis. In one study (133), oral nystatin was given to all patients receiving antibiotics, and no cases of FP occurred. In a second study (135), antifungal prophylaxis with fluconazole, given during antibiotic therapy, resulted in a significant decline in the rate of FP: In 1832 patient–months without treatment in a historical cohort, 12 episodes of secondary FP occurred; in 1705 patient–months in a fluconazole-treated cohort, only 2 episodes occurred. In a third study (138), 70 PD patients received no antifungal prophylaxis during 1450 patient–months between 1986 and 1995, and 96 patients received antifungal prophylaxis (initially, oral nystatin 500 000 U 3 times daily; later on, fluconazole 100 mg daily or 100 mg every other day) during 2269 patient–months between 1996 and 2005. None of 131 peritonitis episodes in patients receiving antifungal prophylaxis were FP, but 8 of 121 episodes in the first 10-year period were FP. In a similar observational study from China (141), the FP rate of the nystatin group was slightly lower than that of the control group (0.011 vs. 0.019 episodes per patient–year), but the difference did not reach statistical significance. However, compared with the control group, the nystatin group experienced a significant decline in the incidence and proportion of antibiotic-related FP.
Mention should also be made of two large, nonrandomized, historically controlled trials that showed no significant decline in FP with nystatin prophylaxis (136,137). A short follow-up period and a high incidence of non-antibiotic-related FP might have masked a beneficial effect of antifungal prophylaxis in those studies.
Finally, recent adult data from the United Kingdom have been derived from an audit of the effect of co-administration of daily oral fluconazole to PD patients being treated with antibiotics for peritonitis (126). Of 3222 total episodes of peritonitis, 49 (1.47%) were FP episodes (>90% Candida species). The incidence of FP in centers that prescribed antifungal prophylaxis was lower by a factor of 3; however, those centers also had lower overall peritonitis rates. Although the analysis suggested that co-prescription of prophylactic fluconazole produced no overall benefit, an effect could not be excluded because of the low background rate of peritonitis.
Based on the results noted above, fungal prophylaxis with nystatin or fluconazole has now been accepted as a part of routine prophylactic therapy in many large pediatric centers (1). The small number of FP episodes that occur in any single pediatric dialysis program preclude the use of FP incidence data to help determine the likely benefit of the therapy. It should be emphasized, however, that prevention of FP is not a simple routine of giving fluconazole or nystatin with each antibiotic prescription, but should involve a strategy of detection and management of potential risk factors in both the host and the environment. Each program must examine its population and identify the patients felt to be at high risk for FP, including those experiencing frequent bacterial peritonitis, those on prolonged courses of antibiotics, and those with impaired immune systems (58,142,143).
Guideline 6.2: Contamination at the time of an exchange procedure can lead to peritonitis, and an effluent sample for culture should be obtained, if possible. Touch contamination before the infusion of dialysate can be treated with a sterile transfer set change alone if the clamp on the transfer set remains closed and if no fluid has been infused. There is no need for prophylactic antibiotics in the latter case.
If contamination occurs by accidental disconnection during a PD treatment or if equipment failure occurs (for example, a hole in the solution bag), with associated potential contamination, treatment should consist of both a sterile transfer set change and antibiotic prophylaxis as soon as possible to reduce the risk of peritonitis (18).
No RCTs or observational data on the impact of antibiotic prophylaxis after a break in dialysis technique are available, but the use of a first-generation cephalosporin by the intraperitoneal route for 1 – 3 days is typically recommended in this setting (18). A glycopeptide should be used only in the setting of a patient previously known to be colonized with methicillin-resistant bacteria. In conditions specific to infant patients (such as the PD catheter being contaminated by stool from a diaper), prophylaxis with cefepime or a first-generation cephalosporin combined with ceftazidime or an aminoglycoside may be most appropriate. A culture of the effluent, if positive, and associated susceptibility data will determine subsequent therapy.
Guideline 6.3: Although prophylactic antibiotic therapy is suggested in the setting of invasive procedures despite a lack of evidence based on properly conducted RCTs, recommendations for antibiotic prophylaxis should be based on the risks related to specific procedures and on patient factors that may predispose to the development of an ESI or peritonitis. Because of the development of resistant species, the American Heart Association, in their most recent guidelines, limited their indications for antibiotic prophylaxis to certain high-risk conditions (144). Indeed, recent data from an IPPR study showed that chronic systemic antibiotic prophylaxis is an independent risk factor for relapsing peritonitis. However, recent American Heart Association recommendations for subacute bacterial endocarditis prophylaxis do recommend prophylactic antibiotic therapy for certain dental procedures that involve manipulation of gingival tissue or the periapical region of the teeth, or perforation of the oral mucosa (144). Procedures associated with significant bleeding include dental extractions, dental implant placement, endodontic “root canal” instrumentation, periodontal surgery, and professional scaling or tooth cleaning (145). The same approach can be applied in PD patients. Antibiotics should be given 30 – 60 minutes before the procedure. Oral amoxicillin; intravenous or intramuscular ampicillin, cefazolin, or ceftriaxone (if oral medication is not possible); oral clindamycin; and oral clarithromycin (in the case of allergy to penicillin or ampicillin) are recommended as options for prophylactic therapy.
Guideline 6.4: The rates of bacteremia after gastrointestinal procedures are generally lower than those seen after routine daily activities such as chewing food, brushing and flossing teeth, and using toothpicks. Such bacteremia seldom results in clinically evident infection. However, for several procedures carrying a high risk for bacteremia [esophageal stricture dilation, treatment of varices, endoscopic retrograde cholangio-pancreatography, and percutaneous endoscopic gastrostomy (PEG)], prophylactic antibiotic therapy may prove particularly beneficial (146,147).
No specific recommendations have been made regarding antibiotic prophylaxis for genitourinary or gastrointestinal procedures other than PEG placement in patients undergoing PD. However, the 2005 adult PD guidelines recommended, and we agree, that the abdomen should be emptied of fluid before any procedure involving the abdomen or pelvis. Intravenous cefoxitin or cefotetan just before the procedure is recommended as prophylaxis for invasive gastrointestinal procedures; cefazolin should be adequate for PEG placement. Such prophylaxis is discussed further in the guideline for gastrostomy placement (guideline 7.4).
LIMITATIONS
No well-controlled studies permitting the development of specific recommendations regarding antibiotic prophylaxis in patients undergoing PD are available. Also, very few observational data on the impact of antifungal prophylaxis provided during a course of antibiotic therapy are available.
RESEARCH RECOMMENDATIONS
Prospective, randomized, double-blind multicenter studies of antimicrobial prophylaxis (antibiotic with or without antifungal prophylaxis) in PD patients who undergo a potential bacteremia-producing procedure (dental, gastrointestinal, or genitourinary) are needed.
Multicenter studies should be conducted to better identify the patients who would benefit most from antifungal prophylaxis.
GUIDELINE 7 – OSTOMY PATIENTS
7.1 The PD catheter exit site should be placed as far as possible from an ostomy site (not graded).
7.2 We recommend that gastrostomy placement should preferentially take place either before or at the time of PD catheter placement (1C).
7.3 We recommend the preferential use of an open surgical procedure for gastrostomy placement in patients who are already receiving PD. In patients not yet receiving PD, gastrostomy placement can be performed by either open surgical technique or laparoscopically (1C).
7.4 We suggest administration of prophylactic antibiotic and antifungal therapy during gastrostomy placement (2C).
7.5 We suggest withholding PD for 1 or more days after gastrostomy placement (2D).
RATIONALE
Guideline 7.1: Ostomy sites (colostomy, ureterostomy, nephrostomy, gastrostomy) are inherently prone to bacterial and fungal growth and to local infection because of constant moisture and the organic content of the drainage. In addition, secretions from a colostomy are loaded with intestinal flora. The presence of a PD catheter exit site in close proximity to an ostomy is, in turn, likely a risk factor for peritonitis.
Ramage et al. (148) reported that, compared with control subjects, patients with a gastrostomy experienced a significantly higher peritonitis rate (1 infection every 7.8 months vs every 18.4 months, p < 0.001). More recently, the IPPR data revealed an association approaching significance (p = 0.06) between gram-negative peritonitis and the presence of a gastrostomy in children (4). A significant association between FP and gastrostomy feedings has also been reported in children receiving PD if their course was complicated by malnutrition (149). In contrast, a later report by Warady et al. (130) involving 51 PD patients with a history of FP did not reveal any significant relationship between the fungal infection and the presence of a gastrostomy. Nevertheless, it seems most reasonable to create the PD catheter exit site as far as pos sible from the ostomy site to lower the potential risk of peritoneal infection.
In the case of infants, the right upper quadrant of the abdomen should usually be the preferred site for the PD catheter exit site because a gastrostomy may be placed in the left upper quadrant if one is not already there. In infants with a colostomy, in whom the risk of soiling is high, the PD catheter exit site can actually be created over the lower portion of the chest wall and at a distance from the site of the colostomy, ensuring that it is also at considerable distance from the nipple, especially in female infants (36).
Guidelines 7.2 and 7.3: The dextrose content of the dialysate in the setting of contamination of the peritoneum secondary to gastrostomy placement encourages the proliferation of organisms, increases the risk for peritonitis, and should therefore prompt gastrostomy placement before or at the time of PD catheter placement if at all possible. When earlier or simultaneous placement is not possible, the technique used to place the gastrostomy can influence the risk of peritonitis. A gastrostomy can be placed using either an open surgical procedure or a PEG technique, and the former procedure is preferred in patients who are already receiving PD (150). The open procedure theoretically limits contamination of the peritoneal cavity by securing the stomach to the abdominal wall with sutures. A report by Ledermann et al. (150) revealed a high risk of peritonitis developing after PEG placement in children who were already receiving PD. Similarly, in a recent retrospective survey carried out by von Schnakenburg (151) that included 27 pediatric patients who had a gastrostomy placed (25 by PEG) while already receiving PD, peritonitis occurred in 10 of the 27 (37%) within 7 days of PEG insertion, and FP occurred in 7 of the 27 (26%). Furthermore, 8 of the 27 required replacement of their PD catheter, 4 were transferred to HD, and another 2 experienced late deaths because of the associated problems.
The risk associated with PEG placement in patients on PD is likely a result of the small amount of leakage of gastric contents that occurs during and possibly after gastrostomy placement (152). The risk likely persists until an adequate seal develops between the stomach and the abdominal wall because there are no sutures to secure the stomach to the abdominal wall. Instead, a relatively rigid device, usually in the form of a “mushroom tip” at the end of gastrostomy tube, keeps the stomach and abdominal wall apposed until they heal.
Guideline 7.4: To lower the significant risk of peritonitis, prophylactic therapy is strongly recommended at the time of gastrostomy placement (open surgical or PEG), especially in a patient already receiving PD. Patients are typically given a single dose of parenteral cefazolin, with completion of the infusion within 60 minutes before initiation of the surgical procedure. If the risk for MRSA is high, vancomycin infused over 60 – 90 minutes to conclude within 60 minutes before the gastrostomy placement is recommended. Antifungal prophylaxis can be provided by giving fluconazole every other day.
In a meta-analysis of 10 randomized controlled trials involving more than 1000 patients undergoing PEG placement for variety of indications (147), patients who received antibiotic prophylaxis showed a significantly reduced rate of peristomal infection compared the rate in patients who did not receive prophylaxis (crude wound infection rate: 8% vs 26%; RR reduction: 64%). The study by von Schnakenburg (151) also demonstrated that the lowest rate of infectious complications after PEG placement was observed in patients who received both antifungal and antibiotic prophylaxis (no FP and no catheter loss). Anecdotal reports have also provided evidence for a higher risk of fungal infection when a gastrostomy is placed in patients with advanced malnutrition (149). Malnourished patients should therefore ideally receive a period of nasogastric feeding to improve their nutrition and immune status before gastrostomy insertion, and antifungal prophylaxis should be given at the time of the surgical procedure.
Guideline 7.5: Regardless of the gastrostomy placement technique used, PD should preferably be withheld for a period of time after the gastrostomy placement. The optimal duration that the patient should be maintained off PD is not known, but Ledermann et al. (150) recommended a period of 1 – 4 days after surgery. It is important to reinitiate dialysis with a lower exchange volume and then gradually to increase it to the maintenance volume over the next 5 – 7 days. In the patient receiving CAPD, consideration may also be given to temporarily changing the PD modality to APD, with a diminished or absent daytime exchange volume.
LIMITATIONS
No randomized studies have compared the risk for complications between PEG and the open surgical procedure for gastrostomy placement. Also, no studies have defined the optimal time to withhold PD after placement of a gastrostomy.
RESEARCH RECOMMENDATIONS
Controlled studies to establish the benefit of antibiotic and antifungal prophylaxis are difficult to justify in the setting of a high-risk procedure such as gastrostomy placement, but the risk of peritonitis with various treatment modifications might be compared prospectively. Such randomized studies could compare PEG with open gastrostomy placement and various PD break times after ostomy placement.
GUIDELINE 8 – DIAGNOSIS OF PD-RELATED PERITONITIS
8.1 We recommend that a diagnosis of peritonitis be considered in the presence of cloudy peritoneal effluent (1A).
8.2 We recommend that cloudy peritoneal effluent be sent for cell count, differential count, and culture to confirm the diagnosis of peritonitis (1A).
8.3 We recommend that an empiric diagnosis of peritonitis be made if the effluent white blood cell count is greater than 100/mm3, and at least 50% of the WBCs are polymorphonuclear leukocytes (1A).
8.4 We recommend that the effluent be centrifuged, and the resulting sediment be cultured if possible. Blood-culture bottles should be used as an alternative culture technique (1B).
RATIONALE
Guideline 8.1: Patients on PD who have peritonitis usually present with cloudy effluent and abdominal pain (153–157). Other symptoms include fever, chills and rigors, anorexia, vomiting, abdominal distension, and in late cases, septic shock. To make the diagnosis early, peritonitis should be considered whenever the peritoneal effluent is cloudy. Other causes of cloudy effluent include chemical peritonitis, eosinophilic peritonitis, hemoperitoneum, specimen taken from a “dry” abdomen, and rarely, malignancy and chylous effluent.
In the PD patient with abdominal pain and clear fluid, peritonitis must also be excluded. Some of the peritonitis episodes collected by the IPPR were associated with clear effluent at presentation (4). In such cases, a repeat assessment of the effluent for cloudiness should also be conducted with subsequent exchanges. Other causes of abdominal pain in children include constipation, acute gastritis, gastroenteritis, and acute appendicitis or pancreatitis.
The abdominal pain in peritonitis is typically generalized, and it is often associated with guarding and rebound tenderness. The degree of pain is variable, being mild to moderate in CNS peritonitis and more severe in infections involving Streptococcus, gram-negative rods, and S. aureus. If the pain and tenderness are localized, acute appendicitis must be considered. If subsequent peritoneal fluid cultures grow multiple organisms, viscus perforation must be excluded.
A Disease Severity Score (see guideline 21), defined by the sum of points for pain (0 = no pain; 1 = moderate pain, or nausea not requiring specific therapy; 2 = severe pain usually requiring analgesic therapy, or vomiting; 3 = peritoneal pain with a tense abdomen or paralytic bowel) and for fever based on oral temperature (0 = <37.5°C; 1 = 37.5°C – 38.9°C; 2 = >38.9°C) has been used to objectively evaluate the severity of the clinical status (158,159).
Guidelines 8.2 and 8.3: Investigations of patients suspected of having peritonitis should include a peritoneal fluid cell count, differential count, gram stain, and culture. A blood culture should also be obtained if the patient appears toxic.
Microscopy is essential to confirm the presence of white blood cells (WBCs), because cloudy fluid can also be a result of the presence of chyle, fibrin, or red blood cells. As an early screening test for the presence of WBCs, leukocyte esterase reagent test strips have been used at some centers in patients suspected of having peritonitis (160,161).
For patients on CAPD or APD with a daytime exchange, the first cloudy bag or the manual drain should be sent for cell count, differential count, gram stain, and culture. After a dwell time of at least 2 hours, a peritoneal effluent WBC count of more than 100/mm3 in an uncentrifuged specimen, with a differential count of at least 50% neutrophils, is highly suggestive of peritonitis.
For the child on APD without a day dwell, the fill volume should be instilled for a minimum of 1 – 2 hours, with the subsequent effluent being sent for cell count, differential count, and culture. The absolute WBC count may not fulfill the standard diagnostic criteria if the dwell time is too short; in this case, the presence of 50% or more neutrophils, even if the total cell count is less than 100/mm3, is highly suggestive of peritonitis. In equivocal cases, or in patients with systemic or abdominal symptoms in whom the effluent appears clear, a second exchange with a dwell time of at least 2 hours is performed.
In a recent IPPR report, 2.8% of clinical peritonitis episodes had WBC counts less than 100/mm3, and 8.5% of cases had less than 50% neutrophils (4). If the eosinophil count exceeds 10%, a diagnosis of eosinophilic peritonitis should be considered, especially if the peritoneal fluid cultures are negative (162).
To guide empiric therapy, it is useful to perform gram staining on all samples; however, the sensitivity of a gram stain is low. Despite large numbers of WBCs, micro-organisms may not be visible or may be low in yield because of their sequestration within phagocytes. Still, the gram stain could be the first clue to a fungal infection, because budding yeast may be seen.
Guideline 8.4: Obtaining the sample correctly and using proper culture techniques are crucial in establishing the diagnosis of peritonitis and in determining the proper choice of antibiotics. Patients who reside in areas far from medical facilities should be taught the recommended technique for collecting the cloudy peritoneal effluent and placing it in blood culture bottles or for refrigerating (not freezing) the effluent bag until the sample can be brought to the dialysis center for transport to the laboratory.
Specimens should be sent to the laboratory and processed within 6 hours. Should there be any delay in either transport or processing for culture, effluent samples must be refrigerated at 4°C until processed, but blood-culture bottles should be incubated at 37°C. A delay of more than 12 hours is unacceptable and will likely generate spurious results (57,163).
The optimum culture technique involves centrifuging a large volume (50 mL) of the peritoneal effluent at 3000g for 15 minutes to obtain sediment for culture. The sediment is resuspended in 5 – 10 mL of sterile normal saline and inoculated directly onto solid-culture media and into standard blood-culture media. The solid-culture media should be incubated in aerobic, anaerobic, and microaerophilic conditions. Concentration techniques such as this one should yield a culture-negative rate of less than 5% (164,165). An alternative culture method involves injecting 20 – 30 mL of peritoneal effluent from the sample bag into 3 – 4 blood-culture bottles. The latter technique will result in a culture-negative rate of less than 20% (57). The rate of culture-negative peritonitis should not exceed 20% of peritonitis episodes in any center, and in an ideal setting, the goal is to achieve a culture-negative peritonitis rate of less than 10% (57).
Rapid blood culture techniques such as Bactec (Becton–Dickinson, Franklin Lakes, NJ, USA), Septi-Chek (Becton–Dickinson), and BacT/Alert (bioMérieux, Marcy l’Etoile, France) are useful in reducing the time to identification of the micro-organism causing the peritonitis. Bedside-inoculated bottles have not been shown to be significantly better than laboratory-inoculated bottles, and high-volume bottles were not significantly better than low-volume bottles for the detection of patients positive for micro-organisms; however, the total number of micro-organisms recovered was significantly better from inoculated blood culture bottles than from routine culture (166). Two recent prospective studies also support the routine use of the broth culture technique compared with the water lysis technique (167,168). Preliminary organism identification by gram staining was 70.6% with the broth culture method, a rate significantly greater than the 17.6% achieved with the water lysis method. The broth culture method, with BacT/Alert blood-culture bottles, also detected organisms faster than the water lysis method, facilitating early streamlining of empiric antibiotic therapy.
Using the foregoing culture techniques and associated concentration methods, most cultures will become positive within 24 hours. A microbiologic diagnosis can be obtained in more than 75% of specimens by 72 hours. If cultures remain negative after 3 – 5 days in an automated culture system but the clinical picture is highly suggestive of peritonitis, further subculturing of blood-culture bottles onto media in aerobic, anaerobic, and microaerophilic environments for an additional 3 – 4 days may be necessary to identify slow-growing bacteria and yeasts.
Polymerase chain reaction can be a sensitive method for identifying causative organisms. Broad-spectrum polymerase chain reaction with RNA sequencing, and quantitative bacterial DNA polymerase chain reaction assays can complement, but not replace, culture methods in the diagnosis of peritonitis, especially if the patient is receiving antibiotic therapy (169,170).
LIMITATIONS
Pediatric data on which to recommend use of the effluent WBC differential counts as a means to diagnose peritonitis when the total effluent WBC count is low in patients receiving APD are limited.
FUTURE RESEARCH
The factors contributing to elevated rates of culture-negative peritonitis (>20%) in pediatric centers should be explored.
Prospective trials should be used to compare the sensitivity and specificity of the various diagnostic technologies available in the setting of PD-related peritonitis.
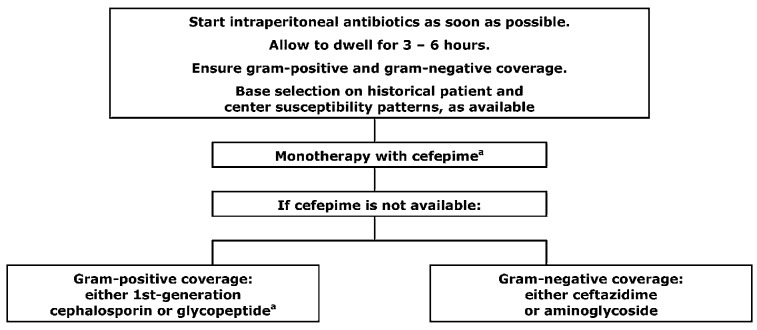
GUIDELINE 9 – ADMINISTRATION OF ANTIBIOTICS
9.1 We recommend that antibiotics for the treatment of bacterial peritonitis be administered by the intraperitoneal route (1B).
9.2 In non-anuric patients receiving intermittent intraperitoneal doses of glycopeptide antibiotics (vancomycin or teicoplanin), we recommend monitoring blood levels of the antibiotics (2A).
9.3 We recommend that beta-lactam antibiotics be administered continuously (1B).
RATIONALE
Guideline 9.1: In PD-associated peritonitis, intraperitoneal instillation is the administration route of choice for most antibiotics, because high bactericidal concentrations are immediately established at the site of infection. In addition, most antibiotics are readily absorbed from the peritoneal cavity, leading to therapeutic blood levels. For most currently used antibiotics, the doses required to be delivered to the peritoneal space to achieve adequate blood levels have been established in pharmacokinetic studies (Table 5). An additional advantage of intraperitoneal administration is the capacity for the home-based provision of antibiotic therapy after proper training.
TABLE 5.
Antibiotic Dosing Recommendationsa for the Treatment of Peritonitis
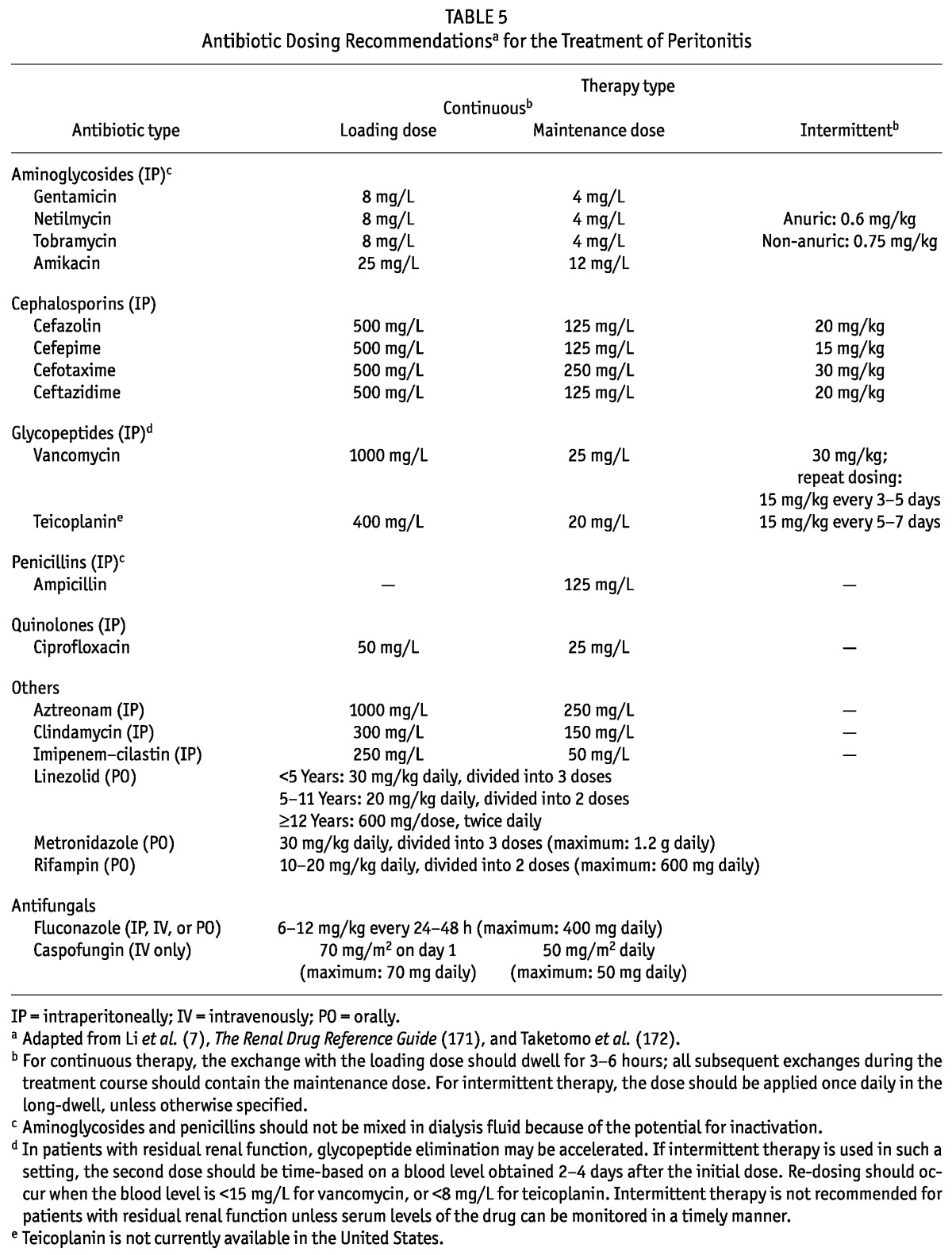
Guidelines 9.2 and 9.3: Antibiotics given by the peritoneal route can be administered either continuously or intermittently. Continuous dosing ensures constant therapeutic antibiotic concentrations locally. Most dosing schemes include an initial extended-dwell cycle with a higher antibiotic concentration (to saturate the distribution space), followed by maintenance dosing.
