Abstract
Objective
To describe India’s National Antimalarial Drug Resistance Monitoring System, measure the efficacy of first-line malaria treatments, and determine risk factors for treatment failure.
Methods
In 2009–2010, prospective studies with 28 days of follow-up were conducted at 25 sentinel sites. Patients infected with Plasmodium falciparum were given artesunate plus sulfadoxine-pyrimethamine (AS+SP); those infected with P. vivax were given chloroquine. Polymerase chain reaction was used to distinguish post-treatment reinfection from treatment failure. Isolates of P. falciparum were checked for dhfr and dhps mutations.
Findings
Overall, 1664 patients were enrolled. Kaplan–Meier survival analysis showed an efficacy of 98.8% for AS+SP. Most patients with P. falciparum parasitaemia cleared their parasitaemias within 24 hours of treatment initiation, but six, including four with treatment failure, remained parasitaemic after 72 hours. Double mutants in dhfr were found in 68.4% of the genotyped isolates. Triple or quadruple mutants in dhfr and mutations in dhps were rare. A daily dose of artesunate of < 3 mg per kg of body weight, age of less than 5 years, and fever at enrolment were associated with an increased risk of treatment failure. Chloroquine remained 100% efficacious and generally cleared P. vivax parasitaemias within 48 hours. Vomiting (seen in 47 patients) was the most common adverse event.
Conclusion
India’s National Antimalarial Drug Resistance Monitoring System provides wide coverage. The first-line antimalarials used in the country remain safe and efficacious. The treatment of malaria in young children and the relative benefits of age- and weight-based dosing need further exploration.
Résumé
Objectif
Décrire le système national indien de surveillance de la résistance aux médicaments antipaludiques, mesurer l'efficacité des traitements antipaludiques de première ligne et déterminer les facteurs de risque de l'échec du traitement.
Méthodes
En 2009-2010, des études prospectives, avec 28 jours de suivi, ont été menées dans 25 sites sentinelles. On a administré aux patients infectés par le Plasmodium falciparum de l'artésunate et de la sulfadoxine-pyriméthamine (AS+SP). Ceux qui étaient infectés par le P. vivax ont reçu de la chloroquine. La réaction en chaîne par polymérase a été utilisée pour distinguer la réinfection après traitement de l'échec du traitement. Des isolats de P. falciparum ont été vérifiés pour déceler les mutations de dhfr et de dhps.
Résultats
Un total de 1664 patients ont participé à l'étude. L'analyse de survie de Kaplan-Meier a montré une efficacité de 98,8% de l'AS+SP. La plupart des patients infectés par le P. falciparum ont vu disparaître leurs parasitémies dans les 24 heures suivant le début du traitement, mais 6, dont 4 avec échec du traitement, sont restés parasitémiques après 72 heures. Des doubles mutations de dhfr ont été trouvées dans 68,4% des isolats génotypés. Les triples ou quadruples mutations de dhfr et les mutations de dhps étaient rares. Une dose quotidienne d'artésunate inférieure à 3 mg par kg de poids corporel, un âge inférieur à 5 ans, et de la fièvre au moment de l'enregistrement ont été associés à un risque accru d'échec thérapeutique. La chloroquine restait efficace à 100% et faisait généralement disparaître les parasitémies de P. vivax dans les 48 heures. Les vomissements (observés chez 47 patients) étaient l'effet indésirable le plus fréquent.
Conclusion
En Inde, le système national de surveillance de la résistance aux médicaments antipaludiques offre une large couverture. Les antipaludéens de première ligne utilisés dans le pays restent sûrs et efficaces. Le traitement du paludisme chez les jeunes enfants et les avantages relatifs de la posologie en fonction de l'âge et du poids nécessitent une étude plus poussée.
Resumen
Objetivo
Describir el sistema nacional de seguimiento de la resistencia a los fármacos contra el paludismo en India, medir la eficacia de los tratamientos de primera línea contra el paludismo y determinar los factores de riesgo en el fracaso del tratamiento.