For drugs with efficient peritoneal absorption and a long pharmacologic or biologic half-life (or both), intermittent dosing is an option. After systemic absorption, the body acts as a reservoir for continuous back-diffusion of the antibiotic into the peritoneal cavity. Ideally, dialysate concentrations at the end of the antibiotic-free dwell will exceed the minimal inhibitory concentration (MIC) of the infecting organism (173–175). Administration intervals depend on the half-life of the drug, which is determined mainly by protein binding and residual renal and extrarenal metabolic clearance. Long-standing experience with intermittent antibiotic dosing is available for the glycopeptides vancomycin and teicoplanin (administered at 5- to 7-day intervals) and for aminoglycosides and cephalosporins applied once daily (57,159,173,175).
The concept of intermittent antibiotic administration appears intriguing because of its practicality and cost efficiency, but the efficacy and safety of intermittent dosing depend on several factors (176). Most importantly, the dialysate flow rate strongly affects both absorption and elimination of the drug (174). Given that the loading doses of most antibiotics have been established using extended (6- to 8-hour) dwells, the use of long-dwell periods (nighttime cycle in CAPD, daytime cycle in APD) for intermittent dose administration is recommended (57).
A more significant issue with intermittent antibiotic dosing in APD may be how to maintain therapeutic antibiotic levels in dialysate in the post-dosing interval (173). Frequent short cycles in APD may prevent accumulation of antibiotic in the peritoneal cavity to reach concentrations exceeding the MIC. A pharmacokinetic study that evaluated the disposition of intraperitoneal vancomycin in children suggested that enhanced total-body vancomycin elimination in children (relative to adults), coupled with slow peritoneal transfer, might be associated with inadequate time to achieve therapeutic intraperitoneal levels by the re-entry mechanism (159,177). Moreover, transperitoneal drug movement may be less effective in the post-acute phase of peritoneal infection when inflammation-related capillary hyperperfusion subsides. Also, given that the metabolic clearance of antibiotics such as vancomycin and aminoglycosides is closely correlated with residual renal function, the efficacy of intermittent therapy will depend on appropriate adaptation of dosing intervals and may require monitoring of blood levels. Finally, the clinical efficacy of intermittent dosing may also depend on post-exposure antibiotic properties. A post-exposure bacteriostatic effect is well established for aminoglycosides, but beta-lactam antibiotics lack a post-antibiotic effect (178).
In clinical practice, intermittent antibiotic dosing has been mostly, but not unequivocally successful in eradicating bacterial growth (33,159,179–182). In a pediatric trial comparing intermittent and continuous intraperitoneal administration of empiric glycopeptide and ceftazidime, persistent growth of the causative organism in dialysate was found in 33% and 10% of the intermittently treated episodes and in 6% and 1% of the continuously treated episodes after 60 hours and after 7 days of treatment respectively (159). Delayed eradication with intermittent therapy was found not to affect overall treatment outcomes, but a higher rate of clinical treatment failure was observed in patients with gram-negative peritonitis receiving intermittent ceftazidime. The inferior efficacy of once-daily ceftazidime in gram-negative peritonitis was confirmed in the IPPR registry, where intermittent therapy was independently associated with a risk of empiric treatment failure that was higher by a factor of 14 (33).
Based on the foregoing findings, we do not recommend intermittent administration of beta-lactam antibiotics (176). Furthermore, we recommend monitoring the blood levels of antibiotics (within 2 – 4 days of first administration) in patients receiving glycopeptides intermittently—at least in patients with significant residual renal function. Although not established in routine clinical practice, monitoring of dialysate concentrations may provide even more relevant information.
LIMITATIONS
The pharmacokinetic and pharmacodynamic basis for intermittent drug dosing, particularly in patients undergoing APD with frequent short cycles, is limited to a few adult and even fewer pediatric studies.
The usefulness of therapeutic drug monitoring in patients receiving intermittent glycopeptide therapy has not been demonstrated in clinical studies.
RESEARCH RECOMMENDATIONS
The kinetics of drug disposition after intermittent intraperitoneal administration should be studied in all pediatric age groups for all antibiotics listed in the present guideline.
The effects of PD prescription modifications (fill volume, number and duration of cycles) on peritoneal drug resorption and clearance should be assessed by computer simulation, using experimentally established pharmacokinetic characteristics.
The predictive value of plasma and dialysate antibiotic levels for bacterial eradication and clinical outcomes should be studied.
GUIDELINE 10 – EMPIRIC ANTIBIOTIC THERAPY
10.1 We suggest that the center-specific antibiotic susceptibility pattern should help to guide the selection of empiric antibiotic therapy (2B).
10.2 We suggest intraperitoneal cefepime monotherapy for the empiric treatment of peritonitis in centers in which that antibiotic is available and affordable (2C).
10.3 We recommend intraperitoneal administration of a first-generation cephalosporin combined with ceftazidime or an aminoglycoside if cefepime is not available (1C).
10.4 We suggest the addition of an intraperitoneal glycopeptide to cefepime, or replacement of a first-generation cephalosporin with an intraperitoneal glycopeptide, if the center-specific resistance rate of S. aureus isolates to methicillin or oxacillin exceeds 10% or if the patient has a history of MRSA (2B).
RATIONALE
Guideline 10.1: Empiric antibiotic therapy (Figure 1) should be effective in treating most gram-positive and -negative bacteria that cause peritonitis in children requiring PD. Recent reviews of the bacteriology of PD related peritonitis reveal that, in adults, a selective decline in the incidence of gram-positive peritonitis is occurring as a result of prophylactic measures such as those addressing S. aureus nasal carriage. That decline is associated with a relative rise in the gram-negative peritonitis rates (53,92,183). A similar decline has been seen in the pediatric population, with gram-positive bacterial infections comprising as few as 44% of all culture-positive peritonitis episodes (158). In the adult dialysis population, CNS have been found to be three times more common than S. aureus, but the IPPR reported that staphylococcal peritonitis was almost evenly distributed between those two bacterial species at 22% and 21% respectively (5,158,183). Moreover, data from the IPPR showed significant variation in the distribution of bacteria in various global regions, with the distribution of gram-negative organisms being particularly variable between centers. The local distribution pattern of causative organisms and their antibiotic susceptibilities (antibiogram), and the patient’s history of infections and colonization pattern (for example, MRSA, VRE), should therefore be taken into account when empiric therapy is chosen.
Figure 1.
— Empiric therapy.
a If the center’s rate of methicillin-resistant Staphylococcus aureus (MRSA) exceeds 10%, or if the patient has history of MRSA infection or colonization, glycopeptide (vancomycin or teicoplanin) should be added to cefepime or should replace the first-generation cephalosporin for gram-positive coverage. Glycopeptide use can also be considered if the patient has a history of severe allergy to penicillins and cephalosporins.
Antibiograms and resistograms show the bacteria commonly isolated at a center and provide information on the percentage of isolates that are susceptible to individual antibiotics. Antibiograms and resistograms are usually developed annually by the microbiology laboratory. Although no data are available on the level of resistance that should be used to guide empiric therapy, an antibiotic should not be used for empiric peritonitis therapy if the institution-specific resistance rate of a potential bacterial pathogen is greater than 10%, except for CNS (see guideline 10.4).
Guideline 10.2: Cefepime is a fourth-generation cephalosporin that can treat gram-positive bacteria, including methicillin-susceptible S. aureus, and most gram-negative bacteria, including P. aeruginosa. In one study, intraperitoneal cefepime was as effective as the combination of vancomycin–netilmycin in the treatment of CAPD-associated bacterial peritonitis (184). Li and colleagues reported an 81% favorable primary response when using cefepime as monotherapy (185). And unlike ceftazidime and other third-generation cephalosporins, cefepime is not associated with the development of resistant gram-negative rods as a result of induction of extended-spectrum beta-lactamase (ESBL) production.
Notable reports of cefepime side effects include an increase in mortality and neurotoxicity. A meta-analysis suggested that intravenous cefepime was associated with an increase in all-cause mortality (186). However, subsequent studies, including a meta-analysis conducted by the US Food and Drug Administration, concluded that cefepime is not associated with an increase in mortality and is safe to use (187,188). Neurotoxicity has been reported in patients with renal failure receiving intravenous cefepime and in 2 patients given intraperitoneal cefepime (189,190); however, in a study of 87 episodes of peritonitis treated with intraperitoneal cefepime, only 1 patient discontinued the drug (because of epigastric pain), and no patient was reported to have experienced neurotoxicity (185).
Guideline 10.3: The previous ISPD guideline for the treatment of peritonitis in children recommended vancomycin or a first-generation cephalosporin (for example, cefazolin, cephalothin) to treat gram-positive bacteria, and a third-generation cephalosporin (for example, ceftazidime) to treat gram-negative bacteria (3). Early studies showed that glycopeptide-based regimens had higher complete cure rates than regimens that included a first-generation cephalosporin (80% vs 65%), but no significant differences were observed in the primary failure or relapse rates (185–188). In fact, when higher doses of cefazolin (125 mg/L) were used, no difference in the cure rates for vancomycin and cefazolin were found (191). More recently, Warady and colleagues reported that, when initiating treatment for children with peritonitis, no difference in the initial response was noted when a regimen of cefazolin plus ceftazidime was compared with a regimen of glycopeptide plus ceftazidime (4). However, recent data from the IPPR demonstrated an increase in relapse episodes among children who received cefazolin monotherapy (32).
Additional data from the IPPR demonstrated that 20% of the gram-negative isolates were resistant to ceftazidime, with a risk of an insufficient early clinical response that was higher by a factor of 7 (32). Therefore, if a center’s gram-negative resistance to ceftazidime exceeds 10%, an aminoglycoside should be used, provided that its gram-negative resistance rate is lower. Studies have shown that, in terms of complete cure, treatment failure, and its effect on residual renal function, empiric intraperitoneal administration of cefazolin–netilmycin did not differ from that of cefazolin–ceftazidime (192). However, extended courses of aminoglycoside therapy are associated with vestibular and ototoxicity and potential loss of residual kidney function, mandating the discontinuation of empiric aminoglycosides once susceptibilities are known and an alternative antibiotic to which the organism will respond is available (193).
Guideline 10.4: Studies have demonstrated that MRSA causes a more severe peritonitis, as indicated by a lower initial response rate, a lower cure rate, a higher frequency of catheter removal, and a higher death rate (194,195). Interestingly, in a study of 245 episodes of S. aureus peritonitis, 45 of which were MRSA, no significant difference in outcome (defined as the primary response rate) was observed between episodes empirically treated with cefazolin and those treated with vancomycin or other antibiotics. Even in the subgroup of patients treated with cefazolin as empiric therapy before being changed to vancomycin after results of susceptibility testing were available, episodes that were caused by methicillin-resistant and methicillin-susceptible bacteria had similar primary response rates and complete cure rates (195). Additionally, in a recent study involving 503 cases of S. aureus peritonitis, of which 102 were MRSA, no increase in relapse, hospitalization, catheter removal, permanent HD transfer, or death was observed in patients empirically treated with a regimen not including vancomycin (194). However, because of the potential severity of S. aureus peritonitis, empiric therapy with a glycopeptide is suggested if a center’s MRSA constitutes more than 10% of all S. aureus isolates. This figure of 10% is based on opinion and the clinical experience of the workgroup members.
Because of the increased risk for a subsequent infection with MRSA, children who have a history of MRSA infection or colonization should also be empirically treated with a glycopeptide (196).
The resistance rate of CNS is not recommended for use as a factor in determining an institution’s empiric therapy, because CNS causes a mild, indolent illness for which cure rates are high (197).
Vancomycin is not recommended for all patients because of concerns related to the development of resistant bacteria. A greater than 20% incidence of VRE has been reported in nosocomial infections, and significant morbidity is associated with VRE-related infections. These bacteria have, in fact, been reported as a cause of a significant proportion of peritonitis episodes in some centers (198–200).
LIMITATIONS
Variability in susceptibilities and resources in different centers make it impossible to provide one recommendation for empiric therapy that would be useful for all centers. It is therefore imperative that centers utilize their antibiograms and maintain awareness of the bacteria that commonly cause peritonitis in patients receiving PD. Furthermore, the percentage of resistance that should guide a change in empiric antibiotic recommendations is not clear.
No data are available on the use of cefepime monotherapy as treatment for peritonitis in children.
RESEARCH RECOMMENDATIONS
Additional information is needed on the outcomes associated with various empiric antibiotic regimens. Further studies into an understanding of the epidemiology and risk factors associated with specific bacterial causes of peritonitis would be useful in providing the best empiric regimens based on clinical presentation. The impact of empiric cefepime monotherapy on early treatment response needs to be monitored.
GUIDELINE 11 – MODIFICATION OF THERAPY FOR GRAM-POSITIVE PERITONITIS
11.1 Use susceptibility data to guide the post-empiric antibiotic selection in patients with gram-positive peritonitis (not graded; Figure 2).
11.2 We recommend continuing empiric cefepime or cefazolin when the gram-positive bacteria identified is susceptible to one of those antibiotics (1B).
11.3 In patients receiving empiric ceftazidime or an aminoglycoside, we recommend discontinuing those antibiotics if a gram-positive bacteria is isolated (1B).
11.4 In patients with S. aureus or coagulase-negative Staphylococcus who have a delayed response to initial therapy (>72 hours), we suggest adding rifampin (2B).
Figure 2.
— Gram-positive organism on culture.
MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive S. aureus; VRE = vancomycin-resistant enterococci.
RATIONALE
Guidelines 11.1 and 11.2: Data from the IPPR suggest that gram-positive bacteria are identified in 62% of pediatric cases in which an organism is isolated. The most common organisms are CNS and S. aureus, occurring in 22% and 21% of all cases respectively (5). Peritonitis secondary to CNS is typically a result of touch contamination and characteristically presents as a mild infection that responds readily to antibiotic therapy. In contrast, infections involving S. aureus are commonly associated with a PD catheter TI or ESI and may be associated with S. aureus nasal carriage and severe symptomatology.
In general, the susceptibility of the causative bacteria should guide antimicrobial therapy, regardless of the initial clinical response to empiric therapy. Empiric cefepime or cefazolin should be continued if the bacteria that are identified are susceptible. Cefepime or cefazolin can also be used to treat methicillin-susceptible S. aureus peritonitis, and if a glycopeptide was empirically started, it should be discontinued and replaced with an alternative agent. The recommended length of therapy for CNS is 2 weeks. A 3-week course of treatment appears appropriate for S. aureus–related peritonitis because of the greater likelihood of cuff and tunnel involvement.
Guidelines 11.3 and 11.4: When ceftazidime or an aminoglycoside is used as part of the empiric antibiotic regimen, it can be discontinued if a gram-positive organism is cultured, because those agents are not recommended for treatment of gram-positive bacteria. Methicillin resistance indicates that the organism is resistant to beta-lactam–related antibiotics, including penicillins, cephalosporins, and carbapenems. Treatment of MRSA should be based on the susceptibility of the bacteria. Clindamycin, vancomycin or teicoplanin can be used to treat PD-associated peritonitis caused by MRSA. The recommended length of therapy for PD-associated peritonitis caused by MRSA is 3 weeks.
In general, children with gram-positive peritonitis do well. Data from the IPPR demonstrated that only 5% of S. aureus and 1% of CNS episodes had a delayed response to empiric treatment (4). “Delayed response” is defined as no improvement after 3 days of empiric antibiotics. In cases in which a delayed response occurs, the addition of rifampin is suggested. Rifampin should never be used as monotherapy because resistance develops rapidly in that setting.
ADDITIONAL RATIONALE
Enterococcal peritonitis frequently originates from intra-abdominal pathology, but it can also result from touch contamination or an ESI or TI. A recent experience with 116 episodes of enterococcal peritonitis in adults highlighted the risk of a poor outcome when other pathogens were isolated along with the Enterococcus species (201). Recent data from the IPPR identified Enterococcus species as the cause of 6% of peritonitis episodes in children. In that study, all patients had a full recovery, and only 1 catheter had to be exchanged (202). These favorable outcomes occurred despite the fact that, in most cases of Enterococcus peritonitis, the organism was not susceptible in vitro to the empiric choice of antibiotic. Therefore, empiric antibiotic coverage specific for Enterococcus species is not required.
When an Enterococcus species is identified, continuation of vancomycin or teicoplanin (if used as a component of empiric therapy) until susceptibilities are known and modification of therapy can be instituted appears justified. Likewise, if glycopeptides are not part of the empiric treatment protocol, but the patient is improving clinically, the IPPR data support the continuation of current empiric therapy until susceptibility data are available.
The addition of an aminoglycoside can be considered when the Enterococcus species is susceptible, because that antibiotic has been shown to be synergistic when combined with ampicillin or penicillin or a glycopeptide. However, in the IPPR, Sutherland and colleagues noted that all patients with enterococcal peritonitis experienced clinical improvement, and none received combination therapy with an aminoglycoside (202). Moreover, it should be emphasized that, because of chemical incompatibility, aminoglycosides should not be combined in the same exchange with a penicillin.
If a patient has previously been infected with an ampicillin-resistant VRE, linezolid should be started empirically until culture and susceptibility data are available. Prolonged therapy with linezolid (>14 days) can result in bone-marrow suppression (203).
Like peritonitis caused by Enterococcus species, peritonitis caused by Streptococcus species can produce severe pain and may be secondary to an ESI or TI, or originate from the mouth in a patient with poor oral hygiene. Ampicillin, a first-generation cephalosporin, or cefepime can treat Streptococcus infection, and susceptibilities should ultimately guide the maintenance antibiotic choice.
Table 6 summarizes the information presented in this guideline section.
TABLE 6.
Gram-Positive Bacteria: Recommended Antibiotics and Length of Therapy
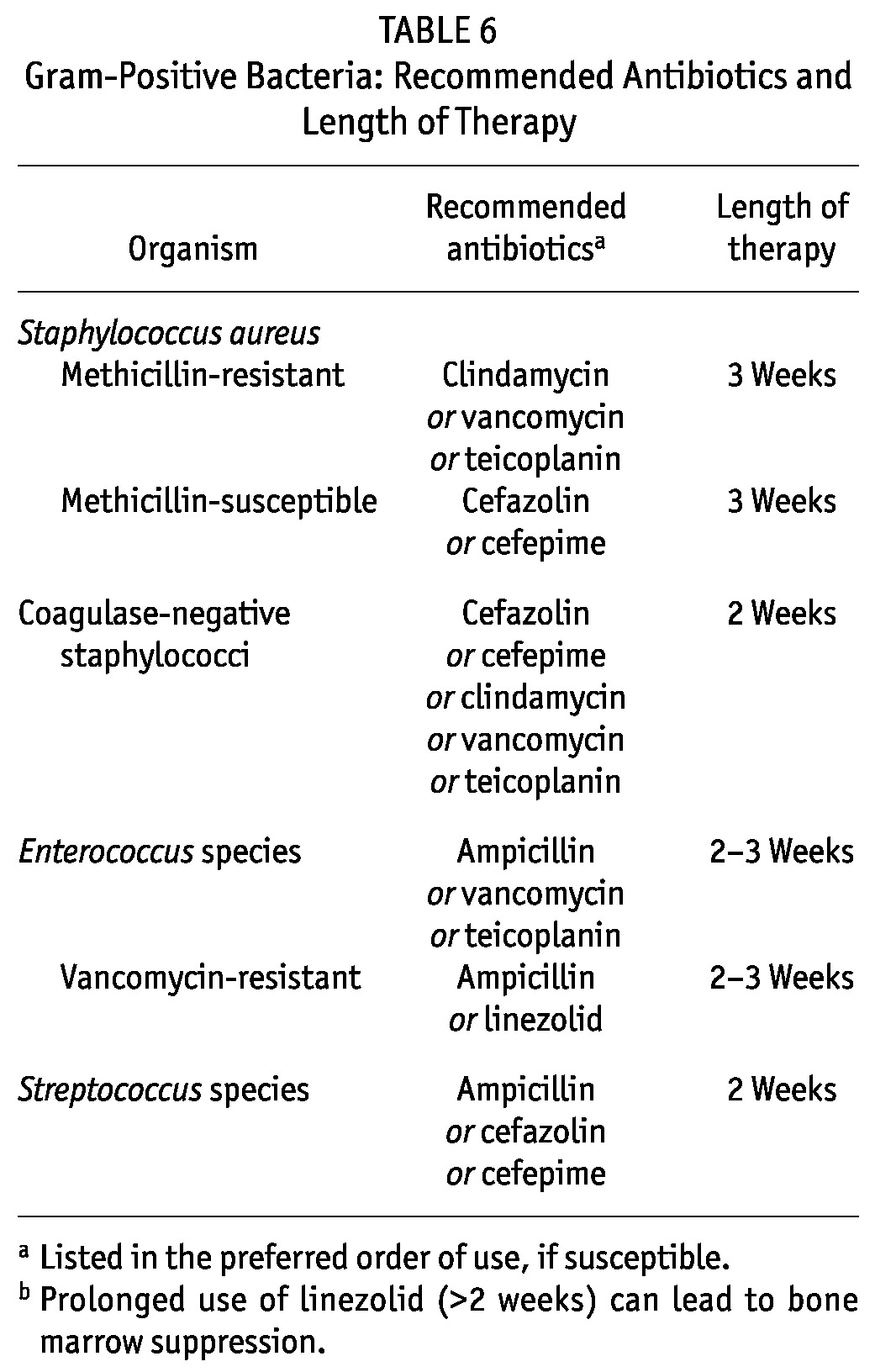
LIMITATIONS
Lengths of therapy are based on expert opinion; studies have not been conducted to determine the shortest amount of time required to sufficiently treat peritonitis in children on chronic PD.
Noteworthy is the recent finding from the IPPR that cefazolin monotherapy for the treatment of susceptible bacteria is associated with an increased risk for relapsing peritonitis (32). However, whether the post-empiric use of a first-generation cephalosporin is a general risk factor for relapsing peritonitis or whether the problem of incomplete eradication is limited to certain bacteria (such as CNS), is currently unclear.
RESEARCH RECOMMENDATIONS
Studies should be conducted to determine the most appropriate length of therapy for peritonitis caused by certain bacteria. Identifying shorter lengths of therapy without compromising efficacy would lower overall antibiotic use and help to minimize the emergence of antibiotic resistance.
GUIDELINE 12 – MODIFICATION OF THERAPY FOR GRAM-NEGATIVE PERITONITIS
12.1 Use susceptibility data to guide the post-empiric antibiotic selection in patients with gram-negative peritonitis (not graded; Figure 3).
12.2 In patients receiving empiric glycopeptide therapy (vancomycin or teicoplanin), we recommend discontinuing those antibiotics if a gram-negative organism is isolated (1B).
12.3 If the gram-negative bacteria are susceptible to empiric cefepime or ceftazidime, we recommend the use of either one as a single agent unless a Pseudomonas species is identified (1B).
12.4 If an aminoglycoside is used for empiric treatment, we recommend discontinuing the aminoglycoside after the species and susceptibilities of the bacteria are known, unless a Pseudomonas species is identified (1B).
12.5 We suggest the addition of a second agent, with a different mechanism of action, when a Pseudomonas species is identified (2C).
Figure 3.
— Gram-negative organism on culture.
RATIONALE
Guidelines 12.1 – 12.3: Susceptibility data should guide the selection of the antibiotic or antibiotics used to treat gram-negative peritonitis. When a glycopeptide (vancomycin or teicoplanin) is used as part of the empiric antibiotic regimen, the antibiotic can be discontinued if a gram-negative organism is cultured, because those glycopeptides are not effective in treating gram-negative bacteria. Generally, cefepime or ceftazidime monotherapy can be used to treat peritonitis caused by gram-negative bacteria that are susceptible to those antibiotics.
Cefepime has been used successfully in treating CAPD-associated peritonitis in adults (184,185). In a report on gram-negative peritonitis from the IPPR, a substantial percentage of organisms (20%) were resistant to ceftazidime, and a multivariate analysis identified intermittent dosing (compared with continuous dosing) of intraperitoneal ceftazidime as an independent predictor of a worse initial response to therapy, prompting a preference for the addition of this antibiotic to all dialysis bags (33). A possible reason for the failure of intermittent ceftazidime might be the lack of a post-antibiotic effect of beta-lactam antibiotics against gram-negative organisms (178).
If the causative bacteria are identified as Escherichia coli or Klebsiella species, cefazolin, ceftazidime, ceftriaxone, or cefotaxime can be used if the bacteria are susceptible. The use of cefazolin alone requires caution. Lane and colleagues noted that peritonitis relapsed in 23% of patients treated with cefazolin alone; however, the type of bacteria associated with those relapses remains unclear (32).