Métodos
En los años 2009-2010, se llevaron a cabo estudios prospectivos con un seguimiento de 28 días en 25 sitios centinela. Se proporcionó artesunato con sulfadoxina-pirimetamina (AS+SP) a los pacientes infectados con Plasmodium falciparum, y cloroquina a los pacientes infectados con P. vivax . Se empleó la reacción en cadena de la polimerasa para distinguir la reinfección postratamiento del fracaso del tratamiento y se examinaron aislados de P. falciparum en busca de mutaciones de DHFR y DHPS.
Resultados
Se inscribieron un total de 1664 pacientes. Los análisis de supervivencia Kaplan-Meier mostraron una eficacia del 98,8% para el AS+SP. En la mayoría de pacientes con parasitemia por P. falciparum, ésta despareció en las 24 horas posteriores al inicio del tratamiento. Solo seis, incluidos los cuatro en los que el tratamiento fracasó, siguieron teniendo parasitemia pasadas 72 horas. Se encontraron mutantes dobles en DHFR en el 68,4% de los aislados genotipados. No fue frecuente encontrar mutantes en DHPS ni mutantes triples o cuádruples en DHFR. Una dosis diaria de artesunato de < 3 mg por kg de peso corporal, una edad inferior a 5 años y fiebre en el momento de la inscripción se asociaron con un riesgo superior de fracaso del tratamiento. La cloroquina fue eficaz en el 100% de los casos y eliminó, por lo general, las parasitemias por P. vivax en un plazo de 48 horas. El acontecimiento adverso más frecuente fueron los vómitos (presentes en 47 pacientes).
Conclusión
El sistema nacional de seguimiento de la resistencia a los fármacos contra el paludismo en India proporciona una cobertura amplia. Los fármacos antipalúdicos empleados en primera línea en el país continúan siendo seguros y eficaces. Se debe seguir examinando el tratamiento del paludismo en los niños pequeños y las ventajas relativas de la edad y las dosis basadas en el peso corporal.
ملخص
الغرض
وصف النظام الوطني لرصد مقاومة الأدوية المضادة للملاريا في الهند وقياس نجاعة علاجات الخط الأول للملاريا وتحديد عوامل الاختطار الخاصة بفشل العلاج.
الطريقة
في الفترة من 2009 إلى 2010، تم إجراء دراسات استطلاعية بلغت مدة متابعتها 28 يوماً في 25 موقعاً خافراً. وتم إعطاء المرضى المصابين بعدوى المتصورة المنجلية الأرتيسونات بالإضافة إلى السلفاديوكسين – بيريميثامين (AS+SP)؛ وتم إعطاء المصابين بالمتصورة النشيطة الكلوروكين. وتم استخدام تفاعل البوليميراز المتسلسل لتمييز معاودة الإصابة عقب العلاج عن الفشل في العلاج. وتم فحص مستفردات المتصورة المنجلية من أجل طفرات هيدروفوليت ريدكتيز وسينثاز دايهيدوربتيرويت.
النتائج
بشكل عام، تم تسجيل 1664 مريضاً. وتبين من تحليل كابلان – ماير للبقاء على قيد الحياة نجاعتها بنسبة 98.8 % بالنسبة للأرتيسونات بالإضافة إلى مادة السلفاديوكسين – بيريميثامين. وتم التخلص من تطفلن الدم لدى معظم المرضى المصابين بتطفلن دم المتصورة المنجلية في غضون 24 ساعة من بدء العلاج ولكن ظل تطفلن الدم لدى ستة أشخاص، من بينهم أربعة فشلوا في العلاج بعد 72 ساعة. ووجدت طافرات مضاعفة في إنزيم هيدروفوليت ريدكتيز لدى 68.4 % من المستفردات ذات الأنماط الجينية. وكانت الطافرات الثلاثية أو الرباعية في إنزيم هيدروفوليت ريدكتيز والطافرات في إنزيم سينثاز دايهيدوربتيرويت نادرة. وكانت جرعة الأرتيسونات اليومية الأقل من 3 ملجم لكل كيلوجرام من وزن الجسم، والسن الأقل من 5 سنوات، والحمى لدى التسجيل، مرتبطة بزيادة خطورة الفشل في العلاج. وظل الكلوروكين ناجعاً بنسبة 100 %، وعموماً تم التخلص من تطفلن دم المتصورة النشيطة في غضون 48 ساعة. وكان القيء (الذي شوهد لدى 47 مريضاً) أكثر الأحداث الضائرة شيوعاً.
الاستنتاج
يوفر النظام الوطني لرصد مقاومة الأدوية المضادة للملاريا في الهند تغطية واسعة. وتظل أدوية الخط الأول المضادة للملاريا المستخدمة في البلد آمنة وناجعة. ويحتاج علاج الملاريا لدى صغار الأطفال والفوائد النسبية للجرعة المستندة على العمر والوزن إلى مزيد من البحث..
摘要
目的
描述印度国家抗疟药物耐药性的监测系统,测量一线疟疾治疗的疗效,并确定治疗失败的风险因素。
方法
2009-2010 年,在25 个监测哨点开展前瞻性研究,并进行28 天的随访。对感染恶性疟原虫的患者给予青蒿琥酯联合乙胺嘧啶(AS+SP)药物治疗;对感染间日疟原虫的患者提供氯喹药物治疗。使用聚合酶链反应区分治疗后的再感染和治疗失败。检查恶性疟原虫菌株,了解是否有dhfr和dhps突变。
结果
总计有1664 例患者参加。Kaplan-Meier生存分析显示,AS+SP治疗具有98.8%的疗效。多数感染恶性疟原虫的患者在开始治疗的24 小时内消除血症,但有6 例(其中包括4 例治疗失败的患者)在74 小时后血症依然不消。在68.4%的基因型菌株中发现dhfr双突变。三重或四重dhfr突变和dhps突变则罕见。每千克体重<3 毫克的青蒿琥酯每日剂量、年龄小于5 岁以及在参加时发热等因素与更高的治疗失败风险有关。氯喹保持100%疗效,一般在48 小时内清除间日疟原虫血症。呕吐(47 例)是最常见的副作用。
结论
在印度,监测抗疟药物耐药性的国家系统覆盖面广。国内一线抗疟药物依然保持安全和有效。幼儿疟疾治疗和基于年龄及体重计算剂量的相对好处需要进一步探索。
Резюме
Цель
Описание национальной системы мониторинга устойчивости к противомалярийным препаратам в Индии, оценка эффективности первой линии лечения малярии, определение факторов риска неэффективности лечения.
Методы
В 2009-2010 годах на 25 участках эпиднадзора были проведены перспективные исследования, включающие принятие последующих мер в течение 28 дней. Пациентам, инфицированным Plasmodium falciparum, назначался артезунат в сочетании с сульфадоксином-пириметамином (AS+SP); пациентам, инфицированным P. vivax , назначался хлорохин. Для различения между повторным заражением после проведенного лечения и неэффективным лечением был использован метод полимеразной цепной реакции. Изоляты вируса P. falciparum были проверены на мутации dhfr и dhps.
Результаты
Всего в обследование было включено 1664 пациентов. Анализ выживаемости Каплана-Мейера для лечения препаратами AS+SP показал эффективность 98,8%. У большинства пациентов паразитемия типа P. falciparum была устранена в течение 24 часов после начала лечения, а 6 пациентов, в том числе 4 с неудачным лечением, остались зараженными после 72 часов. Двойные мутации в dhfr были обнаружены в 68.4% генотипированных изолятов. Тройные и четвертные мутации в dhfr и мутации в dhps встречались редко. Суточная доза артезуната <3 мг на кг массы тела, возраст менее 5 лет и лихорадка при начале лечения были связаны с повышенным риском неудачи лечения. Хлорохин продолжал показывать эффективность 100% и обычно позволял устранить паразитамию P. vivax в течение 48 часов. Рвота (наблюдалась у 47 пациентов) была самым частым побочным эффектом.