Ciprofloxacin has also been shown to be an effective therapy for gram-negative peritonitis (204,205). Data from the IPPR noted that 96% of gram-negative bacteria were susceptible to ciprofloxacin (33). Oral quinolones such as levofloxacin or pefloxacin appear to have gram-negative coverage comparable to that achieved using aminoglycosides, and satisfactory levels of those agents in the peritoneum are able to be achieved even in patients receiving APD (175,206,207). It should be noted that resistance to ciprofloxacin is a strong marker of in vitro multidrug resistance and poor clinical outcomes in patients with peritonitis (208). Moreover, previous exposure to quinolones has been shown to be a risk factor for infection with resistant micro-organisms (209,210). Finally, when quinolones are used, attention should be paid to the concomitant administration of sevelamer, multivalent cations (calcium), oral iron, zinc preparations, sucralfate, magnesium–aluminum antacids, or milk, because chelation interactions that can reduce quinolone absorption may occur. Quinolones should therefore be given at least 2 hours before the administration of the foregoing substances.
Fluoroquinolones have been used extensively in pediatrics, and to date, experimental data in humans have not substantiated the cartilage damage that was observed in beagle puppies (211–214). In general, the overall safety profile of ciprofloxacin in children has not been substantially different from that in adults (215). In children 3 months to 13 years of age given ciprofloxacin for 9 – 16 days, no evidence of any orthopedic complications was observed (216).
Carbapenems such as imipenem have been used successfully in combination with tobramycin and vancomycin (217). However, these broad-spectrum antibiotics should be reserved for situations in which a highly resistant gram-negative bacteria is identified.
Aztreonam, a monobactam, has activity against most gram-negative bacteria and is useful in patients allergic to cephalosporins. It could therefore be effective in combination with a gram-positive antibiotic such as cefazolin or vancomycin. In a study involving 34 episodes of gram-negative peritonitis, an 84% cure rate was observed. Furthermore, no adverse reactions were observed (218).
Guideline 12.4: If cefazolin plus an aminoglycoside is used for empiric therapy, the aminoglycoside should be discontinued if the organism is susceptible to a less-toxic antibiotic—provided that the causative bacteria are not Pseudomonas species. Aminoglycosides alone are not routinely recommended for maintenance therapy because of the risk of ototoxicity and nephrotoxicity (and the potential loss of residual renal function) (219,220).
Guideline 12.5: Infections secondary to Pseudomonas species are difficult to treat because of that bacteria’s capacity to generate a biofilm that lowers the likelihood of successful treatment without catheter removal. In many cases, a catheter TI accompanies the peritonitis episode and increases the likelihood of treatment failure. Antibiotic use in the preceding 30 days has been identified as a risk factor for the development of peritonitis attributable to Pseudomonas species in patients receiving PD (221). Data from the IPPR noted that 45% of children with Pseudomonas peritonitis were currently receiving or had recently received antibiotics (33). The risk of Pseudomonas peritonitis was also found to be increased in patients receiving prophylactic exit-site care with mupirocin as prophylaxis for S. aureus colonization (5).
Combination therapy with cefepime or ceftazidime and a second agent that has a different mechanism of action (for example, fluoroquinolone or aminoglycoside) and to which the bacteria are susceptible is suggested (222). Fluoroquinolones (for example, ciprofloxacin) can be used to treat Pseudomonas peritonitis. Fluoroquinolones have been used extensively in pediatrics, and as noted earlier, experimental data in humans have not substantiated the cartilage damage that was observed in beagle puppies (211,212). The recommended length of therapy for peritonitis caused by Pseudomonas species is 3 weeks.
ADDITIONAL RATIONALE
Multidrug-resistant gram-negative bacteria have emerged as important pathogens in many health care–related infections. Notably, many gram-negative bacteria have developed the ability to inactivate third-generation cephalosporins such as cefotaxime, ceftriaxone, and ceftazidime through various mechanisms, including production of ESBLs. The use of third-generation cephalosporins, specifically ceftazidime, has been associated with induction of ESBL-producing bacteria (223–226). In PD-related peritonitis, risk factors for ESBL-producing E. coli include prior use of cephalosporin and gastric acid inhibitors (227). Furthermore, patients infected with ESBL-producing gram-negative bacteria have been found to experience more treatment failures and lethal outcomes than do patients with non-ESBL-producing E. coli. For that reason, cefepime, which can be used to treat infections secondary to certain ESBL-producing organisms (for example, those caused by AmpC enzyme–producing species such as Citrobacter and Enterobacter), has been prioritized over ceftazidime for empiric therapy in the present guidelines; however, carbapenems are recommended as the first choice for post-empiric antibiotic therapy of infections secondary to ESBL-producing bacteria, because those agents have generally shown superior clinical results in severe nosocomial infections (228). Alternatively, fluoroquinolones such as ciprofloxacin can be used—unless the antibiogram indicates resistance to multiple antibiotic classes. However, with the emerging treatment-related complexities that have arisen with respect to gram-negative resistance, especially as a result of ESBL-producing bacteria, consultation with an infectious disease clinician should be considered to aid in determining the antibiotic options that are preferred to limit resistance and to maximize clinical response.
Table 7 summarizes the information presented in this guideline section.
TABLE 7.
Gram-Negative Bacteria: Recommended Antibiotics and Length of Therapy
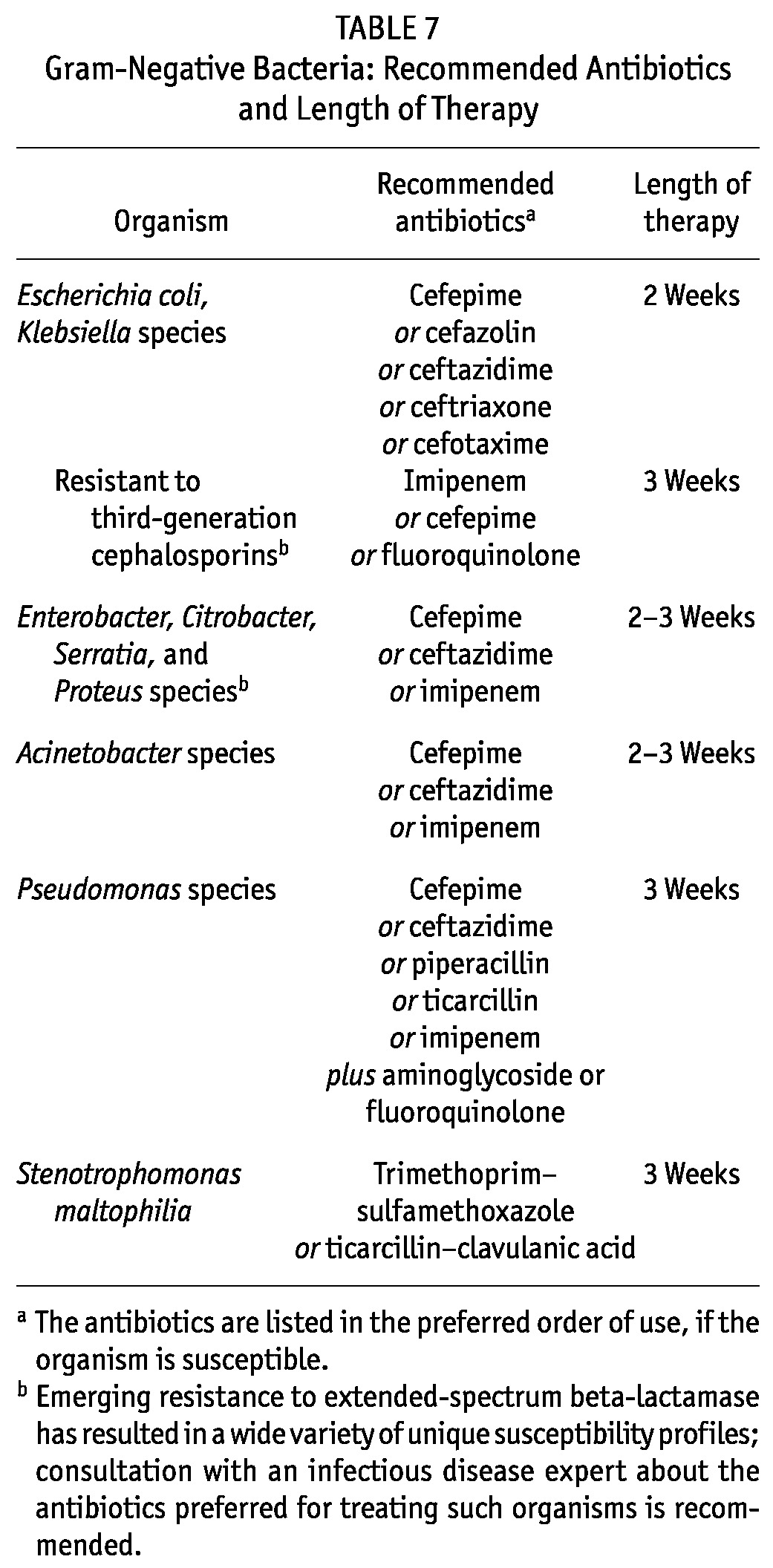
LIMITATIONS
Data on the risk factors associated with the development of resistant gram-negative bacteria are limited. Furthermore, the recommended lengths of therapy are based on expert opinion and do not take into account potential complications that could occur with the infections being treated.
RESEARCH RECOMMENDATIONS
As antibiotic resistance continues to increase, it will be imperative to further understand the factors that are associated with multidrug-resistant gram-negative peritonitis and the therapies that not only provide the best outcomes but also limit the negative consequences (for example, antibiotic resistance, antibiotic side effects).
Studies determining the most appropriate lengths of therapy for gram-negative peritonitis have the potential to minimize antibiotic exposure time and to attenuate the development of antibiotic resistance.
GUIDELINE 13 – MODIFICATION OF THERAPY FOR CULTURE-NEGATIVE PERITONITIS
13.1 If the initial cultures remain sterile at 72 hours and if signs and symptoms of peritonitis are improved, we suggest that empiric antibiotic therapy consisting of cefepime, ceftazidime, cefazolin, or a glycopeptide be continued for 2 weeks (2B).
13.2 We suggest that the administration of an aminoglycoside be discontinued at 72 hours in patients with a sterile culture and clinical improvement (2B).
RATIONALE
Guideline 13.1: Culture-negative peritonitis poses a therapeutic dilemma, because the absence of a positive culture does not allow the clinician to differentiate between an infection with a low infection dose; a slowly replicating, poorly culturable, or efficiently opsonized bacteria; a nonbacterial (that is, viral or fungal) infection; and even a noninfectious cause of peritoneal leukocytosis (for example, chemical or eosinophilic peritonitis).
In the IPPR, 151 peritonitis episodes (31% of all episodes) were culture-negative (4). Of those 151 episodes, 97% showed a good primary response to empiric antibiotic therapy at 72 hours, without a difference between the two monitored treatment protocols—that is, ceftazidime combined with either cefazolin or a glycopeptide (unpublished results). Treatment was continued with both antibiotics for 14 days in 91% of patients; 97% of those patients showed full functional recovery 4 weeks after completion of therapy.
The minimum effective duration of antibiotic treatment in culture-negative peritonitis is unknown. In light of the IPPR results and in the absence of outcomes data after early termination of antibiotic therapy in culture-negative episodes, it appears safe to continue the initial empiric therapy, which provides gram-positive and gram-negative coverage, for a complete treatment course to potentially lower the risk of relapsing infection.
Guideline 13.2: One modification to the recommendation to maintain empiric therapy may apply to empiric antibiotic protocols that include an aminoglycoside. Gram-negative peritonitis is characteristically associated with a severe clinical course and persistence of significant signs and symptoms at 72 hours of treatment (33). Most gram-negative bacteria also grow well in culture. Hence, negative culture results associated with rapid clinical improvement make a gram-negative cause less likely. Extended aminoglycoside administration, on the other hand, increases the risk of drug-induced ototoxicity and nephrotoxicity (and potential loss of residual renal function) and requires an assessment of plasma drug levels (219,220). Given the excellent outcomes observed in the IPPR series with extended ceftazidime administration, it seems advisable to discontinue the aminoglycoside at 72 hours in patients with a negative culture who are clinically improved. The initiation of ceftazidime should be considered for gram-negative coverage.
Patients with culture-negative peritonitis who fail to demonstrate clinical improvement after 72 hours should undergo a repeat PD effluent cell count, differential, and culture as recommended (see guideline 22). If the culture continues to be negative and if the PD effluent cell count has not improved, special culture techniques should be used for the isolation of unusual or fastidious organisms, including fungi, mycobacteria, and Legionella. Patients with culture-negative peritonitis who fail to improve after 5 days of therapy should undergo catheter removal, per the recommendations for refractory peritonitis (see guideline 17).
In centers in which culture-negative peritonitis represents more than 20% of peritonitis episodes, sampling and culture techniques should be reviewed with the dialysis staff and the laboratory (see guideline 8).
LIMITATIONS
An unknown fraction of culture-negative peritonitis episodes may be not be of bacterial origin, but may be caused by toxic, allergic, or traumatic peritoneal damage or gastrointestinal infection that cannot be differentiated from bacterial peritonitis because of poor culture conditions.
RESEARCH RECOMMENDATIONS
Controlled or uncontrolled prospective studies evaluating the safety of short and long courses of empiric antibiotic treatment in culture-negative peritonitis should be performed. Alternatively, such studies could address the risk–benefit ratio of reducing the spectrum of antibacterial coverage in patients with a prompt good clinical response.
GUIDELINE 14 – MODIFICATION OF THERAPY FOR FUNGAL PERITONITIS
14.1 We recommend that, if fungi are identified by gram stain or culture of peritoneal effluent, therapy should consist of treatment with an antifungal agent and early catheter removal (1B).
14.2 We suggest that, after catheter removal, antimycotic therapy be administered for 2 weeks or longer after complete resolution of the clinical symptoms of infection (2D).
RATIONALE
Guideline 14.1: Fungal peritonitis is an infrequent but potentially serious complication of PD, often resulting in hospitalization and a change of dialysis modality, and sometimes, in patient death (123). In pediatrics, FP represents fewer than 2% of all peritonitis episodes (4,58). In a pediatric study of 51 patients with FP, prompt therapy resulted in preservation of the peritoneal membrane and continued PD in most patients (130). A somewhat similar experience has also been documented in adults (128).
Several factors appear to predispose patients to the development of FP, the most common of which is the prior use of antibiotics to treat bacterial peritonitis or a catheter-related infection (123,128,131). However, Warady et al. found that, in nearly 50% of children who developed FP, no history of a prior peritoneal infection was documented (130). The risk associated with the presence of a gastrostomy remains controversial (4). The provision of antifungal prophylaxis during periods of antibiotic use has been advocated in programs characterized by high rates of FP (57,123,126,139) (see guideline 6).
Amphotericin B has historically been recommended as treatment for FP in patients receiving PD, but data collected in children and adults provide evidence that the peritoneal penetration of amphotericin B with systemic administration is poor (229). Moreover, intraperitoneal administration of this drug is characteristically irritating to the peritoneum and may result in severe abdominal pain. In contrast, fluconazole is characterized by excellent bioavailability and peritoneal penetration, and it is the treatment of choice for most Candida species. Alternative agents to be considered based on species identification and MIC values include echinocandins (for example, caspofungin, micafungin, and anidulafungin), posaconazole, and voriconazole. Posaconazole or voriconazole have been preferentially used to treat peritonitis secondary to filamentous fungi such as Aspergillus, and the echinocandins have been used on occasion for the treatment of Aspergillus and non-responding non-albicans Candida (230).
The recommendation for prompt catheter removal after a diagnosis of FP arises from the propensity of fungi to colonize the PD catheter and to prevent eradication of the infection. Electron microscopy of removed catheters has shown organisms in an amorphous matrix on the surface of the catheter. Candida albicans can grow on Silastic surfaces, and biofilm production has been associated with the development of C .albicans peritonitis (231). Most significant is the fact that, compared with patients treated with either antifungal therapy or catheter removal alone, the risk of repeat FP and death was lowest in patients treated with both interventions simultaneously (123,128,232) (see guideline 17).
Guideline 14.2: The recommendation that the duration of antifungal treatment after catheter removal be 2 weeks or longer after complete clinical resolution of the symptoms of peritonitis takes into consideration both the absence of pertinent evidence and the treatment goal of maintaining peritoneal membrane function over the long term. In addition, in other invasive Candida infections, such as bloodstream infections, the recommended length of therapy is 2 weeks after a negative culture is obtained (233).
LIMITATIONS
Published pediatric experiences upon which to base these recommendations are limited. Data in adult patients treated for FP that address the preferred timing of PD catheter removal and replacement (for example, immediate or early) are also limited.
RESEARCH
A review of data on the timing of catheter replacement after treatment for FP in children on PD and the relationship of that timing to the continued ability to perform effective PD is needed.
GUIDELINE 15 – RELAPSING PERITONITIS
15.1 We recommend that a diagnosis of relapsing peritonitis be made if peritonitis recurs with the same organism as in the preceding episode, according to antibiotic susceptibilities, within 4 weeks of completion of antibiotic treatment (1A).
-
15.2 (a) We recommend that empiric therapy in accordance with guideline 9 be reinitiated for relapsing peritonitis, with consideration of the susceptibilities of the original bacteria (1C).
(b) We suggest that post-empiric antibiotic therapy of relapsing peritonitis be guided by in vitro susceptibility results, choosing an antibiotic other than cefazolin (2B).
15.3 We suggest intraluminal instillation of a fibrinolytic agent be considered after diagnosis of a first peritonitis relapse that is not explained by extraluminal pathology such as a tunnel infection or intra-abdominal abscess (2C).
15.4 We recommend removal of the PD catheter as soon as peritonitis is controlled by antibiotic therapy in the setting of relapsing peritonitis associated with a persistent or recurrent tunnel infection, or of a second peritonitis relapse (1C).
RATIONALE
Guideline 15.1: Relapsing peritonitis follows approximately 10% – 20% of primary peritonitis episodes (4,159,234). Repeated bouts of peritonitis can lead to loss of peritoneal membrane function, causing reduced PD efficacy over time and eventual PD technique failure. Findings from the IPPR suggest that, relative to sporadic peritonitis, relapsing infections are associated with a risk of incomplete functional recovery that is increased by a factor of 3 and a risk of permanent PD technique failure that is increased by a factor of 2.5 (32).
The definition of relapsing peritonitis as given is based on consensus in the adult and pediatric PD community (7). The 4-week time window is believed to cover most cases of endogenous reinfection with incompletely eradicated bacteria.
Because of the important therapeutic implications, diagnosis of a relapse should not rely solely on genus and species, but also on the antibiotic susceptibilities of the cultured organism. In sophisticated laboratory settings, strain identity can be confirmed by DNA genotype analysis (235).
The relapse definition has also been extended to culture-negative infections when 2 such infections occur in succession, the second occurring within 4 weeks of completion of therapy for the prior episode. Culture-negative relapses have been found to be preceded by a delayed treatment response at the time of initial infection (32). It is possible that infections arising from loci poorly accessible to antibiotics (such as the catheter tunnel, fibrin clots, or biofilm on the catheter surface) may predispose to a poor initial treatment response and an increased likelihood of relapse of the culture-negative episode despite a low yield of organisms from bacterial culture. Hence, catheter exchange should be considered with a low threshold in relapsing culture-negative peritonitis.
Guideline 15.2(a): Because the causative organism is not known at the time of clinical presentation of a repeat peritonitis episode within the relapse time window, it appears reasonable to re-start empiric treatment with the center-specific protocol until the causative bacteria are known. However, because the cause of relapsing peritonitis might be the same bacteria that caused the first episode of peritonitis, it is prudent to empirically start an antibiotic that would treat the first bacteria identified based on the previously determined antibiotic susceptibilities.
Guideline 15.2(b): In the IPPR study, an elevated relapse rate (23%) was noted when, based on culture and in vitro susceptibility results, patients were switched to monotherapy with a first-generation cephalosporin rather than to other final monotherapies or combination therapies (32). It is possible that bacteria responsible for peritonitis recurrence have different virulence factors—including formation of biofilm—that are less responsive to first-generation cephalosporins than to glycopeptides or combinations of antibiotics (32). Although that observation in a nonrandomized study was felt not to provide sufficient evidence to generally advise against the use of a first-generation cephalosporin in PD-associated peritonitis, we do not recommend repeated use of such agents in relapsing peritonitis. Also, a recent study in adults by Szeto et al. suggested that therapy of relapsing or repeat CNS peritonitis be 3 weeks in duration (197). In their retrospective evaluation of 232 peritonitis episodes, they found that, compared with the conventional 2-week treatment course, 3 weeks of antibiotic therapy was associated with a significantly higher complete cure rate.
Guideline 15.3: Slime-forming bacteria are believed to survive antibiotic therapy in a biofilm matrix or in fibrinous adhesions on the catheter surface. Fibrinolytic activity induced by urokinase may expose sequestered bacteria and render them susceptible to antibiotic activity. Hence, the concept of intraluminal catheter decontamination by local instillation of fibrinolytic agents sounds appealing.
Several controlled and uncontrolled studies with widely varying inclusion criteria and procedural protocols have assessed the efficacy of fibrinolytic agents in resolving resistant and preventing recurrent infections (236–243). A placebo-controlled trial showed no significant effect of urokinase when administered intraperitoneally (30 000 IU/L) as adjuvant therapy in antibiotic-resistant bacterial peritonitis (242). But although intraluminal urokinase administration showed no effect on the clinical course and relapse risk when applied to patients with a first peritonitis episode in that placebo-controlled trial, studies using high-dose intraluminal fibrinolytic agents selectively in patients with resistant or relapsing peritonitis showed more promising results (241). Also, Klaus and colleagues (238) used intraluminal high-dose urokinase (5000 IU/mL) and antibiotic instillation in 9 children with relapsing peritonitis. No second relapse occurred in the treated patients. In contrast, 75% second relapses occurred in an untreated historical control group. In a double-blind placebo-controlled study in patients with resistant or relapsing peritonitis, resolution of peritonitis occurred within 4 days of intraluminal urokinase instillation (1000 IU/mL), and there was no recurrence with the same organism for 6 months in 8 of 12 patients, an effect significantly better than that achieved with placebo (239). Likewise, intraluminal administration of high-dose recombinant tissue plasminogen activator (1 mg/mL) was found to be efficacious in anecdotal reports of patients with relapsing peritonitis (244,245).
Guideline 15.4: Catheter exchange has been shown to be superior to urokinase in lowering treatment failure rates in relapsing or persistent peritonitis (237,243). Not surprisingly, that finding is especially true with respect to relapsing peritonitis related to a TI or intra-abdominal abscess, which always should be carefully ruled out in patients with relapsing peritonitis. If a second relapse occurs secondary to any bacteria or after a culture-negative episode and if no other pathology is identified, these circumstances are considered to be an indication for catheter removal (see guideline 17).
LIMITATIONS
A limited number of children have received fibrinolytic therapy as part of treatment for relapsing peritonitis.
RESEARCH RECOMMENDATIONS
The definition of relapsing peritonitis should be corroborated by systematic analyses of the distribution of time intervals between repeated episodes caused by the same organism.
The specificity of diagnosing relapsing peritonitis according to resistograms should be evaluated by DNA genotyping studies.
A randomized clinical trial evaluating the usefulness of intraluminal recombinant tissue plasminogen activator instillation in relapsing peritonitis should be performed.
GUIDELINE 16 – ADJUNCTIVE THERAPY
16.1 Reduce the peritoneal fill volume during the initial 24 – 48 hours of therapy in patients with significant abdominal discomfort (not graded).
16.2 We suggest the intraperitoneal administration of 500 – 1000 IU/L heparin until complete resolution of dialysate cloudiness (2B).
16.3 We suggest that the provision of intravenous immunoglobulin G be considered in selected patients with frequent or refractory peritonitis episodes or in infants with documented hypogammaglobulinemia and peritonitis or sepsis (2D).
RATIONALE
Guideline 16.1: Abdominal pain is frequently noted in children who develop peritonitis. Early in the course of treatment, the pain may be worsened by the presence of the routine exchange volume. Accordingly, the peritoneal volume can be slightly (<25%) lowered during the initial 24 – 48 hours of therapy until clinical symptoms improve. In this setting, no alteration in the intraperitoneal antibiotic concentration is necessary because the very high local antibiotic levels are well above the MIC of susceptible organisms. The exchange volume should subsequently be increased to the normal prescription to prevent a prolonged period of underdialysis (3). Pain not improved by alteration in exchange volumes and antibiotic therapy, or complicated by emesis and volume depletion, may mandate hospitalization. For recommendations pertaining to the alteration of exchange frequency, see guideline 20.
Guideline 16.2: Although the efficacy of intraperitoneal heparin has not been formally proved, its inhibitory effect on fibrin clot formation is believed to contribute to catheter patency in cases of severe peritonitis with massive protein exudation. Heparin also has effects beyond anticoagulation, having antiangiogenic and anti-inflammatory properties (246). However, the rationale for routine intraperitoneal heparin use in PD peritonitis is not strong because of limited clinical evidence.
Hypercoagulability and hypofibrinolysis were shown during CAPD peritonitis in a small pediatric patient group. Plasminogen activator inhibitor type 1 antigen and thrombin–antithrombin III complexes were increased, and D-dimer and plasmin-α2–antiplasmin complexes were decreased in 7 children with peritonitis (247). In a larger adult study, Nadig et al. looked at the major coagulant and fibrinolytic proteins in a total of 194 dialysate samples (approximately 60 being from patients with peritonitis). Those authors demonstrated that, in nearly all samples (with or without peritonitis), a parallel increase occurred in both the procoagulant and fibrinolytic pathways, suggesting that heparin was not required in those patients even during peritonitis (248). Nonetheless, in 15 samples from 3 patients, that relationship did not hold true, and in those samples (11 of the 15 being from peritonitis episodes), the D-dimer levels were very low in the PD effluent, suggesting a block in fibrinolysis and a requirement for heparin in this subset of patients (248). Accordingly, patients with cloudy effluent may benefit from the addition of low-dose heparin (500 – 1000 U/L) into the dialysate, because the heparin can help to prevent occlusion of the catheter by fibrin, which is often present as a result of the inflammatory process (247).
Guideline 16.3: The high peritonitis rate in a subgroup of patients on PD may be a result of alterations in peritoneal defense mechanisms—that is, opsonization, phagocytosis, and bacterial killing (249). An inverse relationship has been reported between the frequency of peritonitis and opsonic activity or the immunoglobulin G (IgG) concentration of dialysate, or both (250–252). Decreased macrophage bactericidal activity is found in patients with a high incidence of peritonitis. A disturbance in the release of lymphokines and monokines in some PD patients may also reduce the ability of peritoneal macrophages to kill bacteria (249).
Hypogammaglobulinemia, hypocomplementemia, and a decreased chemotactic response of peripheral blood neutrophils have been detected in pediatric patients undergoing CAPD (253–255). Given those defects, it may be possible to lower the frequency of peritonitis in some patients by providing supplemental IgG. This issue has been evaluated in several nonrandomized clinical trials.
Keane et al. found evidence of increased opsonizing activity and a significant decline in the peritonitis rate associated with the daily intraperitoneal infusion of 250 mg IgG in individuals with low pre-study dialysate IgG levels (250,256). Lamperi and Carozzi detected a significant reduction in the peritonitis rate from 1 episode in 6.2 patient–months to 1 episode in 21.6 patient–months associated with a rise in peritoneal effluent IgG levels achieved by infusing 12 g IgG intraperitoneally every 3 weeks (257). Those studies were conducted during the early years of PD, when peritonitis rates were exceptionally high. In a more recent study, Dursun et al. (258) found similar results characterized by a positive effect of low-dose intraperitoneal IgG (0.5 g in each exchange bag, four times daily for 7 days) in conjunction with antibiotics for the treatment of refractory or relapsing peritonitis. In addition, in the same study, a significant reduction in the long-term peritonitis rate was observed. In a small randomized adult study, dialysate leukocyte count decreased more rapidly in patients who were given IgG intraperitoneally in conjunction with antibiotics (259). Intraperitoneal infusion of a single dose of IgG (100 mg/kg) has also resulted in a significant increase in chemotaxis in a small pediatric population on chronic PD (260). Finally, positive correlations have been found between low serum IgG levels and the peritonitis rate, malnutrition, and duration of PD in pediatric CAPD patients (260). However, other studies have reported no relationship between dialysate IgG concentration and peritonitis incidence (253,254,261).
Given that infants receiving chronic PD with and without peritonitis can lose substantial amounts of gamma globulin across the peritoneum, they may benefit from IgG therapy, especially if they have low measured serum IgG levels or if they appear septic (262,263).
It is therefore the opinion of the workgroup that the use of intraperitoneal or intravenous IgG as an adjunctive agent (where available) may be a reasonable option in selected individuals—that is, patients with frequent or refractory peritonitis episodes, or infants with documented hypogammaglobulinemia and clinical evidence of peritonitis or sepsis.
LIMITATIONS
No well-designed placebo-controlled randomized trial has evaluated the effect of intraperitoneal IgG on the resolution of resistant peritonitis episodes in critically ill infants or children, or on the frequency of subsequent infections. No dosing recommendations have been developed for the intraperitoneal administration of IgG.
RESEARCH RECOMMENDATIONS
Determination of serum and dialysate IgG levels before and during peritonitis episodes is needed.
A placebo-controlled trial evaluating the effects of varying doses and durations of intraperitoneal IgG in the setting of sepsis or resistant peritonitis episodes is needed.
Evaluations of the clinical impact of regular intravenous IgG infusions on the rate of peritonitis in infants with documented hypogammaglobulinemia on PD are needed.
GUIDELINE 17 – CATHETER REMOVAL AND REPLACEMENT
17.1 We recommend removal of the peritoneal catheter for refractory bacterial peritonitis (1C).
17.2 We recommend removal of the peritoneal catheter when a diagnosis of fungal peritonitis is established (1B).
17.3 We recommend catheter removal in patients with an exit-site or tunnel infection in conjunction with peritonitis with the same bacteria (particularly S. aureus and P. aeruginosa), except CNS (1C).
17.4 We suggest simultaneous catheter removal and replacement for a refractory exit-site or tunnel infection (2C).
17.5 We suggest simultaneous removal and replacement of the peritoneal catheter after clearing of the peritoneal effluent (white blood cells < 100/mm3) in repeated relapsing bacterial peritonitis (2C).
17.6 We suggest a minimum period of 2 – 3 weeks between catheter removal and insertion of a new catheter for fungal, enteric, and refractory bacterial peritonitis (2C).
RATIONALE
Guideline 17.1: Catheter removal is indicated to prevent morbidity and mortality from peritonitis and to protect the peritoneal membrane for future use. It is well recognized that prolonged attempts to treat refractory peritonitis and to “save the catheter” must be avoided to prevent a poor patient outcome (57,143,264). However, no properly conducted RCTs appear to have addressed definitive indications for, and timing of, catheter removal. In addition, the available evidence fails to indicate the optimal timing for insertion of a new PD catheter to replace a catheter that has been removed because of peritonitis (243).
As demonstrated by the IPPR, treatment of most cases of peritonitis will result in marked clinical improvement within several days. Bacterial peritonitis that fails to resolve after 5 days of appropriate antibiotic treatment in PD patients (so-called refractory peritonitis, see Appendix A) is unlikely to respond to continued medical management and often responds to removal of the catheter. Catheter removal in this situation is recommended as a means of protecting the peritoneal membrane for future use (265,266). The same recommendation holds true in the setting of refractory culture-negative peritonitis (57).
Guideline 17.2: Most clinicians agree that prompt catheter removal after a diagnosis of FP, in combination with antifungal therapy for at least 2 weeks, is essential to successfully eradicate FP. In fact, some have argued that catheter removal may be the only therapy needed.
Evidence from the adult PD population also suggests that prompt catheter removal is associated with a lesser risk of death (7,124,128,131,142,143,232,264). Thus, the ISPD adult guidelines published in 2010 recommend that the catheter should be removed immediately after fungi are identified by either microscopy or culture (7). A Dutch study suggested that early—but not immediate—catheter removal in children is indicated, because peritoneal lavage with antimycotics has been hypothesized to minimize peritoneal damage. However, that experience has not been universal, and early removal in children has been associated with preservation of PD capacity (130,267). In those rare patients in whom the catheter is not initially removed for reasons that include difficulty achieving vascular access for HD, the catheter should be removed immediately if improvement does not occur within 3 days of treatment initiation. Notably, Miles et al. (123) found that the chance of returning to PD was no different in patients who underwent catheter removal early (<5 days) than in those who underwent catheter removal late (>5 days) as treatment for FP.
Guideline 17.3: If peritonitis attributable to the same bacteria infecting the exit site or tunnel develops, particularly if the infection is caused by S. aureus or P. aeruginosa, catheter removal should be strongly considered, because the development of peritonitis indicates that infection has developed along the length of the catheter, a situation extremely difficult to treat successfully with antimicrobial agents alone (78,221,268–272). In a large series of Pseudomonas peritonitis episodes, the presence of an ESI secondary to Pseudomonas was demonstrated to be a predictor of poor therapeutic response of the peritonitis to antibiotics (221).
Although catheter removal should be promptly considered when both an ESI or TI and peritonitis occur secondary to S. aureus and P. aeruginosa, the same approach does not need to be taken with a catheter-related infection attributable to CNS. A CNS peritonitis is generally a mild form of infection, characteristically readily responsive to antibiotic treatment. In contrast, relapsing CNS peritonitis suggests colonization of the intra-abdominal portion of the catheter with biofilm, a condition best treated by replacing the catheter (237).
Guideline 17.4: Simultaneous removal and replacement of the catheter is an acceptable and safe procedure in refractory bacterial ESIs or TIs (including infections attributable to Pseudomonas species), because timely replacement of the catheter can lower the risk of peritonitis (270,273,274). In general, the catheter should be removed if the infection does not respond or if it progresses after 2 – 3 weeks of antibiotic therapy.
If P. aeruginosa ESI or TI persists or progresses despite prolonged and appropriate dual antibiotic treatment, simultaneous removal and replacement of the PD catheter under antibiotic coverage is the treatment of choice. In a series comprising 37 adult patients with refractory P. aeruginosa ESI, simultaneous catheter removal and insertion of a new PD catheter without interruption of PD was successful in all patients. Late recurrence of P. aeruginosa ESI occurred in only 8% of the patients within the first year after the procedure (270). Although cuff-shaving for refractory infection may be tried before catheter removal (275), peritonitis is a distinct risk if the tunnel is infected (see guideline 19).
Guideline 17.5: Simultaneous removal and replacement of the catheter under antibiotic coverage has also been performed successfully in the setting of relapsing bacterial peritonitis once the effluent clears with antibiotic therapy (276). The technique may be particularly beneficial with relapsing peritonitis secondary to CNS or S. aureus, because those infections may be a result of sequestration of bacteria in biofilm surrounding the intra-abdominal portion of the catheter (277). When removal and replacement is performed, the infected catheter can be removed and a new catheter placed simultaneously in the opposite lower quadrant—an approach that eliminates the need for central venous access and a prolonged period on HD (270,278). Some patients, especially those using a cycler for PD, can avoid HD altogether by dialyzing only in the supine position for several days post catheter reimplantation to avoid or minimize the increase in intraperitoneal pressure and the risk for leaks and hernias, with the subsequent addition of a daytime exchange.
The removal and replacement procedure has been performed successfully in pediatric and adult patient populations (38,242,267,274,276,279–281). In data collected by the Italian pediatric PD registry, simultaneous removal and replacement procedures were, in fact, performed in 76% of catheter removals (38). In an adult study, simultaneous catheter placement and removal was successful in 30 of 36 patients (274). In a small randomized study, a recurrence rate of only 5% was associated with the use of this approach to treat recurrent CNS and culture-negative peritonitis (237). However, this treatment regimen should not be attempted in patients with FP or enteric peritonitis, with active or refractory peritonitis, or with intra-abdominal adhesions (273).
Regardless of the indication for simultaneous catheter removal and replacement, antibiotic therapy should be continued for 1 – 2 weeks after the surgical procedure (282). Most clinicians have chosen to insert the new catheter before removing the old catheter, but the alternative approach has been taken with similar results (267,270,282).
Guideline 17.6: The optimal period between catheter removal for infection and insertion of a new catheter is not known (58). The empiric recommendation for a period of at least 2 – 3 weeks in both the present guidelines and the ISPD adult guidelines takes into consideration the absence of data and the goal of long-term peritoneal membrane function in children (7). Many clinicians recommend catheter removal and delay of catheter replacement for at least 6 weeks in patients treated for Mycobacterium tuberculosis peritonitis, but some patients have been successfully treated and have continued PD without catheter removal (7). In all cases, recommendations regarding the duration of antibiotic therapy and the timing of catheter replacement may require modification based on clinical response in the particular patient.
Table 8 summarizes the information presented in this guideline section.
TABLE 8.
Indications for Catheter Removal for Peritoneal Dialysis (PD)–Associated Infections
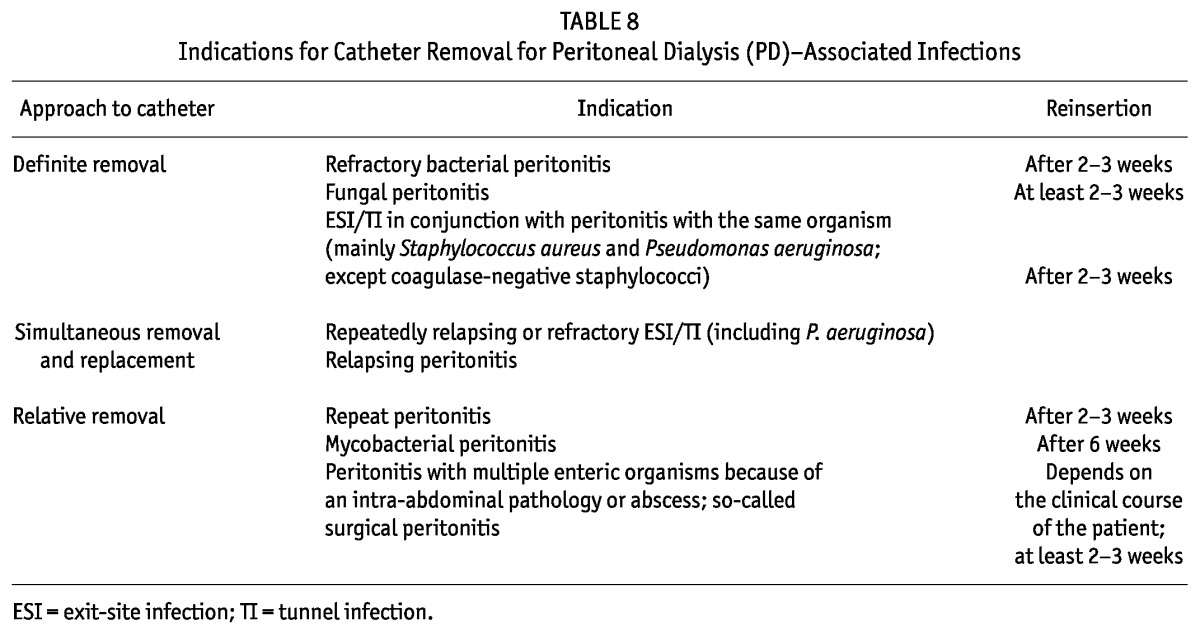
LIMITATIONS
No RCTs in adults or children have provided definitive indications for catheter removal because of peritonitis or for optimal timing of the procedure, including appropriate timing for insertion of a new catheter.
No data in the pediatric or adult literature are available to support an evidence-based recommendation for the length of antibiotic treatment after catheter removal.
RESEARCH RECOMMENDATIONS
Registry data should explore the time interval between a diagnosis of peritonitis and PD catheter removal and the relationship between that interval and subsequent peritoneal membrane function.
GUIDELINE 18 – DIAGNOSIS OF CATHETER-RELATED INFECTION
18.1 We suggest that an objective scoring system be used to monitor the status of the PD catheter exit site (2B).
18.2 We suggest that a diagnosis of a catheter exit-site infection be made in the presence of pericatheter swelling, redness, and tenderness (exit-site score of 2 or greater in the presence of a pathogenic organism and 4 or greater regardless of culture results) (2B).
18.3 We suggest that a tunnel infection be defined by the presence of redness, edema, and tenderness along the subcutaneous portion of the catheter, with or without purulent drainage from the exit site (exit-site score of 6 or greater) (2B).
RATIONALE
Guideline 18.1: Subjective assessment of the exit site may vary widely, and so it is imperative that objective criteria be used to diagnose an ESI. Work by Twardowski has led to a better classification of exit-site morphology and a more uniform approach to the diagnosis of infection (42). A scoring system developed by pediatric nephrologists (Table 9) has also proved useful in monitoring the exit-site status on a regular and recurrent basis, and most importantly, in complementing—but not substituting for—clinical judgment (159). It should therefore be emphasized that the subsequent guideline statements 18.2 and 18.3 are meant to provide guidance, but may not account for all possible scenarios.
TABLE 9.
Exit-Site Scoring Systema
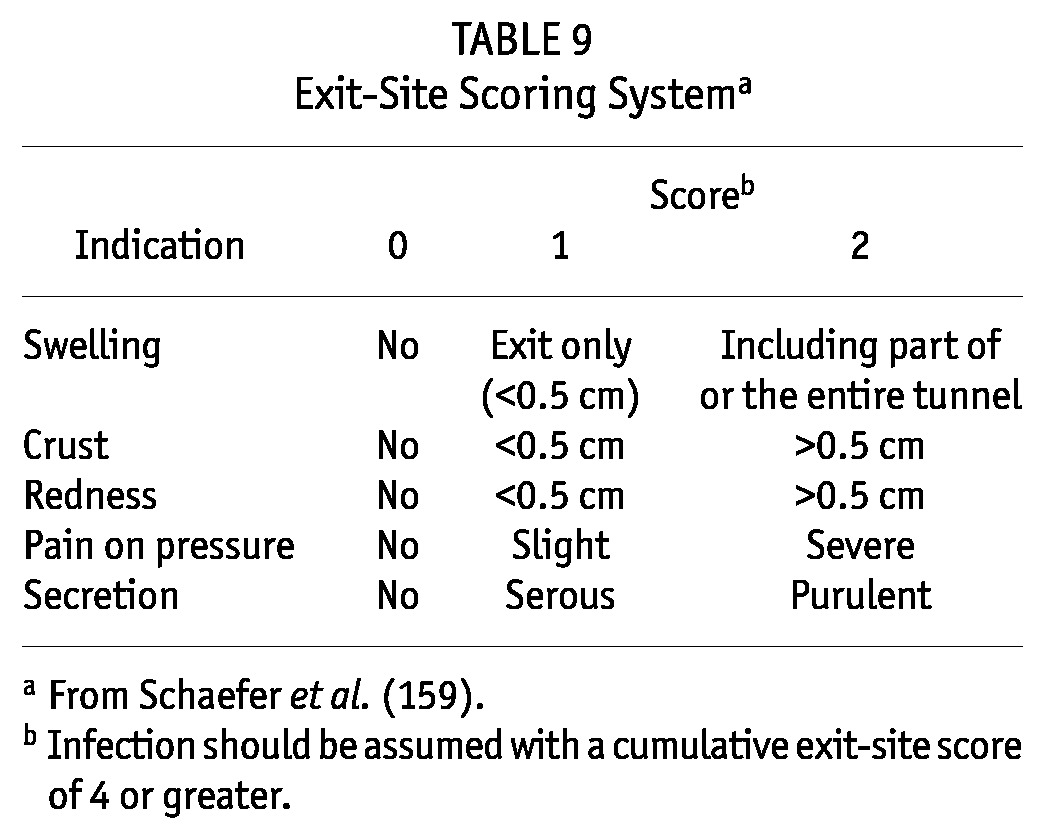
The importance of closely monitoring the status of the catheter exit site and of promptly diagnosing infection is emphasized by the finding that, compared with children not having an ESI or TI, those with such an infection have twice the risk of developing peritonitis or requiring peritoneal catheter revision, and the risk of hospitalization for catheter-related complications that is increased by a factor of 3 (34). In the IPPR experience (4), an exit-site score greater than 2 in patients with gram-positive peritonitis was associated with an increased likelihood of empiric antibiotic treatment response failure 3 days after treatment initiation (odds ratio: 5.46; p < 0.05).
Guideline 18.2: S. aureus accounts for most catheter ESIs, followed by Pseudomonas species, Enterococcus species, E. coli, Klebsiella species, and other gram-negative species. S. epidermidis is frequently cultured, but it is not usually causative of ESI. A positive culture is not required for the diagnosis of an ESI (which can be diagnosed on clinical appearance alone), but a positive culture from an exit site that is not inflamed often indicates colonization and not infection—thus, the requirement of a score of 2 or greater in that setting to diagnosis an ESI.
The decision about whether to initiate therapy or to follow carefully should be based on the combination of clinical judgment and repeated assessment (58). At the same time, in the latest IPPR report, the same micro-organism (most commonly Pseudomonas species) was retrieved from the peritoneal fluid and exit site in 12% of children with gram-negative peritonitis, and fewer than half of those patients showed symptoms of a concurrent ESI (33).
Guideline 18.3: Exit-site infections attributable to S. aureus and P. aeruginosa are often associated with a concomitant TI and subsequent peritonitis related to the catheter infection; in those cases, treatment of the catheter-related infection should be particularly aggressive, because the associated peritonitis episode can be extremely resistant to treatment (4,33,57,158). These particular organisms may also form a biofilm on the catheter, thereby precluding successful antibiotic management without catheter removal (277).
Although most catheter TIs can be diagnosed by clinical exam alone, ultrasonographic examination of the catheter tunnel may be helpful in detecting a clinically occult TI, in delineating the extent of the infection, and in evaluating response to antibiotic therapy (142,283–285).
LIMITATIONS
Evaluation of the exit site, even by means of the pediatric scoring system, remains dependent on the observer and has not been validated by clinical trials.
The use of ultrasonography to diagnose and follow catheter-related infections has not been studied in pediatric patients.
RESEARCH RECOMMENDATIONS
An evaluation of the diagnosis of ESI or TI based on the exit-site scoring system, with particular reference to outcomes by causative organism, is needed.
GUIDELINE 19 – TREATMENT OF CATHETER-RELATED INFECTION
19.1 We suggest that oral antibiotic therapy of uncomplicated catheter exit-site infections (exit-site score of 4 or greater, or 2 or greater with pathogenic organism on culture, and no tunnel involvement) be initiated upon receipt of culture results and susceptibilities, and that treatment be continued for a minimum of 2 weeks and for at least 7 days after complete resolution of the infection. Treatment for at least 3 weeks is recommended for exit-site infections caused by S. aureus or P. aeruginosa (2B).
19.2 We suggest that antibiotic therapy for catheter tunnel infections be initiated after culture and susceptibility results have been obtained unless signs of severe infection or a history of S. aureus or P. aeruginosa is present, for which initiation of empiric therapy should be considered. The route of antibiotic administration can be oral, intraperitoneal, or intravenous unless MRSA is the causative agent, in which case intraperitoneal or intravenous glycopeptide therapy is indicated. Treatment duration should be 2 – 4 weeks (2B).
RATIONALE
Guideline 19.1: Successful treatment of catheter ESIs is important because failure of therapy may result in catheter removal or peritonitis. In most cases, oral antibiotic therapy will prove effective (see Table 10).
TABLE 10.
Oral Antibiotics Used in Exit-Site and Tunnel Infectiona
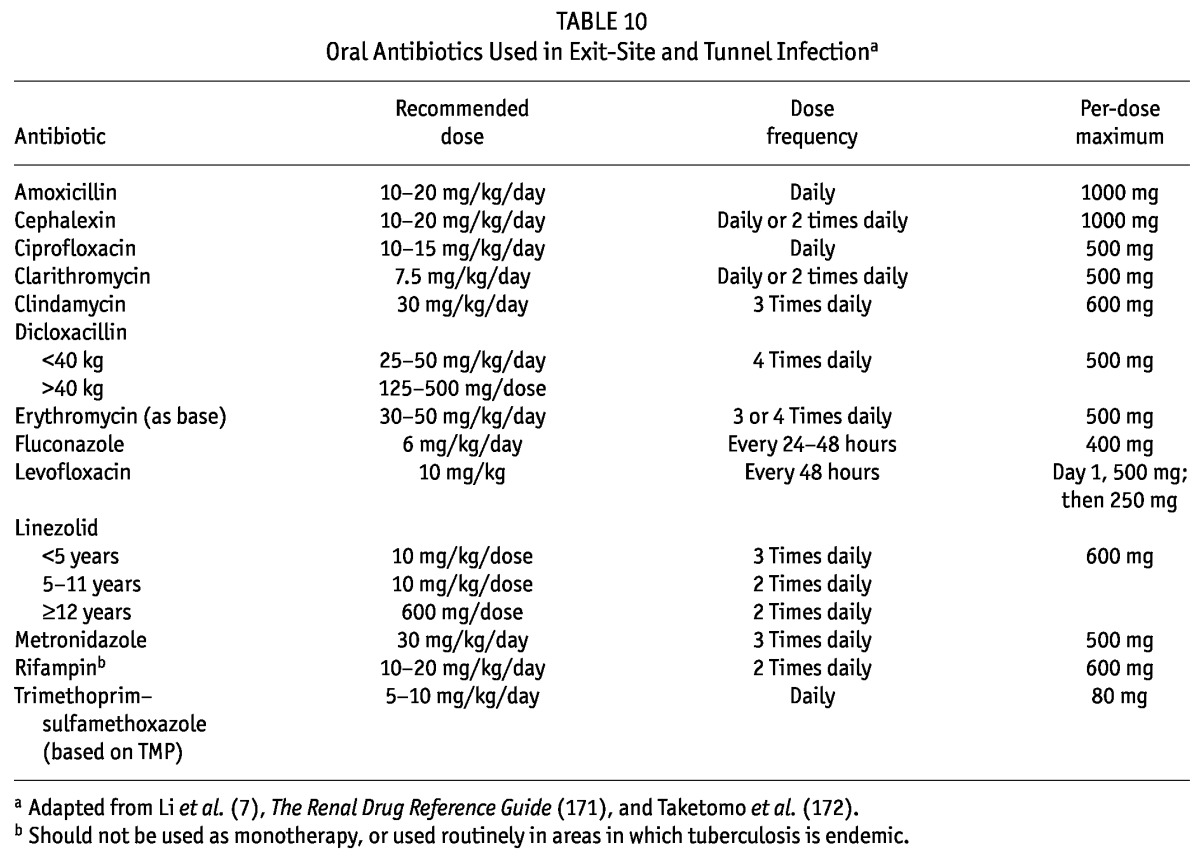
Infections caused by gram-positive bacteria should be treated with a first-generation cephalosporin or a penicillinase-resistant penicillin, with the addition of rifampin in S. aureus infections that fail to improve or resolve promptly. Because of concerns of emerging bacterial resistance, glycopeptides (for example, vancomycin or teicoplanin) should be avoided for the routine treatment of ESIs secondary to gram-positive bacteria; those agents should be reserved for MRSA infections.
Gram-negative infections secondary to P. aeruginosa should be treated with oral ciprofloxacin because of the efficacy of that agent against an organism that frequently results in the development of peritonitis. As noted earlier (see guideline 12), fluoroquinolones have been used extensively in pediatrics, and to date, experimental data have not substantiated the cartilage damage observed in beagle puppies (211–214). The use of ciprofloxacin monotherapy can be complicated by the development of antibiotic resistance.
P. aeruginosa ESIs are particularly difficult to treat; if resolution of the infection is slow or if there is recurrence, a second anti-Pseudomonas drug such as cefepime, piperacillin, or meropenem should be added (57). In patients in whom the exit-site culture and gram-stain results are negative, or in a patient whose infection is severe and whose culture and gram-stain results are not yet available, empiric therapy with either a first-generation cephalosporin or oral ciprofloxacin should be initiated. Close monitoring of this patient group is essential, with modification of the antibiotic regimen contingent on the early response to therapy. In patients receiving prophylactic therapy (by application of an antibiotic ointment or cream at the exit site), slower growth of the causative organism is possible, and the potential for resistance to any prophylactic antibiotic used should be considered in the choice of empiric therapy.
Screening for S. aureus nasal (or even rectal) carriage may be helpful in this situation to detect the possible causative organism. When quinolones are used, attention should be paid to the concomitant administration of sevelamer, multivalent cations (calcium), oral iron, zinc preparations, sucralfate, magnesium–aluminum antacids, or milk, because chelation interactions may occur that can reduce quinolone absorption. Quinolones should therefore be given at least 2 hours before the administration of the foregoing substances.
Adjunctive therapy should include the use of daily or twice-daily dressing changes as long as significant discharge from the sinus tract is present. Exuberant granulation tissue (“proud flesh”) should be cautiously removed by cauterization with silver nitrate. The catheter should be immobilized and protected from trauma.
Sonographic examination may help to evaluate the extent of infection along the tunnel and the likely efficacy of antibiotic therapy (142,284,286). Involvement of the external cuff is associated with poor clinical outcomes (287). Shaving of the external cuff, followed by re-tunnelization, as an alternative to catheter removal for treatment of a persistent ESI has been proposed if the inner cuff is not involved. Only two single-center pediatric experiences on this approach have been reported (288–290). Antibiotic treatment should be continued after cuff shaving.
Treatment should continue for a minimum of 2 weeks and for at least 7 days after complete clinical resolution of the infection—that is, until the exit-site appears entirely normal. Treatment for at least 3 weeks is recommended for ESIs caused by S. aureus or P. aeruginosa. Data from a survey conducted by the Japanese Study Group of Pediatric Peritoneal Dialysis among 130 patients less than 15 years of age showed a relapse rate of 15%; the relapse rate was 40% among infection episodes caused by MRSA (66). Close follow-up of the exit-site and tunnel conditions is therefore necessary after completion of therapy. Failure to achieve clinical improvement in 2 weeks or complete resolution of the infection after 4 weeks, or development of peritonitis secondary to the same bacteria (particularly S. aureus and P. aeruginosa), are all indications for catheter removal (see guideline 17).
Guideline 19.2: In the case of a catheter TI, the bacteriology is the same as that with an ESI, with particular concern when the causative organism is S. aureus or P. aeruginosa; however, the severity of the infection is greater and it is frequently complicated by the development of a peritoneal infection. Empiric therapy is therefore often indicated, with modification subsequent to receipt of culture and susceptibility results. Sonographic examination may help in the evaluation of the extent of infection along the tunnel and the likely efficacy of antibiotic therapy (142,284–286). Catheter removal and replacement is often required when continued medical management appears futile.
LIMITATIONS
No prospective RCTs on the treatment of catheter ESIs and TIs in pediatric PD patients are available; thus, most recommendations in this section are based on expert consensus or the results of retrospective observational studies.
RESEARCH RECOMMENDATIONS
An evaluation of the outcome of ESI or TI treatment based on the use of the present recommendations, with particular reference to organism-specific outcomes, the occurrence of subsequent peritonitis episodes, and the need for catheter removal or replacement is needed.
GUIDELINE 20 – MODIFICATION OF APD
In patients who receive nocturnal APD with short dwell times as routine therapy:
20.1 We suggest prolongation of the dialysate dwell time to 3 – 6 hours, until the peritoneal effluent clears (2C).
20.2 We suggest use of an automated PD prescription characterized by prolongation of the dialysate dwell time throughout the treatment of peritonitis if the intraperitoneal antibiotic is being given by intermittent dosing (2C).
RATIONALE
Guideline 20.1: Many children who receive APD characteristically receive dialysis exchanges with short dwell times (≤2 hours) to enhance solute and fluid removal. However, the cellular components and opsonins of local host defense mechanisms are depleted by frequent exchanges (291–294). Furthermore, the inherent cytotoxicity of fresh conventional dialysis solution (because of low pH, hyperosmolality, and high glucose and lactate content) compromises the function of peritoneal macrophages, leukocytes, and mesothelial cells (294–297). A recent study comparing the new biocompatible bicarbonate/lactate–based solution Physioneal (Baxter Healthcare Corporation) with the standard low-pH lactate-buffered PD solution Dianeal (Baxter Healthcare Corporation) revealed a better state of the peritoneal macrophages with the former solution (298). Therefore, prolongation of the dwell time, such that at least partial normalization of the peritoneal “milieu” and, ideally, improved bacterial killing occurs, is a reasonable alteration to the dialysis prescription to aid in early peritonitis treatment. When the effluent clears, which typically occurs within the initial 48 – 72 hours of treatment, the patient may return to a more standard APD regimen. Prolongation of the dwell time may not be necessary or beneficial for patients with mild symptoms and clear peritoneal effluent or for those using a biocompatible dialysis solution; it may not be feasible for patients who require frequent exchanges because of higher ultrafiltration needs.
Guideline 20.2: In intermittent dosing, the antibiotic-containing dialysis solution must be allowed to dwell for at least 6 hours to allow for adequate absorption of the antibiotic into the systemic circulation, which permits its subsequent re-entry into the peritoneal cavity during ensuing fresh dialysis solution exchanges. Most antibiotics have significantly enhanced absorption from the peritoneum during peritonitis (for example, vancomycin is normally about 50% absorbed, but closer to 90% during peritonitis), and adequate levels are likely achieved in the systemic circulation; however, the rapid exchanges that often characterize APD in children may be associated with an inadequate time to achieve therapeutic intraperitoneal antibiotic levels by the re-entry mechanism (177). As noted in guideline 9, intermittent dosing of vancomycin or teicoplanin appears to be as efficacious as continuous dosing, but a pediatric study that evaluated the disposition of intraperitoneal vancomycin in children suggested that enhanced total-body vancomycin elimination in children (relative to adults), coupled with slow peritoneal transfer, might be associated with inadequate time to achieve therapeutic intraperitoneal levels by the re-entry mechanism (159,177). That finding holds particularly true in patients receiving a prescription characterized by short dwell times, a situation that should prompt reevaluation of the recommendations for intermittent vancomycin therapy, with consideration of longer dwell times or higher intraperitoneal concentrations. Accordingly, dwell times may need to be prolonged in those situations for the entire duration of peritonitis treatment. Logically, patients receiving CAPD do not require any change in their exchange frequency.
LIMITATIONS
No studies have assessed the optimal dialysate dwell times associated with the most favorable outcomes in patients with peritonitis. Similarly, little is known about dialysate dwell times that result in therapeutic intraperitoneal antibiotic levels achieved by the re-entry mechanism.
RESEARCH RECOMMENDATIONS
Prospective trials are needed to compare various dialysate dwell times and their association with the most favorable outcomes in patients treated with intermittent antibiotic therapy.
GUIDELINE 21 – EVALUATION OF PRIMARY RESPONSE
21.1 We suggest that, in addition to visual inspection of the dialysis effluent for cloudiness, an objective standardized measure such as a Disease Severity Score be used to monitor clinical response for at least 72 hours after initiation of appropriate antibiotic therapy (2C).
RATIONALE
Guideline 21.1: Monitoring the clinical response to antibiotic therapy may provide important information to help guide therapy and further evaluation while culture results are awaited, or in the face of culture-negative peritonitis. This monitoring should include daily inspection of the dialysis effluent for a reduction in cloudiness. Improvement in patient symptoms (for example, a decline in the pain and fever) and clearing of effluent cloudiness at 72 hours is, in most cases, evidence of successful therapy. In fact, data from the IPPR, based on more than 500 episodes of peritonitis in pediatric PD patients, demonstrated that 94% of patients showed clinical improvement and 55% experienced complete resolution of symptoms after 72 hours of antibiotic therapy (5).
In some cases, use of objective standardized response criteria can be helpful to avoid unnecessary premature changes of treatment and delayed recognition of an insufficient treatment response. A prospective randomized study of 168 episodes of peritonitis in 152 pediatric PD patients used a Disease Severity Score (DSS) to monitor clinical response. The DSS was defined as the sum of points for pain and fever (Table 11). The DSS score was recorded at diagnosis and after 60 hours of antibiotic treatment (159). The study demonstrated excellent agreement between improvement in the DSS score, defined as a reduction in the DSS to less than 2, and final outcome (159). Those findings have been supported by data from the IPPR, where improvement in the DSS score correlated with an absence of S. aureus, Pseudomonas species, and other gram-negative infections, and with the likelihood of full functional recovery after gram-negative peritonitis (5,33).
TABLE 11.
Disease Severity Scorea
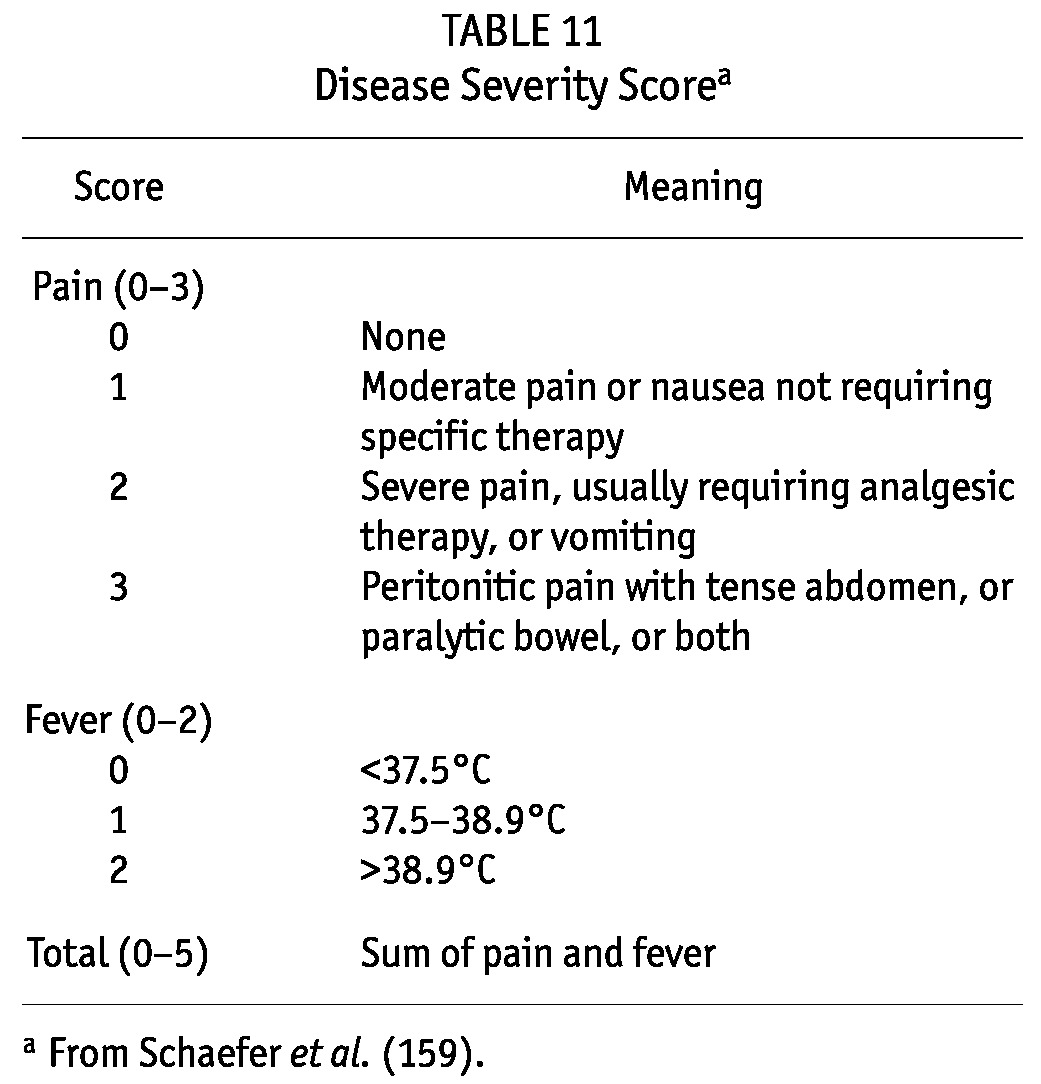
LIMITATIONS
Although meant to provide an objective assessment of disease severity, instruments such as the DSS are not without subjectivity. In the IPPR, the DSS at presentation varied significantly by region (5).
RESEARCH RECOMMENDATIONS
Further evaluation and validation of an objective scoring system may help to eliminate regional variability and improve the reliability of the instrument.
GUIDELINE 22 – FAILURE TO DEMONSTRATE IMPROVEMENT
22.1 We suggest that patients who fail to demonstrate improvement in clinical response within 72 hours of initiation of appropriate antibiotic therapy should undergo a repeat PD effluent cell count, differential, and culture (2B).
22.2 We suggest that the adequacy of empiric therapy be assessed and that evaluation for potential sources of persistent infection be performed in patients who fail to demonstrate improvement within 72 hours of antibiotic initiation (2B).
RATIONALE
Guideline 22.1: Most pediatric patients demonstrate prompt clinical improvement soon after initiation of successful treatment for peritonitis. In one pediatric study, Schaefer et al. found that 74% of all peritonitis episodes were free of any associated clinical symptoms after 60 hours of antibiotic treatment (159). Those findings were confirmed by IPPR data, which showed that nearly all patients demonstrated clinical improvement and that more than half experienced complete resolution of symptoms after 72 hours of antibiotic therapy (5). Data from the IPPR also demonstrated a correlation between initial response to treatment and final outcome (33). Among patients with gram-negative peritonitis, the likelihood of full functional recovery was influenced independently by the initial response to treatment (odds ratio: 5.39; 95% CI: 1.75 to 16.6; p < 0.01) (33). Among patients with culture-negative peritonitis, a significantly higher rate of early treatment response failure (DSS > 2) was noted for relapsing episodes (18%) than for non-relapsing episodes (2.2%, p < 0.005) (32).
Accordingly, it is reasonable to further investigate a patient who has not demonstrated any improvement after 3 days of appropriate therapy. Earlier evaluation may be warranted in patients whose symptoms worsen after initiation of antibiotics. In all cases, the re-evaluation should include a repeat assessment of the peritoneal effluent cell count, gram stain, and effluent culture. In some cases (for example, tuberculosis, Capnocytophaga), special culture techniques may be necessary.
In a prospective trial of PD-associated peritonitis in pediatric patients, a decline in the effluent WBC count by 50% 3 days after initiation of treatment was a helpful diagnostic indicator of treatment response (159). A relative shift from polymorphonuclear to mononuclear cells should also start at this time, but that shift occurs with greater temporal variability than does the absolute decline in the number of WBCs (159). The same study found persistent bacterial growth in 20% of peritonitis episodes 60 hours after treatment initiation. After 7 days of continued therapy, the eradication rate was 95%; eradication by treatment day 3 or 7 did not predict the risk for peritonitis relapse. Incomplete eradication of micro-organisms from the peritoneal cavity after 3 days of antibiotic therapy should therefore not be considered treatment failure.
Guideline 22.2: Ultimately, antibiotic choice should be guided by results of the PD effluent culture and antibiotic susceptibility testing; however, inadequate treatment response, defined by failure to demonstrate clinical improvement after 72 hours of antibiotic therapy, should prompt reassessment of the appropriateness of the antibiotic therapy and evaluation for potential sources of persistent infection. The evaluation should include careful assessment for the presence of a PD catheter TI, which may be made by a combination of clinical evaluation and ultrasonography in most cases (see guideline 18) (286).
As suggested in guideline 11.4, in the setting of treatment-resistant infections with CNS and S. epidermidis, a brief (48- to 72-hour) trial with the addition of oral rifampin therapy may be considered. If the patient is receiving a first-generation cephalosporin and the organism is methicillin-resistant, the cephalosporin should be discontinued, and therapy with a glycopeptide (for example, vancomycin or teicoplanin) or clindamycin should be instituted. Continued treatment failure, especially with S. aureus or Pseudomonas species, may be the result of a concomitant catheter TI (299). In this case, successful eradication may require catheter removal. This indication for catheter removal, and others, are discussed in guideline 17. In patients with treatment resistant peritonitis secondary to anaerobic bacteria or multiple gram-negative organisms, the possibility of intraperitoneal pathology (for example, a ruptured appendix) should be considered, the catheter should be removed, and intravenous therapy should be prescribed (300).
LIMITATIONS
Although prospective studies have demonstrated an association between improvement in DSS, reduction in the PD effluent WBC count, and ultimate outcome in pediatric PD-associated peritonitis, those findings are not without subjectivity and variability, and thus, no absolute thresholds define treatment failure.
RESEARCH RECOMMENDATIONS
Further evaluation and validation of an objective DSS and prospective evaluation of effluent WBC counts in response to antibiotic treatment may improve the reliability of those tools to guide antibiotic therapy.
DISCLOSURES
BAW has acted as an advisor to Baxter Healthcare and has received research support from Baxter Healthcare and from Fresenius Medical Care. MC has acted as an advisor to Baxter Healthcare. FS has received research support from Baxter Healthcare and Fresenius Medical Care. SB, JN, EV, AN, VC, and HKY have no financial conflicts of interest to declare.
APPENDIX A – TERMINOLOGY FOR PERITONITIS
Use of consistent terminology when characterizing peritonitis episodes is imperative, because only in this manner can uniform treatment recommendations be made. In addition, appropriate characterization is necessary for proper determination of peritonitis rates. The common terminology incorporated into both the adult and the pediatric guidelines is outlined in Table A.1.
TABLE A.1.
Terminology for Peritonitis

Relapsing episodes should not be counted as another peritonitis when peritonitis rates are calculated; recurrent and repeat episodes should be counted.
APPENDIX B – REPORTING OF PERITONITIS RATES
Every program should regularly monitor infection rates—at a minimum, annually (301–303).
Programs should carefully monitor all peritoneal dialysis (PD)–related infections, both exit-site infections and peritonitis, including the presumed cause and the organisms cultured, as part of a continuous quality improvement program.
Causative organisms and their antibiotic susceptibilities, and the presumed origin of each episode of infection, must be reviewed in a regular fashion by the PD team, including the nurses and the physicians alike, and if appropriate, the physician assistant or nurse practitioner. In this way, interventions can be implemented if infection rates are rising or are unacceptably high.
Table B.1 sets out an easy method for calculating infection rates. Infection rates for individual organisms should also be calculated and compared with those reported in the literature.
TABLE B.1.
Methods for Reporting Peritoneal Dialysis (PD)–Related Infections (Peritonitis, Exit-Site Infections)a

The calculation for peritonitis episodes per year at risk is done by totaling all the days on PD for the center’s patient population, converting that total to years, and using the converted value as the denominator of the fraction. The number of peritonitis episodes serves as the numerator of the fraction. As an example, assume that a pediatric program has 15 patients, all of whom received PD for 6 months. The 90 months of dialysis (15 patients × 6 months) equates to 2736 days (90 months × 30.4 days/month). The 2736 days are then divided by the 365 days in a typical year to convert the time on dialysis to years. In this case, the result is 7.5. If 4 episodes of peritonitis occurred during that time, the rate is 4 / 7.5—that is, 0.53 episodes per year at risk.
Likewise, calculating the months between episodes involves totaling all the days on PD for the center’s patient population, converting the days to months, and using the resulting value as the numerator of the fraction. The number of peritonitis episodes serves as the denominator of the fraction. Based on the foregoing example, the 2736 days equates to 90 months. Given the 4 episodes of peritonitis that occurred during that time, the rate is 90 / 4—that is, 1 episode every 22.5 patient–months.
APPENDIX C – DRUG DELIVERY AND STABILITY
Vancomycin, aminoglycosides, and cephalosporins can be mixed in the same dialysis solution bag without loss of bioactivity. However, an aminoglycoside should not be added to an exchange that also contains a penicillin because of chemical incompatibility (aminoglycoside and cephalosporin can be added to the same bag). For any antibiotics that are to be admixed, separate syringes must be used for adding them to the bag. Even though vancomycin and ceftazidime are compatible when added to dialysis solutions (1 L or more), they are incompatible if combined in the same syringe or added to an empty dialysate bag for reinfusion into the patient. Such an approach is not recommended.
Antibiotics should be added using sterile technique: povidone iodine should be placed, and alcohol or chlorhexidine should be rubbed on the medication port for 5 minutes before insertion of the needle through the port.
Drug stability is determined by the degradation of the drug in solution, which is influenced by temperature and humidity and should generally be less than 10% for a drug to be considered stable (304). Data suggest that some antibiotics are stable for variable times when added to dextrose-containing dialysis solution. Vancomycin (25 mg/L) is stable for 28 days in dialysis solution stored at room temperature, although high ambient temperatures will reduce the duration of stability. Gentamicin (8 mg/L) is stable for 14 days, but the duration of stability is reduced by admixture with heparin. Cefazolin (500 mg/L) is stable for at least 8 days at room temperature, or for 14 days refrigerated; addition of heparin has no adverse influence. Ceftazidime is less stable: concentrations of 125 mg/L are stable for 4 days at room temperature or 7 days refrigerated, and concentrations of 200 mg/L are stable for 10 days refrigerated. Cefepime is stable in dialysis solution for 14 days if the solution is refrigerated (305).
These data are derived from duration-of-stability studies. However, it is important to recognize that because of significant differences in the constitution of current PD solutions in terms of buffers, osmotic agents, and pH, stability data cannot be extrapolated in all cases from one PD solution to another (306). Icodextrin-containing dialysis solutions are compatible with vancomycin, cefazolin, ampicillin, cloxacillin, ceftazidime, gentamicin, and amphotericin (307). Nonetheless, data on the stability of individual antibiotics in various new PD solutions are limited.
Finally, compatibility is a broader concept that includes drug stability, drug–drug interactions, and drug–container interactions. There is evidence that polyvinylchloride container material adsorbs drugs more readily than does nonpolar polyolefin container material (306).
The most comprehensive collection of drug stability data has been collected by de Vin et al. (306). Tables C.1 and C.2 present a partial list of their data.
TABLE C.1.
Stability of Single Drugs in Peritoneal Dialysis (PD) Solutions in Polyvinylchloride Containersa
TABLE C.2.
Stability of Single Drugs in Peritoneal Dialysis (PD) Solutions in Polyolefin Containersa
Acknowledgments
The authors thank Chelsey Jensen, PharmD BCPS, and Leslie Stach, PharmD BCPS, for their valuable input concerning the antibiotic dosing recommendations and Cynthia Kiel for her outstanding administrative assistance.
REFERENCES
- 1. Auron A, Simon S, Andrews W, Jones L, Johnson S, Musharaf G, et al. Prevention of peritonitis in children receiving peritoneal dialysis. Pediatr Nephrol 2007; 22:578–85 [DOI] [PubMed] [Google Scholar]
- 2. The EMMES Corporation North American Pediatric Renal Trials and Collaborative Studies: NAPRTCS 2008 Annual Report. Renal Transplantation, Dialysis, Chronic Renal Insufficiency. Rockville, MD: The EMMES Corporation; 2008. [Available online at: https://web.emmes.com/study/ped/annlrept/Annual%20Report%20-2008.pdf; accessed April 2012] [Google Scholar]
- 3. Warady BA, Schaefer F, Holloway M, Alexander S, Kandert M, Piraino B, et al. Consensus guidelines for the treatment of peritonitis in pediatric patients receiving peritoneal dialysis. Perit Dial Int 2000; 20:610–24 [PubMed] [Google Scholar]
- 4. Warady BA, Feneberg R, Verrina E, Flynn JT, Müller–Wiefel DE, Besbas N, et al. Peritonitis in children who receive long-term dialysis: a prospective evaluation of therapeutic guidelines. J Am Soc Nephrol 2007; 18:2172–9 [DOI] [PubMed] [Google Scholar]
- 5. Schaefer F, Feneberg R, Aksu N, Donmez O, Sadikoglu B, Alexander SR, et al. Worldwide variation of dialysis-associated peritonitis in children. Kidney Int 2007; 72:1374–9 [DOI] [PubMed] [Google Scholar]
- 6. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9(Suppl 3):S1–155 [DOI] [PubMed] [Google Scholar]
- 7. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis–related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [DOI] [PubMed] [Google Scholar]
- 8. Bernardini J, Price V, Figueiredo A. on behalf of the International Society for Peritoneal Dialysis (ISPD) Nursing Liaison Committee Peritoneal dialysis patient training, 2006. Perit Dial Int 2006; 26:625–32 [PubMed] [Google Scholar]
- 9. Bernardini J, Price V, Figueiredo A, Riemann A, Leung D. International survey of peritoneal dialysis training programs. Perit Dial Int 2006; 26:658–63 [PubMed] [Google Scholar]
- 10. Holloway M, Mujais S, Kandert M, Warady BA. Pediatric peritoneal dialysis training: characteristics and impact on peritonitis rates. Perit Dial Int 2001; 21:401–4 [PubMed] [Google Scholar]
- 11. Gunasekara WD, Ng KH, Chan YH, Aragon E, Foong PP, Lau YW, et al. Specialist pediatric dialysis nursing improves outcomes in children on chronic peritoneal dialysis. Pediatr Nephrol 2010; 25:2141–7 [DOI] [PubMed] [Google Scholar]
- 12. Bordin G, Casati M, Sicolo N, Zuccherato N, Eduati V. Patient education in peritoneal dialysis: an observational study in Italy. J Ren Care 2007; 33:165–71 [DOI] [PubMed] [Google Scholar]
- 13. Hall G, Bogan A, Dreis S, Duffy A, Greene S, Kelley K, et al. New directions in peritoneal dialysis patient training. Nephrol Nurs J 2004; 31:149–54,159–63 [PubMed] [Google Scholar]
- 14. Coles G, Uttley L. Recommendations of the International Society for Peritoneal Dialysis for training requirements of nephrology trainees and nurses. Perit Dial Int 1994; 14:115–16 [PubMed] [Google Scholar]
- 15. Chow KM, Szeto CC, Law MC, Fun Fung JS, Kam–Tao Li P. Influence of peritoneal dialysis training nurses’ experience on peritonitis rates. Clin J Am Soc Nephrol 2007; 2:647–52 [DOI] [PubMed] [Google Scholar]
- 16. Jones L, Aldridge M. Organization and management of a pediatric dialysis program. In: Warady BA, Schaefer FS, Alexander SR, eds. Pediatric Dialysis. New York, NY: Springer; 2011. [Google Scholar]
- 17. Winterbottom A, Conner M, Mooney A, Bekker HL. Evaluating the quality of patient leaflets about renal replacement therapy across UK renal units. Nephrol Dial Transplant 2007; 22:2291–6 [DOI] [PubMed] [Google Scholar]
- 18. Bender FH, Bernardini J, Piraino B. Prevention of infectious complications in peritoneal dialysis: best demonstrated practices. Kidney Int Suppl 2006; (103):S44–54 [DOI] [PubMed] [Google Scholar]
- 19. Miller TE, Findon G. Touch contamination of connection devices in peritoneal dialysis—a quantitative microbiologic analysis. Perit Dial Int 1997; 17:560–7 [PubMed] [Google Scholar]
- 20. Firanek C, Guest S. Hand hygiene in peritoneal dialysis. Perit Dial Int 2011; 31:399–408 [DOI] [PubMed] [Google Scholar]
- 21. Pittet D, Allegranzi B, Boyce J. on behalf of the World Health Organization World Alliance for Patient Safety First Global Patient Safety Challenge Core Group of Experts The World Health Organization Guidelines on hand hygiene in health care and their consensus recommendations. Infect Control Hosp Epidemiol 2009; 30:611–22 [DOI] [PubMed] [Google Scholar]
- 22. Watson AR, Gartland C. on behalf of the European Paediatric Peritoneal Dialysis Working Group Guidelines by an ad hoc European committee for elective chronic peritoneal dialysis in pediatric patients. Perit Dial Int 2001; 21:240–4 [PubMed] [Google Scholar]
- 23. Chua AN, Warady BA. Adherence of pediatric patients to automated peritoneal dialysis. Pediatr Nephrol 2011; 26:789–93 [DOI] [PubMed] [Google Scholar]
- 24. Schiller B, Alcaraz M, Hadley K, Moran J. Peritonitis and zoonosis: your best friend sometimes isn’t! Perit Dial Int 2011; 31:127–30 [DOI] [PubMed] [Google Scholar]
- 25. Chadha V, Warady BA. Capnocytophaga canimorsus peritonitis in a pediatric peritoneal dialysis patient. Pediatr Nephrol 1999; 13:646–8 [DOI] [PubMed] [Google Scholar]
- 26. Russo R, Manili L, Tiraboschi G, Amar K, De Luca M, Alberghini E, et al. Patient re-training in peritoneal dialysis: why and when is it needed? Kidney Int Suppl 2006; (103):S127–32 [DOI] [PubMed] [Google Scholar]
- 27. Bakkaloglu SA. Prevention of peritonitis in children: emerging concepts. Perit Dial Int 2009; 29(Suppl 2):S186–9 [PubMed] [Google Scholar]
- 28. Golper TA, Brier ME, Bunke M, Schreiber MJ, Bartlett DK, Hamilton RW, et al. Risk factors for peritonitis in long-term peritoneal dialysis: the Network 9 peritonitis and catheter survival studies. Academic Subcommittee of the Steering Committee of the Network 9 Peritonitis and Catheter Survival Studies. Am J Kidney Dis 1996; 28:428–36 [DOI] [PubMed] [Google Scholar]
- 29. Eklund B, Honkanen E, Kyllönen L, Salmela K, Kala AR. Peritoneal dialysis access: prospective randomized comparison of single-cuff and double-cuff straight Tenckhoff catheters. Nephrol Dial Transplant 1997; 12:2664–6 [DOI] [PubMed] [Google Scholar]
- 30. Lo WK, Lui SL, Li FK, Choy BY, Lam MF, Tse KC, et al. A prospective randomised study on three different peritoneal dialysis catheters. Perit Dial Int 2003; 23(Suppl 2):S127–31 [PubMed] [Google Scholar]
- 31. Strippoli GF, Tong A, Johnson D, Schena FP, Craig JC. Catheter-related interventions to prevent peritonitis in peritoneal dialysis: a systematic review of randomized controlled trials. J Am Soc Nephrol 2004; 15:2735–46 [DOI] [PubMed] [Google Scholar]
- 32. Lane JC, Warady BA, Feneberg R, Majkowski NL, Watson AR, Fischbach M, et al. Relapsing peritonitis in children who undergo chronic peritoneal dialysis: a prospective study of the International Pediatric Peritonitis Registry. Clin J Am Soc Nephrol 2010; 5:1041–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zurowska A, Feneberg R, Warady BA, Zimmering M, Monteverde M, Testa S, et al. Gram-negative peritonitis in children undergoing long-term peritoneal dialysis. Am J Kidney Dis 2008; 51:455–62 [DOI] [PubMed] [Google Scholar]
- 34. Furth SL, Donaldson LA, Sullivan EK, Watkins SL. on behalf of the North American Pediatric Renal Transplant Cooperative Study Peritoneal dialysis catheter infections and peritonitis in children: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 2000; 15:179–82 [DOI] [PubMed] [Google Scholar]
- 35. Neu AM, Ho PL, McDonald RA, Warady BA. Chronic dialysis in children and adolescents. The 2001 NAPRTCS Annual Report. Pediatr Nephrol 2002; 17:656–63 [DOI] [PubMed] [Google Scholar]
- 36. Chadha V, Jones LL, Ramirez ZD, Warady BA. Chest wall placement of peritoneal dialysis catheters in infants with a colostomy. Adv Perit Dial 2000; 16:318–20 [PubMed] [Google Scholar]
- 37. Warchol S, Ziolkowska H, Roszkowska–Blaim M. Exit-site infection in children on peritoneal dialysis: comparison of two types of peritoneal catheters. Perit Dial Int 2003; 23:169–73 [PubMed] [Google Scholar]
- 38. Rinaldi S, Sera F, Verrina E, Edefonti A, Gianoglio B, Perfumo F, et al. Chronic peritoneal dialysis catheters in children: a fifteen-year experience of the Italian Registry of Pediatric Chronic Peritoneal Dialysis. Perit Dial Int 2004; 24:481–6 [PubMed] [Google Scholar]
- 39. Daschner M, Gfrörer S, Zachariou Z, Mehls O, Schaefer F. Laparoscopic Tenckhoff catheter implantation in children. Perit Dial Int 2002; 22:22–6 [PubMed] [Google Scholar]
- 40. Mattioli G, Castagnetti M, Verrina E, Trivelli A, Torre M, Jasonni V, et al. Laparoscopic-assisted peritoneal dialysis catheter implantation in pediatric patients. Urology 2007; 69:1185–9 [DOI] [PubMed] [Google Scholar]
- 41. Copeland DR, Blaszak RT, Tolleson JS, Saad DF, Jackson RJ, Smith SD, et al. Laparoscopic Tenckhoff catheter placement in children using a securing suture in the pelvis: comparison to the open approach. J Pediatr Surg 2008; 43:2256–9 [DOI] [PubMed] [Google Scholar]
- 42. Twardowski Z, Nichols W. Peritoneal dialysis access and exit site care including surgical aspects. In: Gokal R, Khanna R, Krediet R, Nolph KD, eds. Peritoneal Dialysis. Dordrecht, Netherlands: Kluwer Academic Publishers; 2000: 307–61 [Google Scholar]
- 43. Sojo ET, Grosman MD, Monteverde ML, Bailez MM, Delgado N. Fibrin glue is useful in preventing early dialysate leakage in children on chronic peritoneal dialysis. Perit Dial Int 2004; 24:186–90 [PubMed] [Google Scholar]
- 44. Sardegna KM, Beck AM, Strife CF. Evaluation of perioperative antibiotics at the time of dialysis catheter placement. Pediatr Nephrol 1998; 12:149–52 [DOI] [PubMed] [Google Scholar]
- 45. Strippoli GF, Tong A, Johnson D, Schena FP, Craig JC. Antimicrobial agents to prevent peritonitis in peritoneal dialysis: a systematic review of randomized controlled trials. Am J Kidney Dis 2004; 44:591–603 [PubMed] [Google Scholar]
- 46. Wikdahl AM, Engman U, Stegmayr BG, Sörenssen JG. One-dose cefuroxime i.v. and i.p. reduces microbial growth in PD patients after catheter insertion. Nephrol Dial Transplant 1997; 12:157–60 [DOI] [PubMed] [Google Scholar]
- 47. Lye WC, Lee EJ, Tan CC. Prophylactic antibiotics in the insertion of Tenckhoff catheters. Scand J Urol Nephrol 1992; 26:177–80 [DOI] [PubMed] [Google Scholar]
- 48. Bennett–Jones DN, Martin J, Barratt AJ, Duffy TJ, Naish PF, Aber GM. Prophylactic gentamicin in the prevention of early exit-site infections and peritonitis in CAPD. Adv Perit Dial 1998; 4:147–50 [Google Scholar]
- 49. Gadallah MF, Ramdeen G, Mignone J, Patel D, Mitchell L, Tatro S. Role of preoperative antibiotic prophylaxis in preventing postoperative peritonitis in newly placed peritoneal dialysis catheters. Am J Kidney Dis 2000; 36:1014–19 [DOI] [PubMed] [Google Scholar]
- 50. Berns J. Infection with antimicrobial-resistant microorganisms in dialysis patients. Semin Dial 2003; 16:30–7 [DOI] [PubMed] [Google Scholar]
- 51. Atta MG, Eustace JA, Song X, Perl TM, Scheel PJ., Jr Outpatient vancomycin use and vancomycin-resistant enterococcal colonization in maintenance dialysis patients. Kidney Int 2001; 59:718–24 [DOI] [PubMed] [Google Scholar]
- 52. von Baum H, Schehl J, Geiss HK, Schaefer F. Prevalence of vancomycin-resistant enterococci among children with end-stage renal failure. Mid-European Pediatric Peritoneal Dialysis Study Group. Clin Infect Dis 1999; 29:912–16 [DOI] [PubMed] [Google Scholar]
- 53. Zelenitsky S, Barns L, Findlay I, Alfa M, Ariano R, Fine A, et al. Analysis of microbiological trends in peritoneal dialysis-related peritonitis from 1991 to 1998. Am J Kidney Dis 2000; 36:1009–13 [DOI] [PubMed] [Google Scholar]
- 54. Troidle L, Kliger AS, Gorban–Brennan N, Fikrig M, Golden M, Finkelstein FO. Nine episodes of CPD-associated peritonitis with vancomycin resistant enterococci. Kidney Int 1996; 50:1368–72 [DOI] [PubMed] [Google Scholar]
- 55. Troidle L, Kliger AS, Goldie SJ, Gorban–Brennan N, Brown E, Fikrig M, et al. Continuous peritoneal dialysis–associated peritonitis of nosocomial origin. Perit Dial Int 1996; 16:505–10 [PubMed] [Google Scholar]
- 56. Berns JS, Tokars JI. Preventing bacterial infections and antimicrobial resistance in dialysis patients. Am J Kidney Dis 2002; 40:886–98 [DOI] [PubMed] [Google Scholar]
- 57. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis–related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31 [PubMed] [Google Scholar]
- 58. Flanigan M, Gokal R. Peritoneal catheters and exit-site practices toward optimum peritoneal access: a review of current developments. Perit Dial Int 2005; 25:132–9 [PubMed] [Google Scholar]
- 59. Dombros N, Dratwa M, Feriani M, Gokal R, Heimbürger O, Krediet R, et al. European best practice guidelines for peritoneal dialysis. 3. Peritoneal access. Nephrol Dial Transplant 2005; 20(Suppl 9):ix8–12 [DOI] [PubMed] [Google Scholar]
- 60. Prowant BF, Twardowski ZJ. Recommendations for exit care. Perit Dial Int 1996; 16(Suppl 3):S94–9 [PubMed] [Google Scholar]
- 61. Twardowski ZJ, Prowant BF. Exit-site healing post catheter implantation. Perit Dial Int 1996; 16(Suppl 3):S51–70 [PubMed] [Google Scholar]
- 62. Prowant BF, Warady BA, Nolph KD. Peritoneal dialysis catheter exit-site care: results of an international survey. Perit Dial Int 1993; 13:149–54 [PubMed] [Google Scholar]
- 63. Twardowski ZJ, Prowant BF. Current approaches to exit-site infections in patients on peritoneal dialysis. Nephrol Dial Transplant 1997; 12:1284–95 [DOI] [PubMed] [Google Scholar]
- 64. Luzar MA, Brown CB, Balf D, Hill L, Issad B, Monnier B, et al. Exit-site care and exit-site infection in continuous ambulatory peritoneal dialysis. Perit Dial Int 1990; 10:25–9 [PubMed] [Google Scholar]
- 65. Jones LL, Tweedy L, Warady BA. The impact of exit-site care and catheter design on the incidence of catheter-related infections. Adv Perit Dial 1995; 11:302–5 [PubMed] [Google Scholar]
- 66. Hoshii S, Wada N, Honda M. on behalf of the Japanese Study Group of Pediatric Peritoneal Dialysis A survey of peritonitis and exit-site and/or tunnel infections in Japanese children on PD. Pediatr Nephrol 2006; 21:828–34 [DOI] [PubMed] [Google Scholar]
- 67. Wadhwa NK, Reddy GH. Exit-site care in peritoneal dialysis. Contrib Nephrol 2007; 154:117–24 [DOI] [PubMed] [Google Scholar]
- 68. Mendoza–Guevara L, Castro–Vazquez F, Aguilar–Kitsu A, Morales–Nava A, Rodriguez–Leyva F, Sanchez–Barbosa JL. Amuchina 10% solution, safe antiseptic for preventing infections of exit-site of Tenckhoff catheters, in the pediatric population of a dialysis program. Contrib Nephrol 2007; 154:139–44 [DOI] [PubMed] [Google Scholar]
- 69. Chua AN, Goldstein SL, Bell D, Brewer ED. Topical mupirocin/sodium hypochlorite reduces peritonitis and exit-site infection rates in children. Clin J Am Soc Nephrol 2009; 4:1939–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Piraino B, Bernardini J, Bender FH. An analysis of methods to prevent peritoneal dialysis catheter infections. Perit Dial Int 2008; 28:437–43 [PubMed] [Google Scholar]
- 71. Chu KH, Choy WY, Cheung CC, Fung KS, Tang HL, Lee W, et al. A prospective study of the efficacy of local application of gentamicin versus mupirocin in the prevention of peritoneal dialysis catheter–related infections. Perit Dial Int 2008; 28:505–8 [PubMed] [Google Scholar]
- 72. Bernardini J, Bender F, Florio T, Sloand J, Palmmontalbano L, Fried L, et al. Randomized, double-blind trial of antibiotic exit-site cream for prevention of exit-site infection in peritoneal dialysis patients. J Am Soc Nephrol 2005; 16:539–45 [DOI] [PubMed] [Google Scholar]
- 73. Johnson DW, Clark C, Isbel NM, Hawley CM, Beller E, Cass A, et al. The Honeypot Study protocol: a randomized controlled trial of exit-site application of Medihoney antibacterial wound gel for the prevention of catheter-associated infections in peritoneal dialysis patients. Perit Dial Int 2009; 29:303–9 [PubMed] [Google Scholar]
- 74. Jassal SV, Lok CE. on behalf of the MP3 Study Group A randomized controlled trial comparing mupirocin versus Polysporin Triple for the prevention of catheter-related infections in peritoneal dialysis patients. Perit Dial Int 2008; 28:67–72 [PubMed] [Google Scholar]
- 75. Piraino B. Staphylococcus aureus infections in dialysis patients. Focus on prevention. ASAIO J 2000; 46:S13–17 [DOI] [PubMed] [Google Scholar]
- 76. Blowey DL, Warady BA, McFarland KS. The treatment of Staphylococcus aureus nasal carriage in pediatric peritoneal dialysis patients. Adv Perit Dial 1994; 10:297–9 [PubMed] [Google Scholar]
- 77. Kingwatanakul P, Warady BA. Staphylococcus aureus nasal carriage in children receiving long-term peritoneal dialysis. Adv Perit Dial 1997; 13:281–4 [PubMed] [Google Scholar]
- 78. Gupta B, Bernardini J, Piraino B. Peritonitis associated with exit site and tunnel infections. Am J Kidney Dis 1996; 28:415–19 [DOI] [PubMed] [Google Scholar]
- 79. Swartz R, Messana J, Starmann B, Weber M, Reynolds J. Preventing Staphylococcus aureus infection during chronic peritoneal dialysis. J Am Soc Nephrol 1991; 2:1085–91 [DOI] [PubMed] [Google Scholar]
- 80. Turner K, Uttley L, Scrimgeour A, McKewan A, Gokal R. Natural history of Staphylococcus aureus nasal carriage and its relationship to exit-site infection. Perit Dial Int 1998; 18:271–3 [PubMed] [Google Scholar]
- 81. Amato D, de Jesús Ventura M, Miranda G, Leaños B, Alcántara G, Hurtado ME, et al. Staphylococcal peritonitis in continuous ambulatory peritoneal dialysis: colonization with identical strains at exit site, nose, and hands. Am J Kidney Dis 2001; 37:43–8 [DOI] [PubMed] [Google Scholar]
- 82. Herwaldt LA. Reduction of Staphylococcus aureus nasal carriage and infection in dialysis patients. J Hosp Infect 1998; 40(Suppl B):S13–23 [DOI] [PubMed] [Google Scholar]
- 83. Zimmerman SW, Ahrens E, Johnson CA, Craig W, Leggett J, O’Brien M, et al. Randomized controlled trial of prophylactic rifampin for peritoneal dialysis–related infections. Am J Kidney Dis 1991; 18:225–31 [DOI] [PubMed] [Google Scholar]
- 84. Pérez–Fontán M, García–Falcón T, Rosales M, Rodríguez–Carmona A, Adeva M, Rodríguez–Lozano I, et al. Treatment of Staphylococcus aureus nasal carriers in continuous ambulatory peritoneal dialysis with mupirocin: long-term results. Am J Kidney Dis 1993; 22:708–12 [DOI] [PubMed] [Google Scholar]
- 85. Nasal mupirocin prevents Staphylococcus aureus exit-site infection during peritoneal dialysis. Mupirocin Study Group. J Am Soc Nephrol 1996; 7:2403–8 [DOI] [PubMed] [Google Scholar]
- 86. Thodis E, Bhaskaran S, Pasadakis P, Bargman JM, Vas SI, Oreopoulos DG. Decrease in Staphylococcus aureus exit-site infections and peritonitis in CAPD patients by local application of mupirocin ointment at the catheter exit-site. Perit Dial Int 1998; 18:261–70 [PubMed] [Google Scholar]
- 87. Casey M, Taylor J, Clinard P, Graham A, Mauck V, Spainhour L, et al. Application of the mupirocin cream at the catheter exit-site reduces exit-site infections and peritonitis in peritoneal dialysis patients. Perit Dial Int 2000; 20:566–7 [PubMed] [Google Scholar]
- 88. Bernardini J, Piraino B, Holley J, Johnston JR, Lutes R. A randomized trial of Staphylococcus aureus prophylaxis in peritoneal dialysis patients: mupirocin calcium ointment 2% applied to the exit-site versus cyclic oral rifampin. Am J Kidney Dis 1996; 27:695–700 [DOI] [PubMed] [Google Scholar]
- 89. Davey P, Craig AM, Hau C, Malek M. Cost-effectiveness of prophylactic nasal mupirocin in patients undergoing peritoneal dialysis based on a randomized, placebo controlled trial. J Antimicrob Chemother 1999; 43:105–12 [DOI] [PubMed] [Google Scholar]
- 90. Wong SS, Chu KH, Cheuk A, Tsang WK, Fung SK, Chan HW, et al. Prophylaxis against gram-positive organisms causing exit-site infection and peritonitis in continuous ambulatory peritoneal dialysis patients by applying mupirocin ointment at the catheter exit-site. Perit Dial Int 2003; 23(Suppl 2):S153–8 [PubMed] [Google Scholar]
- 91. Araki Y, Hataya H, Ikeda M, Ishikura K, Honda M. Intranasal mupirocin does not prevent exit-site infections in children receiving peritoneal dialysis. Perit Dial Int 2003; 23:267–9 [PubMed] [Google Scholar]
- 92. Piraino B, Bernardini J, Florio T, Fried L. Staphylococcus aureus prophylaxis and trends in gram-negative infections in peritoneal dialysis patients. Perit Dial Int 2003; 23:456–9 [PubMed] [Google Scholar]
- 93. Ritzau J, Hoffman RM, Tzamaloukas AH. Effect of preventing Staphylococcus aureus carriage on rates of peritoneal catheter-related staphylococcal infections. Literature synthesis. Perit Dial Int 2001; 21:471–9 [PubMed] [Google Scholar]
- 94. Tacconelli E, Carmeli Y, Aizer A, Ferreira G, Foreman MG, D’Agata EM. Mupirocin prophylaxis to prevent Staphylococcus aureus infection in patients undergoing dialysis: a meta-analysis. Clin Infect Dis 2003; 37:1629–38 [DOI] [PubMed] [Google Scholar]
- 95. Crabtree JH, Hadnott LL, Burchette RJ, Siddiqi RA. Outcome and clinical implications of a surveillance and treat program for Staphylococcus aureus nasal carriage in peritoneal dialysis patients. Adv Perit Dial 2000; 16:271–5 [PubMed] [Google Scholar]
- 96. Laupland KB, Conly JM. Treatment of Staphylococcus aureus colonization and prophylaxis for infection with topical intranasal mupirocin: an evidence-based review. Clin Infect Dis 2003; 37:933–8 [DOI] [PubMed] [Google Scholar]
- 97. Piraino B. New insights on preventing and managing peritonitis. Pediatr Nephrol 2004; 19:125–7 [DOI] [PubMed] [Google Scholar]
- 98. Annigeri R, Conly J, Vas S, Dedier H, Prakashan KP, Bargman JM, et al. Emergence of mupirocin-resistant Staphylococcus aureus in chronic peritoneal dialysis patients using mupirocin prophylaxis to prevent exit-site infection. Perit Dial Int 2001; 21:554–9 [PubMed] [Google Scholar]
- 99. Pérez–Fontán M, Rosales M, Rodríguez–Carmona A, Falcón TG, Valdés F. Mupirocin resistance after long-term use for Staphylococcus aureus colonization in patients undergoing chronic peritoneal dialysis. Am J Kidney Dis 2002; 39:337–41 [DOI] [PubMed] [Google Scholar]
- 100. Lobbedez T, Gardam M, Dedier H, Burdzy D, Chu M, Izatt S, et al. Routine use of mupirocin at the peritoneal catheter exit-site and mupirocin resistance: still low after 7 years. Nephrol Dial Transplant 2004; 19:3140–3 [DOI] [PubMed] [Google Scholar]
- 101. Mahaldar A, Weisz M, Kathuria P. Comparison of gentamicin and mupirocin in the prevention of exit-site infection and peritonitis in peritoneal dialysis. Adv Perit Dial 2009; 25:56–9 [PubMed] [Google Scholar]
- 102. Lok CE, Stanley KE, Hux JE, Richardson R, Tobe SW, Conly J. Hemodialysis infection prevention with Polysporin ointment. J Am Soc Nephrol 2003; 14:169–79 [DOI] [PubMed] [Google Scholar]
- 103. McQuillan RF, Chiu E, Nessim S, Lok CE, Roscoe JM, Tam P, et al. A randomized controlled trial comparing mupirocin and Polysporin Triple ointments in peritoneal dialysis patients: the MP3 Study. Clin J Am Soc Nephrol 2012; 7:297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cooper RA, Halas E, Molan PC. The efficacy of honey in inhibiting strains of Pseudomonas aeruginosa from infected burns. J Burn Care Rehabil 2002; 23:366–70 [DOI] [PubMed] [Google Scholar]
- 105. Cooper RA, Molan PC, Harding KG. The sensitivity to honey of gram-positive cocci of clinical significance isolated from wounds. J Appl Microbiol 2002; 93:857–63 [DOI] [PubMed] [Google Scholar]
- 106. Johnson DW, van Eps C, Mudge DW, Wiggins KJ, Armstrong K, Hawley CM, et al. Randomized, controlled trial of topical exit-site application of honey (Medihoney) versus mupirocin for the prevention of catheter-associated infections in hemodialysis patients. J Am Soc Nephrol 2005; 16:1456–62 [DOI] [PubMed] [Google Scholar]
- 107. Daly CD, Campbell MK, MacLeod AM, Cody DJ, Vale LD, Grant AM, et al. Do the Y-set and double-bag systems reduce the incidence of CAPD peritonitis? A systematic review of randomized controlled trials. Nephrol Dial Transplant 2001; 16:341–7 [DOI] [PubMed] [Google Scholar]
- 108. Maiorca R, Cantaluppi A, Cancarini GC, Scalamogna A, Broccoli R, Graziani G, et al. Prospective controlled trial of a Y-connector and disinfectant to prevent peritonitis in continuous ambulatory peritoneal dialysis. Lancet 1983; 2:642–4 [DOI] [PubMed] [Google Scholar]
- 109. Rottembourg J, Brouard R, Issad B, Allouache M, Jacobs C. Prospective randomized study about Y connectors in CAPD patients. Adv Perit Dial 1987; 3:107–13 [Google Scholar]
- 110. Lindholm T, Simonsen O, Krutzen L, van Leusen R. Evaluation of a new take-off system: a prospective randomized multicenter study. Adv Perit Dial 1988; 4:262–5 [Google Scholar]
- 111. Peritonitis in continuous ambulatory peritoneal dialysis (CAPD): a multi-centre randomized clinical trial comparing the Y connector disinfectant system to standard systems. Canadian CAPD Clinical Trials Group. Perit Dial Int 1989; 9:159–63 [PubMed] [Google Scholar]
- 112. Owen JE, Walker RG, Lemon J, Brett L, Mitrou D, Becker GJ. Randomized study of peritonitis with conventional versus O-set techniques in continuous ambulatory peritoneal dialysis. Perit Dial Int 1992; 12:216–20 [PubMed] [Google Scholar]
- 113. Li PK, Chan TH, So WY, Wang AY, Leung CB, Lai KN. Comparisons of Y-set disconnect system (Ultraset) versus conventional spike system in uremic patients on CAPD: outcome and cost analysis. Perit Dial Int 1996; 16(Suppl 1):S368–70 [PubMed] [Google Scholar]
- 114. Monteón F, Correa–Rotter R, Paniagua R, Amato D, Hurtado ME, Medina JL, et al. Prevention of peritonitis with disconnect systems in CAPD: a randomized controlled trial. The Mexican Nephrology Collaborative Study Group. Kidney Int 1998; 54:2123–8 [DOI] [PubMed] [Google Scholar]
- 115. Cheng IK, Chan CY, Cheng SW, Poon JF, Ji YL, Lo WK, et al. A randomized prospective study of the cost-effectiveness of the conventional spike, O-set and UVXD techniques in continuous ambulatory peritoneal dialysis. Perit Dial Int 1994; 14:255–60 [PubMed] [Google Scholar]
- 116. Kiernan L, Kliger A, Gorban–Brennan N, Juergensen P, Tesin D, Vonesh E, et al. Comparison of continuous ambulatory peritoneal dialysis–related infections with different “Y-tubing” exchange systems. J Am Soc Nephrol 1995; 5:1835–8 [DOI] [PubMed] [Google Scholar]
- 117. Ong LM, Lim TO, Hooi LS, Morad Z, Tan PC, Wong HS, et al. A randomized, multicenter, open-label trial to establish therapeutic equivalence between the Carex and Ultra disconnect systems in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 2003; 23(Suppl 2):S139–43 [PubMed] [Google Scholar]
- 118. Wong HS, Ong LM, Lim TO, Hooi LS, Morad Z, Ghazalli R, et al. A randomized multicenter, open-label trial to determine peritonitis rate, product defect, and technique survival between ANDY-Disc and UltraBag in patients on CAPD. Am J Kidney Dis 2006; 48:464–72 [DOI] [PubMed] [Google Scholar]
- 119. Smith CA. Reduced incidence of peritonitis by utilizing “flush before fill” in APD. Adv Perit Dial 1997; 13:224–6 [PubMed] [Google Scholar]
- 120. Warady BA, Ellis EN, Fivush BA, Lum GM, Alexander SR, Brewer ED, et al. “Flush before fill” in children receiving automated peritoneal dialysis. Perit Dial Int 2003; 23:493–8 [PubMed] [Google Scholar]
- 121. Bazzato G, Landini S, Fracasso A, Morachiello P, Righetto F, Scanferla F, et al. Why the double-bag system still remains the best technique for peritoneal fluid exchange in continuous ambulatory peritoneal dialysis. Perit Dial Int 1993; 13(Suppl 2):S152–5 [PubMed] [Google Scholar]
- 122. Cox SD, Steddon S, Mallinder S, Fan SL, Punzalan S. Re-training and switching of PD system to reduce recurrent gram-positive PD peritonitis. J Ren Care 2006; 32:198–201 [DOI] [PubMed] [Google Scholar]
- 123. Miles R, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Predictors and outcomes of fungal peritonitis in peritoneal dialysis patients. Kidney Int 2009; 76:622–8 [DOI] [PubMed] [Google Scholar]
- 124. Wang AY, Yu AW, Li PK, Lam PK, Leung CB, Lai KN, et al. Factors predicting outcome of fungal peritonitis in peritoneal dialysis: analysis of a 9-year experience of fungal peritonitis in a single center. Am J Kidney Dis 2000; 36:1183–92 [DOI] [PubMed] [Google Scholar]
- 125. Raaijmakers R, Schröder C, Monnens L, Cornelissen E, Warris A. Fungal peritonitis in children on peritoneal dialysis. Pediatr Nephrol 2007; 22:288–93 [DOI] [PubMed] [Google Scholar]
- 126. Davenport A, Wellsted D. on behalf of the Pan Thames Renal Audit Peritoneal Dialysis Group Does antifungal prophylaxis with daily oral fluconazole reduce the risk of fungal peritonitis in peritoneal dialysis patients? The Pan Thames Renal Audit. Blood Purif 2011; 32:181–5 [DOI] [PubMed] [Google Scholar]
- 127. Michel C, Courdavault L, al Khayat R, Viron B, Roux P, Mignon F. Fungal peritonitis in patients on peritoneal dialysis. Am J Nephrol 1994; 14:113–20 [DOI] [PubMed] [Google Scholar]
- 128. Goldie SJ, Kiernan–Tridle L, Torres C, Gorban–Brennan N, Dunne D, Kliger AS, et al. Fungal peritonitis in a large peritoneal dialysis population: a report of 55 episodes. Am J Kidney Dis 1996; 28:86–91 [DOI] [PubMed] [Google Scholar]
- 129. Bren A. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis. Eur J Clin Microbiol Infect Dis 1998; 17:839–43 [DOI] [PubMed] [Google Scholar]
- 130. Warady BA, Bashir M, Donaldson LA. Fungal peritonitis in children receiving peritoneal dialysis: a report of the NAPRTCS. Kidney Int 2000; 58:384–9 [DOI] [PubMed] [Google Scholar]
- 131. Prasad KN, Prasad N, Gupta A, Sharma RK, Verma AK, Ayyagari A. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis: a single centre Indian experience. J Infect 2004; 48:96–101 [DOI] [PubMed] [Google Scholar]
- 132. Lo WK, Chan CY, Cheng SW, Poon JF, Chan DT, Cheng IK. A prospective randomized control study of oral nystatin prophylaxis for Candida peritonitis complicating continuous ambulatory peritoneal dialysis. Am J Kidney Dis 1996; 28:549–52 [DOI] [PubMed] [Google Scholar]
- 133. Záruba K, Peters J, Jungbluth H. Successful prophylaxis for fungal peritonitis in patients on continuous ambulatory peritoneal dialysis: six years experience. Am J Kidney Dis 1991; 17:43–6 [DOI] [PubMed] [Google Scholar]
- 134. Robitaille P, Mérouani A, Clermónt MJ, Hébert E. Successful antifungal prophylaxis in chronic peritoneal dialysis: a pediatric experience. Perit Dial Int 1995; 15:77–9 [PubMed] [Google Scholar]
- 135. Wadhwa NK, Suh H, Cabralda T. Antifungal prophylaxis for secondary fungal peritonitis in peritoneal dialysis patients. Adv Perit Dial 1996; 12:189–91 [PubMed] [Google Scholar]
- 136. Thodis E, Vas SI, Bargman JM, Singhal M, Chu M, Oreopoulos DG. Nystatin prophylaxis: its inability to prevent fungal peritonitis in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 1998; 18:583–9 [PubMed] [Google Scholar]
- 137. Williams PF, Moncrieff N, Marriott J. No benefit in using nystatin prophylaxis against fungal peritonitis in peritoneal dialysis patients. Perit Dial Int 2000; 20:352–3 [PubMed] [Google Scholar]
- 138. Moreiras–Plaza M, Vello–Román A, Sampróm–Rodríguez M, Feijóo–Piñeiro D. Ten years without fungal peritonitis: a single center’s experience. Perit Dial Int 2007; 27:460–3 [PubMed] [Google Scholar]
- 139. Restrepo C, Chacon J, Manjarres G. Fungal peritonitis in peritoneal dialysis patients: successful prophylaxis with fluconazole, as demonstrated by prospective randomized control trial. Perit Dial Int 2010; 30:619–25 [DOI] [PubMed] [Google Scholar]
- 140. Lye WC. Nystatin prophylaxis for fungal peritonitis: to be or not to be? Perit Dial Int 2007; 27:511–13 [PubMed] [Google Scholar]
- 141. Wong PN, Lo KY, Tong GM, Chan SF, Lo MW, Mak SK, et al. Prevention of fungal peritonitis with nystatin prophylaxis in patients receiving CAPD. Perit Dial Int 2007; 27:531–6 [PubMed] [Google Scholar]
- 142. Piraino B. Peritoneal infections. Adv Ren Replace Ther 2000; 7:280–8 [DOI] [PubMed] [Google Scholar]
- 143. Prasad N, Gupta A. Fungal peritonitis in peritoneal dialysis patients. Perit Dial Int 2005; 25:207–22 [PubMed] [Google Scholar]
- 144. Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007; 116:1736–54 [DOI] [PubMed] [Google Scholar]
- 145. Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, et al. Prevention of bacterial endocarditis: recommendations by the American Heart Association. J Am Dent Assoc 1997; 128:1142–51 [DOI] [PubMed] [Google Scholar]
- 146. Banerjee S, Shen B, Baron TH, Nelson DB, Anderson MA, Cash BD, et al. on behalf of the ASGE Standards of Practice Committee. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2008; 67:791–8 [DOI] [PubMed] [Google Scholar]
- 147. Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther 2007; 25:647–56 [DOI] [PubMed] [Google Scholar]
- 148. Ramage IJ, Geary DF, Harvey E, Secker DJ, Balfe JA, Balfe JW. Efficacy of gastrostomy feeding in infants and older children receiving chronic peritoneal dialysis. 1999;19:231–6 [PubMed] [Google Scholar]
- 149. Murugasu B, Conley SB, Lemire JM, Portman RJ. Fungal peritonitis in children treated with peritoneal dialysis and gastrostomy feeding. Pediatr Nephrol 1991; 5:620–1 [DOI] [PubMed] [Google Scholar]
- 150. Ledermann SE, Spitz L, Moloney J, Rees L, Trompeter RS. Gastrostomy feeding in infants and children on peritoneal dialysis. Pediatr Nephrol 2002; 17:246–50 [DOI] [PubMed] [Google Scholar]
- 151. von Schnakenburg C, Feneberg R, Plank C, Zimmering M, Arbeiter K, Bald M, et al. Percutaneous endoscopic gastrostomy in children on peritoneal dialysis. Perit Dial Int 2006; 26:69–77 [PubMed] [Google Scholar]
- 152. Rees L, Brandt ML. Tube feeding in children with chronic kidney disease: technical and practical issues. Pediatr Nephrol 2010; 25:699–704 [DOI] [PubMed] [Google Scholar]
- 153. Flanigan MJ, Freeman RM, Lim VS. Cellular response to peritonitis among peritoneal dialysis patients. Am J Kidney Dis 1985; 6:420–4 [DOI] [PubMed] [Google Scholar]
- 154. Gould IM, Casewell MW. The laboratory diagnosis of peritonitis during continuous ambulatory peritoneal dialysis. J Hosp Infect 1986; 7:155–60 [DOI] [PubMed] [Google Scholar]
- 155. Betjes MG, Tuk CW, Visser CE, Zemel D, Krediet RT, Arisz L, et al. Analysis of the peritoneal cellular immune system during CAPD shortly before a clinical peritonitis. Nephrol Dial Transplant 1994; 9:684–92 [DOI] [PubMed] [Google Scholar]
- 156. Koopmans JG, Boeschoten EW, Pannekeet MM, Betjes MG, Zemel D, Kuijper EJ, et al. Impaired initial cell reaction in CAPD-related peritonitis. Perit Dial Int 1996; 16(Suppl 1):S362–7 [PubMed] [Google Scholar]
- 157. Rocklin MA, Teitelbaum I. Noninfectious causes of cloudy peritoneal dialysate. Semin Dial 2001; 14:37–40 [DOI] [PubMed] [Google Scholar]
- 158. Chadha V, Schaefer FS, Warady BA. Dialysis-associated peritonitis in children. Pediatr Nephrol 2010; 25:425–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Schaefer F, Klaus G, Müller–Wiefel DE, Mehls O. Intermittent versus continuous intraperitoneal glycopeptide/ceftazidime treatment in children with peritoneal dialysis-associated peritonitis. The Mid-European Pediatric Peritoneal Dialysis Study Group (MEPPS). J Am Soc Nephrol 1999; 10:136–45 [DOI] [PubMed] [Google Scholar]
- 160. Akman S, Uygun V, Guven AG. Value of the urine strip test in the early diagnosis of bacterial peritonitis. Pediatr Int 2005; 47:523–7 [DOI] [PubMed] [Google Scholar]
- 161. Park SJ, Lee JY, Tak WT, Lee JH. Using reagent strips for rapid diagnosis of peritonitis in peritoneal dialysis patients. Adv Perit Dial 2005; 21:69–71 [PubMed] [Google Scholar]
- 162. Quinlan C, Cantwell M, Rees L. Eosinophilic peritonitis in children on chronic peritoneal dialysis. Pediatr Nephrol 2010; 25:517–22 [DOI] [PubMed] [Google Scholar]
- 163. von Graevenitz A, Amsterdam D. Microbiological aspects of peritonitis associated with continuous ambulatory peritoneal dialysis. Clin Microbiol Rev 1992; 5:36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lye WC, Wong PL, Leong SO, Lee EJ. Isolation of organisms in CAPD peritonitis: a comparison of two techniques. Adv Perit Dial 1994; 10:166–8 [PubMed] [Google Scholar]
- 165. Sewell DL, Golper TA, Hulman PB, Thomas CM, West LM, Kubey WY, et al. Comparison of large volume culture to other methods for isolation of microorganisms from dialysate. Perit Dial Int 1990; 10:49–52 [PubMed] [Google Scholar]
- 166. Blondeau JM, Pylypchuk GB, Kappel JE, Pilkey B, Lawler C. Comparison of bedside- and laboratory-inoculated Bactec high- and low-volume resin bottles for the recovery of microorganisms causing peritonitis in CAPD patients. Diagn Microbiol Infect Dis 1998; 31:281–7 [DOI] [PubMed] [Google Scholar]
- 167. Chow KM, Chow VC, Szeto CC, Law MC, Leung CB, Li PK. Continuous ambulatory peritoneal dialysis peritonitis: broth inoculation culture versus water lysis method. Nephron Clin Pract 2007; 105:c121–5 [DOI] [PubMed] [Google Scholar]
- 168. Azap OK, Timurkaynak F, Sezer S, Cağir U, Yapar G, Arslan H, et al. Value of automatized blood culture systems in the diagnosis of continuous ambulatory peritoneal dialysis peritonitis. Transplant Proc 2006; 38:411–12 [DOI] [PubMed] [Google Scholar]
- 169. Yoo TH, Chang KH, Ryu DR, Kim JS, Choi HY, Park HC, et al. Usefulness of 23S rRNA amplification by PCR in the detection of bacteria in CAPD peritonitis. Am J Nephrol 2006; 26:115–20 [DOI] [PubMed] [Google Scholar]
- 170. Johnson G, Wilks M, Warwick S, Millar MR, Fan SL. Comparative study of diagnosis of PD peritonitis by quantitative polymerase chain reaction for bacterial DNA vs culture methods. J Nephrol 2006; 19:45–9 [PubMed] [Google Scholar]
- 171. Cervelli MJ, ed. The Renal Drug Reference Guide. Adelaide, Australia: Kidney Health Australia; 2007. [Google Scholar]
- 172. Taketomo CK, Hodding JH, Kraus DM. Pediatric Dosage Handbook: Including Neonatal Dosing, Drug Administration, and Extemporaneous Preparations. 17th ed. Hudson, OH: Lexi-Comp; 2010. [Google Scholar]
- 173. Manley HJ, Bailie GR. Treatment of peritonitis in APD: pharmacokinetic principles. Semin Dial 2002; 15:418–21 [DOI] [PubMed] [Google Scholar]
- 174. Manley HJ, Bridwell DL, Elwell RJ, Bailie GR. Influence of peritoneal dialysate flow rate on the pharmacokinetics of cefazolin. Perit Dial Int 2003; 23:469–74 [PubMed] [Google Scholar]
- 175. Cheng IK, Fang GX, Chau PY, Chan TM, Tong KL, Wong AK, et al. A randomized prospective comparison of oral levofloxacin plus intraperitoneal (IP) vancomycin and IP netromycin plus IP vancomycin as primary treatment of peritonitis complicating CAPD. Perit Dial Int 1998; 18:371–5 [PubMed] [Google Scholar]
- 176. Sisterhen LL, Stowe CD, Farrar HC, Blaszak CK, Blaszak RT. Disposition of ceftazidime after intraperitoneal administration in adolescent patients receiving continuous cycling peritoneal dialysis. Am J Kidney Dis 2006; 47:503–8 [DOI] [PubMed] [Google Scholar]
- 177. Blowey DL, Warady BA, Abdel–Rahman S, Frye RF, Manley HJ. Vancomycin disposition following intraperitoneal administration in children receiving peritoneal dialysis. Perit Dial Int 2007; 27:79–85 [PubMed] [Google Scholar]
- 178. De Broe ME, Giuliano RA, Verpooten GA. Choice of drug and dosage regimen. Two important risk factors for aminoglycoside nephrotoxicity. Am J Med 1986; 80:115–18 [DOI] [PubMed] [Google Scholar]
- 179. Golper T. Intermittent versus continuous antibiotics for PD-related peritonitis. Perit Dial Int 1997; 17:11–12 [PubMed] [Google Scholar]
- 180. Bailie GR, Haqqie SS, Eisele G, Gorman T, Low CL. Effectiveness of once-weekly vancomycin and once-daily gentamicin, intraperitoneally, for CAPD peritonitis. Perit Dial Int 1995; 15:269–71 [PubMed] [Google Scholar]
- 181. Vas S, Bargman J, Oreopoulos D. Treatment in PD patients of peritonitis caused by gram-positive organisms with single daily dose of antibiotics. Perit Dial Int 1997; 17:91–4 [PubMed] [Google Scholar]
- 182. Lai MN, Kao MT, Chen CC, Cheung SY, Chung WK. Intraperitoneal once-daily dose of cefazolin and gentamicin for treating CAPD peritonitis. Perit Dial Int 1997; 17:87–9 [PubMed] [Google Scholar]
- 183. Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int Suppl 2006; (103):S55–62 [DOI] [PubMed] [Google Scholar]
- 184. Wong KM, Chan YH, Cheung CY, Chak WL, Choi KS, Leung SH, et al. Cefepime versus vancomycin plus netilmicin therapy for continuous ambulatory peritoneal dialysis-associated peritonitis. Am J Kidney Dis 2001; 38:127–31 [DOI] [PubMed] [Google Scholar]
- 185. Li PK, Ip M, Law MC, Szeto CC, Leung CB, Wong TY, et al. Use of intraperitoneal cefepime as monotherapy in treatment of CAPD peritonitis. Perit Dial Int 2000; 20:232–4 [PubMed] [Google Scholar]
- 186. Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis 2009; 7:338–48 [DOI] [PubMed] [Google Scholar]
- 187. Fisher BT, Aplenc R, Localio R, Leckerman KH, Zaoutis TE. Cefepime and mortality in pediatric acute myelogenous leukemia: a retrospective cohort study. Pediatr Infect Dis J 2009; 28:971–5 [DOI] [PubMed] [Google Scholar]
- 188. Kim PW, Wu YT, Cooper C, Rochester G, Valappil T, Wang Y, et al. Meta-analysis of a possible signal of increased mortality associated with cefepime use. Clin Infect Dis 2010; 51:381–9 [DOI] [PubMed] [Google Scholar]
- 189. Sonck J, Laureys G, Verbeelen D. The neurotoxicity and safety of treatment with cefepime in patients with renal failure. Nephrol Dial Transplant 2008; 23:966–70 [DOI] [PubMed] [Google Scholar]
- 190. Yuen SK, Yong SP, Tsui HS. Neurotoxicity secondary to intraperitoneally administered cefepime: report of two cases. Hong Kong J Nephrol 2004; 6:106–8 [Google Scholar]
- 191. Khairullah Q, Provenzano R, Tayeb J, Ahmad A, Balakrishnan R, Morrison L. Comparison of vancomycin versus cefazolin as initial therapy for peritonitis in peritoneal dialysis patients. Perit Dial Int 2002; 22:339–44 [PubMed] [Google Scholar]
- 192. Lui SL, Cheng SW, Ng F, Ng SY, Wan KM, Yip T, et al. Cefazolin plus netilmicin versus cefazolin plus ceftazidime for treating CAPD peritonitis: effect on residual renal function. Kidney Int 2005; 68:2375–80 [DOI] [PubMed] [Google Scholar]
- 193. Shemin D, Bostom AG, Lambert C, Hill C, Kitsen J, Kliger AS. Residual renal function in a large cohort of peritoneal dialysis patients: change over time, impact on mortality and nutrition. Perit Dial Int 2000; 20:439–44 [PubMed] [Google Scholar]
- 194. Govindarajulu S, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Staphylococcus aureus peritonitis in Australian peritoneal dialysis patients: predictors, treatment, and outcomes in 503 cases. Perit Dial Int 2010; 30:311–19 [DOI] [PubMed] [Google Scholar]
- 195. Szeto CC, Chow KM, Kwan BC, Law MC, Chung KY, Yu S, et al. Staphylococcus aureus peritonitis complicates peritoneal dialysis: review of 245 consecutive cases. Clin J Am Soc Nephrol 2007; 2:245–51 [DOI] [PubMed] [Google Scholar]
- 196. Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis 2003; 36:281–5 [DOI] [PubMed] [Google Scholar]
- 197. Szeto CC, Kwan BC, Chow KM, Lau MF, Law MC, Chung KY, et al. Coagulase negative staphylococcal peritonitis in peritoneal dialysis patients: review of 232 consecutive cases. Clin J Am Soc Nephrol 2008; 3:91–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Perl TM. The threat of vancomycin resistance. Am J Med 1999; 106:26S–7S [DOI] [PubMed] [Google Scholar]
- 199. Servais A, Mercadal L, Brossier F, Venditto M, Issad B, Isnard–Bagnis C, et al. Rapid curbing of a vancomycin-resistant Enterococcus faecium outbreak in a nephrology department. Clin J Am Soc Nephrol 2009; 4:1559–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Barbosa D, Lima L, Silbert S, Sader H, Cendoroglo M, Draibe S, et al. Evaluation of the prevalence and risk factors for colonization by vancomycin-resistant Enterococcus among patients on dialysis. Am J Kidney Dis 2004; 44:337–43 [DOI] [PubMed] [Google Scholar]
- 201. Edey M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Enterococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 116 cases. Nephrol Dial Transplant 2010; 25:1272–8 [DOI] [PubMed] [Google Scholar]
- 202. Sutherland SM, Alexander SR, Feneberg R, Schaefer F, Warady BA. on behalf of the International Pediatric Peritonitis Registry (IPPR) Enterococcal peritonitis in children receiving chronic peritoneal dialysis. Nephrol Dial Transplant 2010; 25:4048–54 [DOI] [PubMed] [Google Scholar]
- 203. Vinh DC, Rubinstein E. Linezolid: a review of safety and tolerability. J Infect 2009; 59(Suppl 1):S59–74 [DOI] [PubMed] [Google Scholar]
- 204. Goffin E, Herbiet L, Pouthier D, Pochet JM, Lafontaine JJ, Christophe JL, et al. Vancomycin and ciprofloxacin: systemic antibiotic administration for peritoneal dialysis–associated peritonitis. Perit Dial Int 2004; 24:433–9 [PubMed] [Google Scholar]
- 205. Lima RC, Barreira A, Cardoso FL, Lima MH, Leite M., Jr Ciprofloxacin and cefazolin as a combination for empirical initial therapy of peritoneal dialysis-related peritonitis: five-year follow-up. Perit Dial Int 2007; 27:56–60 [PubMed] [Google Scholar]
- 206. Lye WC, Lee EJ, van der Straaten J. Intraperitoneal vancomycin/oral pefloxacin versus intraperitoneal vancomycin/gentamicin in the treatment of continuous ambulatory peritoneal dialysis peritonitis. Perit Dial Int 1993; 13(Suppl 2):S348–50 [PubMed] [Google Scholar]
- 207. Yeung SM, Walker SE, Tailor SA, Awdishu L, Tobe S, Yassa T. Pharmacokinetics of oral ciprofloxacin in continuous cycling peritoneal dialysis. Perit Dial Int 2004; 24:447–53 [PubMed] [Google Scholar]
- 208. Fontán MP, Cambre HD, Rodríguez–Carmona A, Muñiz AL, Falcón TG. Treatment of peritoneal dialysis–related peritonitis with ciprofloxacin monotherapy: clinical outcomes and bacterial susceptibility over two decades. Perit Dial Int 2009; 29:310–18 [PubMed] [Google Scholar]
- 209. Carmeli Y, Eliopoulos GM, Samore MH. Antecedent treatment with different antibiotic agents as a risk factor for vancomycin-resistant Enterococcus. Emerg Infect Dis 2002; 8:802–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Oprea SF, Zaidi N, Donabedian SM, Balasubramaniam M, Hershberger E, Zervos MJ. Molecular and clinical epidemiology of vancomycin-resistant Enterococcus faecalis. J Antimicrob Chemother 2004; 53:626–30 [DOI] [PubMed] [Google Scholar]
- 211. Grady R. Safety profile of quinolone antibiotics in the pediatric population. Pediatr Infect Dis J 2003; 22:1128–32 [DOI] [PubMed] [Google Scholar]
- 212. Noel GJ, Bradley JS, Kauffman RE, Duffy CM, Gerbino PG, Arguedas A, et al. Comparative safety profile of levofloxacin in 2523 children with a focus on four specific musculoskeletal disorders. Pediatr Infect Dis J 2007; 26:879–91 [DOI] [PubMed] [Google Scholar]
- 213. Sansone JM, Wilsman NJ, Leiferman EM, Conway J, Hutson P, Noonan KJ. The effect of fluoroquinolone antibiotics on growing cartilage in the lamb model. J Pediatr Orthop 2009; 29:189–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Adefurin A, Sammons H, Jacqz–Aigrain E, Choonara I. Ciprofloxacin safety in paediatrics: a systematic review. Arch Dis Child 2011; 96:874–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Chyský V, Kapila K, Hullmann R, Arcieri G, Schacht P, Echols R. Safety of ciprofloxacin in children: worldwide clinical experience based on compassionate use. Emphasis on joint evaluation. Infection 1991; 19:289–96 [DOI] [PubMed] [Google Scholar]
- 216. Pradhan KM, Arora NK, Jena A, Susheela AK, Bhan MK. Safety of ciprofloxacin therapy in children: magnetic resonance images, body fluid levels of fluoride and linear growth. Acta Paediatr 1995; 84:855–60 [DOI] [PubMed] [Google Scholar]
- 217. Kobayashi K, Nakamoto H, Okada S, Hoshitani K, Uchida K, Arima H, et al. Efficacy and safety of meropenem plus tobramycin followed by meropenem plus vancomycin for treating peritonitis in patients on continuous ambulatory peritoneal dialysis. Adv Perit Dial 2006; 22:65–8 [PubMed] [Google Scholar]
- 218. Dratwa M, Glupczynski Y, Lameire N, Matthys D, Verschraegen G, Vaneechoutte M, et al. Treatment of gram-negative peritonitis with aztreonam in patients undergoing continuous ambulatory peritoneal dialysis. Rev Infect Dis 1991; 13(Suppl 7):S645–7 [DOI] [PubMed] [Google Scholar]
- 219. Shemin D, Maaz D, St Pierre D, Kahn SI, Chazan JA. Effect of aminoglycoside use on residual renal function in peritoneal dialysis patients. Am J Kidney Dis 1999; 34:14–20 [DOI] [PubMed] [Google Scholar]
- 220. Warady BA, Reed L, Murphy G, Kastetter S, Karlsen E, Alon U, et al. Aminoglycoside ototoxicity in pediatric patients receiving long-term peritoneal dialysis. Pediatr Nephrol 1993; 7:178–81 [DOI] [PubMed] [Google Scholar]
- 221. Szeto CC, Chow KM, Leung CB, Wong TY, Wu AK, Wang AY, et al. Clinical course of peritonitis due to Pseudomonas species complicating peritoneal dialysis: a review of 104 cases. Kidney Int 2001; 59:2309–15 [DOI] [PubMed] [Google Scholar]
- 222. Burgess DS, Nathisuwan S. Cefepime, piperacillin/tazobactam, gentamicin, ciprofloxacin, and levofloxacin alone and in combination against Pseudomonas aeruginosa. Diagn Microbiol Infect Dis 2002; 44:35–41 [DOI] [PubMed] [Google Scholar]
- 223. Ariffin H, Navaratnam P, Mohamed M, Arasu A, Abdullah WA, Lee CL, et al. Ceftazidime-resistant Klebsiella pneumoniae bloodstream infection in children with febrile neutropenia. Int J Infect Dis 2000; 4:21–5 [DOI] [PubMed] [Google Scholar]
- 224. Kim YK, Pai H, Lee HJ, Park SE, Choi EH, Kim J, et al. Bloodstream infections by extended-spectrum betalactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob Agents Chemother 2002; 46:1481–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Asensio A, Oliver A, González–Diego P, Baquero F, Pérez–Díaz JC, Ros P, et al. Outbreak of a multiresistant Klebsiella pneumoniae strain in an intensive care unit: antibiotic use as risk factor for colonization and infection. Clin Infect Dis 2000; 30:55–60 [DOI] [PubMed] [Google Scholar]
- 226. Rice LB, Eckstein EC, DeVente J, Shlaes DM. Ceftazidime-resistant Klebsiella pneumoniae isolates recovered at the Cleveland Department of Veterans Affairs Medical Center. Clin Infect Dis 1996; 23:118–24 [DOI] [PubMed] [Google Scholar]
- 227. Yip T, Tse KC, Lam MF, Tang S, Li FK, Choy BY, et al. Risk factors and outcomes of extended-spectrum betalactamase-producing E. coli peritonitis in CAPD patients. Perit Dial Int 2006; 26:191–7 [PubMed] [Google Scholar]
- 228. Zanetti G, Bally F, Greub G, Garbino J, Kinge T, Lew D, et al. on behalf of the Cefepime Study Group. Cefepime versus imipenem–cilastatin for treatment of nosocomial pneumonia in intensive care unit patients: a multicenter, evaluator-blind, prospective, randomized study. Antimicrob Agents Chemother 2003; 47:3442–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Blowey DL, Garg UC, Kearns GL, Warady BA. Peritoneal penetration of amphotericin B lipid complex and fluconazole in a pediatric patient with fungal peritonitis. Adv Perit Dial 1998; 14:247–50 [PubMed] [Google Scholar]
- 230. Matuszkiewicz–Rowinska J. Update on fungal peritonitis and its treatment. Perit Dial Int 2009; 29(Suppl 2):S161–5 [PubMed] [Google Scholar]
- 231. McNeeley DJ, Vas SI, Dombros N, Oreopoulos DG. Fusarium peritonitis: an uncommon complication of CAPD. Perit Dial Bull 1981; 1:94–6 [Google Scholar]
- 232. Chang TI, Kim HW, Park JT, Lee DH, Lee JH, Yoo TH, et al. Early catheter removal improves patient survival in peritoneal dialysis patients with fungal peritonitis: results of ninety-four episodes of fungal peritonitis at a single center. Perit Dial Int 2011; 31:60–6 [DOI] [PubMed] [Google Scholar]
- 233. Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Kavanagh D, Prescott GJ, Mactier RA. Peritoneal dialysis–associated peritonitis in Scotland (1999–2002). Nephrol Dial Transplant 2004; 19:2584–91 [DOI] [PubMed] [Google Scholar]
- 235. Spare MK, Tebbs SE, Lang S, Lambert PA, Worthington T, Lipkin GW, et al. Genotypic and phenotypic properties of coagulase-negative staphylococci causing dialysis catheter-related sepsis. J Hosp Infect 2003; 54:272–8 [DOI] [PubMed] [Google Scholar]
- 236. Pickering SJ, Bowley JA, Fleming SJ, Oppenheim BA, Ralston AJ, Sissons P, et al. Urokinase for recurrent CAPD peritonitis. Lancet 1987; 1:1258–9 [DOI] [PubMed] [Google Scholar]
- 237. Williams AJ, Boletis I, Johnson BF, Raftery AT, Cohen GL, Moorhead PJ, et al. Tenckhoff catheter replacement or intraperitoneal urokinase: a randomized trial in the management of recurrent continuous ambulatory peritoneal dialysis (CAPD) peritonitis. Perit Dial Int 1989; 9:65–7 [PubMed] [Google Scholar]
- 238. Klaus G, Schäfer F, Querfeld U, Soergel M, Wolf S, Mehls O. Treatment of relapsing peritonitis in pediatric patients on peritoneal dialysis. Adv Perit Dial 1992; 8:302–5 [PubMed] [Google Scholar]
- 239. Innes A, Burden RP, Finch RG, Morgan AG. Treatment of resistant peritonitis in continuous ambulatory peritoneal dialysis with intraperitoneal urokinase: a double-blind clinical trial. Nephrol Dial Transplant 1994; 9:797–9 [PubMed] [Google Scholar]
- 240. Worland MA, Radabaugh RS, Mueller BA. Intraperitoneal thrombolytic therapy for peritoneal dialysis-associated peritonitis. Ann Pharmacother 1998; 32:1216–20 [DOI] [PubMed] [Google Scholar]
- 241. Gadallah MF, Tamayo A, Sandborn M, Ramdeen G, Moles K. Role of intraperitoneal urokinase in acute peritonitis and prevention of catheter loss in peritoneal dialysis patients. Adv Perit Dial 2000; 16:233–6 [PubMed] [Google Scholar]
- 242. Tong MK, Leung KT, Siu YP, Lee KF, Lee HK, Yung CY, et al. Use of intraperitoneal urokinase for resistant bacterial peritonitis in continuous ambulatory peritoneal dialysis. J Nephrol 2005; 18:204–8 [PubMed] [Google Scholar]
- 243. Wiggins KJ, Craig JC, Johnson DW, Strippoli GF. Treatment for peritoneal dialysis–associated peritonitis. Cochrane Database Syst Rev 2008;:CD005284 [DOI] [PubMed] [Google Scholar]
- 244. Duch JM, Yee J. Successful use of recombinant tissue plasminogen activator in a patient with relapsing peritonitis. Am J Kidney Dis 2001; 37:149–53 [DOI] [PubMed] [Google Scholar]
- 245. Zorzanello MM, Fleming WJ, Prowant BE. Use of tissue plasminogen activator in peritoneal dialysis catheters: a literature review and one center’s experience. Nephrol Nurs J 2004; 31:534–7 [PubMed] [Google Scholar]
- 246. Margetts P. Heparin and the peritoneal membrane. Perit Dial Int 2009; 29:16–19 [PubMed] [Google Scholar]
- 247. de Boer AW, Levi M, Reddingius RE, Willems JL, van den Bosch S, Schröder CH, et al. Intraperitoneal hypercoagulation and hypofibrinolysis is present in childhood peritonitis. Pediatr Nephrol 1999; 13:284–7 [DOI] [PubMed] [Google Scholar]
- 248. Nadig C, Binswanger U, von Felten A. Is heparin therapy necessary in CAPD peritonitis? Perit Dial Int 1997; 17:493–6 [PubMed] [Google Scholar]
- 249. Lamperi S, Carozzi S. Immunological defenses in CAPD. Blood Purif 1989; 7:126–43 [DOI] [PubMed] [Google Scholar]
- 250. Keane WF, Comty CM, Verbrugh HA, Peterson PK. Opsonic deficiency of peritoneal dialysis effluent in continuous ambulatory peritoneal dialysis. Kidney Int 1984; 25:539–43 [DOI] [PubMed] [Google Scholar]
- 251. Lamperi S, Carozzi S. Defective opsonic activity of peritoneal effluent during continuous ambulatory peritoneal dialysis patients: importance and prevention. Perit Dial Bull 1986; 6:87–92 [Google Scholar]
- 252. McGregor SJ, Brock JH, Briggs JD, Junor BJ. Relationship of IgG, C3 and transferrin with opsonising and bacteriostatic activity of peritoneal fluid from CAPD patients and the incidence of peritonitis. Nephrol Dial Transplant 1987; 2:551–6 [PubMed] [Google Scholar]
- 253. Poyrazoğlu HM, Düşünsel R, Patiroğlu T, Gündüz Z, Utaş C, Güneş T. Humoral immunity and frequency of peritonitis in chronic peritoneal dialysis patients. Pediatr Nephrol 2002; 17:85–90 [DOI] [PubMed] [Google Scholar]
- 254. Krediet RT, Koomen GC, Vlug A, Struijk DG, Buis B, van Olden RW, et al. IgG subclasses in CAPD patients. Perit Dial Int 1996; 16:288–94 [PubMed] [Google Scholar]
- 255. Akman S, Güven AG, Ince S, Yeğin O. IgG and IgG subclasses deficiency in children undergoing continuous ambulatory peritoneal dialysis and its provocative factors. Pediatr Int 2002; 44:273–6 [DOI] [PubMed] [Google Scholar]
- 256. Keane W, Bergeron B, Pence T, Peterson P. Challenges for continuous ambulatory peritoneal dialysis. In: Davison AM, ed. Nephrology: Proceedings of the Xth International Congress of Nephrology. London, UK: Balliere Tindall; 1987: 1255–67 [Google Scholar]
- 257. Lamperi S, Carozzi S. Peritonitis prevention in CAPD by intraperitoneal IgG. Contrib Nephrol 1989; 70:325–9 [DOI] [PubMed] [Google Scholar]
- 258. Dursun B, Tuncer M, Felek R, Ersoy FF. Benefits of low dose immunoglobulin in the treatment of refractory CAPD peritonitis and longevity of technical survival on CAPD. Int Urol Nephrol 2005; 37:565–9 [DOI] [PubMed] [Google Scholar]
- 259. Coban E, Ozdogan M, Tuncer M, Bozcuk H, Ersoy F. The value of low-dose intraperitoneal immunoglobulin administration in the treatment of peritoneal dialysis–related peritonitis. J Nephrol 2004; 17:427–40 [PubMed] [Google Scholar]
- 260. Akman S, Güven AG, Ince S, Yegin O. Effect of intraperitoneal immunoglobulin infusion on neutrophil function in CAPD children with and without peritonitis. Adv Perit Dial 1998; 14:239–42 [PubMed] [Google Scholar]
- 261. Anwar N, Hutchison AJ, Manos J, Uttley L, Brenchley P, Gokal R. Peritoneal dialysate IgG/C3 levels do not predict susceptibility to peritonitis. Perit Dial Int 1996; 16:154–7 [PubMed] [Google Scholar]
- 262. Bouts AH, Davin JC, Krediet RT, van der Weel MB, Schröder CH, Monnens L, et al. Immunoglobulins in chronic renal failure of childhood: effects of dialysis modalities. Kidney Int 2000; 58:629–37 [DOI] [PubMed] [Google Scholar]
- 263. Neu AM, Warady BA, Lederman HM, Furth SL, Fivush BA. Hypogammaglobulinemia in infants and young children maintained on peritoneal dialysis. Pediatric Dialysis Study Consortium. Perit Dial Int 1998; 18:440–3 [PubMed] [Google Scholar]
- 264. Piraino B. Peritoneal dialysis catheter replacement: “save the patient and not the catheter.” Semin Dial 2003; 16:72–5 [DOI] [PubMed] [Google Scholar]
- 265. Choi P, Nemati E, Banerjee A, Preston E, Levy J, Brown E. Peritoneal dialysis catheter removal for acute peritonitis: a retrospective analysis of factors associated with catheter removal and prolonged postoperative hospitalization. Am J Kidney Dis 2004; 43:103–11 [DOI] [PubMed] [Google Scholar]
- 266. Szeto CC, Chow KM, Wong TY, Leung CB, Wang AY, Lui SF, et al. Feasibility of resuming peritoneal dialysis after severe peritonitis and Tenckhoff catheter removal. J Am Soc Nephrol 2002; 13:1040–5 [DOI] [PubMed] [Google Scholar]
- 267. Schröder CH, Severijnen RS, de Jong MC, Monnens LA. Chronic tunnel infections in children: removal and replacement of the continuous ambulatory peritoneal dialysis catheter in a single operation. Perit Dial Int 1993; 13:198–200 [PubMed] [Google Scholar]
- 268. Lye WC, Leong SO, van der Straaten J, Lee EJ. Staphylococcus aureus CAPD-related infections are associated with nasal carriage. Adv Perit Dial 1994; 10:163–5 [PubMed] [Google Scholar]
- 269. Lui SL, Li FK, Lo CY, Lo WK. Simultaneous removal and reinsertion of Tenckhoff catheters for the treatment of refractory exit-site infection. Adv Perit Dial 2000; 16:195–7 [PubMed] [Google Scholar]
- 270. Lui SL, Yip T, Tse KC, Lam MF, Lai KN, Lo WK. Treatment of refractory Pseudomonas aeruginosa exit-site infection by simultaneous removal and reinsertion of peritoneal dialysis catheter. Perit Dial Int 2005; 25:560–3 [PubMed] [Google Scholar]
- 271. Bernardini J, Piraino B, Sorkin M. Analysis of continuous ambulatory peritoneal dialysis–related Pseudomonas aeruginosa infections. Am J Med 1987; 83:829–32 [DOI] [PubMed] [Google Scholar]
- 272. Siva B, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Pseudomonas peritonitis in Australia: predictors, treatment, and outcomes in 191 cases. Clin J Am Soc Nephrol 2009; 4:957–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Mitra A, Teitelbaum I. Is it safe to simultaneously remove and replace infected peritoneal dialysis catheters? Review of the literature and suggested guidelines. Adv Perit Dial 2003; 19:255–9 [PubMed] [Google Scholar]
- 274. Swartz R, Messana J, Reynolds J, Ranjit U. Simultaneous catheter replacement and removal in refractory peritoneal dialysis infections. Kidney Int 1991; 40:1160–5 [DOI] [PubMed] [Google Scholar]
- 275. Scalamogna A, De Vecchi A, Maccario M, Castelnovo C, Ponticelli C. Cuff-shaving procedure. A rescue treatment for exit-site infection unresponsive to medical therapy. Nephrol Dial Transplant 1995; 10:2325–7 [DOI] [PubMed] [Google Scholar]
- 276. Swartz RD, Messana JM. Simultaneous catheter removal and replacement in peritoneal dialysis infections: update and current recommendations. Adv Perit Dial 1999; 15:205–8 [PubMed] [Google Scholar]
- 277. Finkelstein ES, Jekel J, Troidle L, Gorban–Brennan N, Finkelstein FO, Bia FJ. Patterns of infection in patients maintained on long-term peritoneal dialysis therapy with multiple episodes of peritonitis. Am J Kidney Dis 2002; 39:1278–86 [DOI] [PubMed] [Google Scholar]
- 278. Singhal MK, Vas SI, Oreopoulos DG. Treatment of peritoneal dialysis catheter–related infections by simultaneous catheter removal and replacement. Is it safe? Perit Dial Int 1998; 18:565–7 [PubMed] [Google Scholar]
- 279. Majkowski NL, Mendley SR. Simultaneous removal and replacement of infected peritoneal dialysis catheters. Am J Kidney Dis 1997; 29:706–11 [DOI] [PubMed] [Google Scholar]
- 280. Goldraich I, Mariano M, Rosito N, Goldraich N. One-step peritoneal catheter replacement in children. Adv Perit Dial 1993; 9:325–8 [PubMed] [Google Scholar]
- 281. Cancarini GC, Manili L, Brunori G, Camerini C, Zubani R, Colombrita D, et al. Simultaneous catheter replacement-removal during infectious complications in peritoneal dialysis. Adv Perit Dial 1994; 10:210–13 [PubMed] [Google Scholar]
- 282. Posthuma N, Borgstein PJ, Eijsbouts Q, ter Wee PM. Simultaneous peritoneal dialysis catheter insertion and removal in catheter-related infections without interruption of peritoneal dialysis. Nephrol Dial Transplant 1998; 13:700–3 [DOI] [PubMed] [Google Scholar]
- 283. Vychytil A, Lilaj T, Lorenz M, Hörl WH, Haag–Weber M. Ultrasonography of the catheter tunnel in peritoneal dialysis patients: what are the indications? Am J Kidney Dis 1999; 33:722–7 [DOI] [PubMed] [Google Scholar]
- 284. Karahan OI, Taskapan H, Yikilmaz A, Oymak O, Utas C. Ultrasound evaluation of peritoneal catheter tunnel in catheter related infections in CAPD. Int Urol Nephrol 2005; 37:363–6 [DOI] [PubMed] [Google Scholar]
- 285. Stuart S, Booth TC, Cash CJ, Hameeduddin A, Goode JA, Harvey C, et al. Complications of continuous ambulatory peritoneal dialysis. Radiographics 2009; 29:441–60 [DOI] [PubMed] [Google Scholar]
- 286. Vychytil A, Lorenz M, Schneider B, Hörl WH, Haag–Weber M. New criteria for management of catheter infections in peritoneal dialysis patients using ultrasonography. J Am Soc Nephrol 1998; 9:290–6 [DOI] [PubMed] [Google Scholar]
- 287. Kwan TH, Tong MK, Siu YP, Leung KT, Luk SH, Cheung YK. Ultrasonography in the management of exit site infections in peritoneal dialysis patients. Nephrology (Carlton) 2004; 9:348–52 [DOI] [PubMed] [Google Scholar]
- 288. Khanna R, Twardowski ZJ. Recommendations for treatment of exit-site pathology. Perit Dial Int 1996; 16(Suppl 3):S100–4 [PubMed] [Google Scholar]
- 289. Yoshino A, Honda M, Ikeda M, Tsuchida S, Hataya H, Sakazume S, et al. Merit of the cuff-shaving procedure in children with chronic infection. Pediatr Nephrol 2004; 19:1267–72 [DOI] [PubMed] [Google Scholar]
- 290. Macchini F, Testa S, Valadè A, Torricelli M, Leva E, Ardissino G, et al. Conservative surgical management of catheter infections in children on peritoneal dialysis. Pediatr Surg Int 2009; 25:703–7 [DOI] [PubMed] [Google Scholar]
- 291. Brulez HF, Verbrugh HA. First-line defense mechanisms in the peritoneal cavity during peritoneal dialysis. Perit Dial Int 1995; 15(Suppl 7):S24–33 [PubMed] [Google Scholar]
- 292. McGregor SJ, Brock JH, Briggs JD, Junor B. Longitudinal study of peritoneal defense mechanisms in patients on continuous ambulatory peritoneal dialysis (CAPD). Perit Dial Int 1989; 9:115–19 [PubMed] [Google Scholar]
- 293. de Fijter CW, Verbrugh HA, Oe LP, Peters ED, van der Meulen J, Donker AJ, et al. Peritoneal defense in continuous ambulatory versus continuous cyclic peritoneal dialysis. Kidney Int 1992; 42:947–50 [DOI] [PubMed] [Google Scholar]
- 294. Lewis S, Holmes C. Host defense mechanisms in the peritoneal cavity of continuous ambulatory peritoneal dialysis patients. Perit Dial Int 1991; 11:14–21 [PubMed] [Google Scholar]
- 295. Jörres A, Topley N, Witowski J, Liberek T, Gahl GM. Impact of peritoneal dialysis solutions on peritoneal immune defense. Perit Dial Int 1993; 13(Suppl 2):S291–4 [PubMed] [Google Scholar]
- 296. Cameron JS. Host defences in continuous ambulatory peritoneal dialysis and the genesis of peritonitis. Pediatr Nephrol 1995; 9:647–62 [DOI] [PubMed] [Google Scholar]
- 297. Posthuma N, ter Wee P, Donker AJ, Dekker HA, Oe PL, Verbrugh HA. Peritoneal defense using icodextrin or glucose for daytime dwell in CCPD patients. Perit Dial Int 1999; 19:334–42 [PubMed] [Google Scholar]
- 298. Pajek J, Kveder R, Bren A, Gucek A, Ihan A, Osredkar J, et al. Short-term effects of a new bicarbonate/lactate–buffered and conventional peritoneal dialysis fluid on peritoneal and systemic inflammation in CAPD patients: a randomized controlled study. Perit Dial Int 2008; 28:44–52 [PubMed] [Google Scholar]
- 299. Gokal R, Alexander S, Ash S, Chen TW, Danielson A, Holmes C, et al. Peritoneal catheters and exit-site practices toward optimum peritoneal access: 1998 update. (Official report from the International Society for Peritoneal Dialysis). Perit Dial Int 1998; 18:11–33 [PubMed] [Google Scholar]
- 300. Kaplan RA, Alon V, Hellerstein S, Warady BA. Unusual causes of peritonitis in three children receiving peritoneal dialysis. Perit Dial Int 1993; 13:60–3 [PubMed] [Google Scholar]
- 301. Borg D, Shetty A, Williams D, Faber MD. Fivefold reduction in peritonitis using a multifaceted continuous quality initiative program. Adv Perit Dial 2003; 19:202–5 [PubMed] [Google Scholar]
- 302. Diaz–Buxo JA, Wick GS, Pesich AA. Using CQI techniques for managing infections in PD patients. Nephrol News Issues 1998; 12:22–4 [PubMed] [Google Scholar]
- 303. Schaefer F, Kandert M, Feneberg R. Methodological issues in assessing the incidence of peritoneal dialysis–associated peritonitis in children. Perit Dial Int 2002; 22:234–8 [PubMed] [Google Scholar]
- 304. Roberts DM, Fernando G, Singer RF, Kennedy KJ, Lawrence M, Talaulikar G. Antibiotic stability in commercial peritoneal dialysis solutions: influence of formulation, storage and duration. Nephrol Dial Transplant 2011; 26:3344–9 [DOI] [PubMed] [Google Scholar]
- 305. Williamson JC, Volles DF, Lynch PL, Rogers PD, Haverstick DM. Stability of cefepime in peritoneal dialysis solution. Ann Pharmacother 1999; 33:906–9 [DOI] [PubMed] [Google Scholar]
- 306. de Vin F, Rutherford P, Faict D. Intraperitoneal administration of drugs in peritoneal dialysis patients: a review of compatibility and guidance for clinical use. Perit Dial Int 2009; 29:5–15 [PubMed] [Google Scholar]
- 307. Voges M, Faict D, Lechien G, Taminne M. Stability of drug additives in peritoneal dialysis solutions in a new container. Perit Dial Int 2004; 24:590–5 [PubMed] [Google Scholar]