Вывод
Национальная система мониторинга лекарственной устойчивости к противомалярийным препаратам в Индии обеспечивает широкий охват. Противомалярийные препараты первой линии, используемые в стране, показали себя как безопасные и эффективные. Методы лечения малярии у детей раннего возраста и относительные преимущества дозировок, основанных на возрасте и весе, нуждаются в дальнейшем исследовании.
Introduction
Resistance to antimalarial drugs in the parasites that cause malaria is hampering the effective global control of the disease in humans. Plasmodium falciparum rapidly develops resistance to new therapies, and the presence in south-eastern Asia of strains of this parasite that are resistant to many drugs, including artemisinin, is alarming.1 P. vivax can develop resistance to chloroquine and is innately less susceptible to antifolate compounds than P. falciparum, and the sensitivity of the parasite to primaquine during its dormant stages is difficult to assess.2 The long-term public health response to the problem of antimalarial drug resistance must include a reduction in drug pressure through a rational approach to treatment and the creation of a pipeline for the identification and production of alternative drugs. The routine management of parasite resistance is based on the monitoring of the efficacies of current therapies and the subsequent selection of appropriate treatments based on the data collected. Several monitoring techniques are used to inform policy-makers, including in vitro tests of drug sensitivity, pharmacokinetic studies, investigation of the molecular markers of drug resistance, and, as the “gold standard”, in vivo trials of therapeutic efficacy.3
In India the national government has monitored antimalarial drug resistance over many decades. Although chloroquine-resistant P. falciparum was first reported near the India–Myanmar border in 1973,4 chloroquine-resistant P. vivax was unknown in India until 1995, when two cases of infection with resistant P. vivax were detected in Mumbai.5 In 1978, the National Malaria Eradication Programme (now the National Vector Borne Disease Control Programme, or NVBDCP) created six regional monitoring teams.6 For many years, the Malaria Research Centre (now the National Institute of Malaria Research, or NIMR) and other organizations supported a wide range of monitoring efforts in addition to the routine work of these regional teams.7 Between 1978 and 2007, at least 380 in vivo trials of chloroquine and/or sulfadoxine-pyrimethamine for the treatment of P. falciparum malaria were conducted in India, with involvement of almost 19 000 patients.8 Worryingly, the median percentage of cases failing to show an adequate response to sulfadoxine-pyrimethamine within 28 days of treatment increased from 7.7% in 1984–1996 to 25.9% in 1997–2007.8 Indian isolates of P. falciparum were also frequently found to carry mutations in the genes that code for the targets of sulfadoxine and pyrimethamine: dihydropteroate synthase (dhps) and dihydrofolate reductase (dhfr), respectively.9 In 2005, the combination of artesunate with sulfadoxine-pyrimethamine (AS+SP) replaced sulfadoxine-pyrimethamine as the nationally recommended first-line treatment for P. falciparum malaria in India.10 While the efficacy of AS+SP, again measured after 28 days of follow-up, was found to be high (96–100%) in nine studies conducted in India between 2005 and 2007, the numbers of cases investigated were quite small given the large size of the country.3 A major concern is that, since the efficacy and lifespan of artemisinin combination therapies depend largely on the partner drug, any pre-existing resistance to sulfadoxine-pyrimethamine could endanger the new combination.
To address the continued problem of antimalarial drug resistance in India, a joint NVBDCP–NIMR surveillance system – the National Antimalarial Drug Resistance Monitoring System – was set up in 2008. This system has several innovations: (i) only about 50% of the sites are monitored each year (so that each site is monitored every 2 years, there is widespread coverage and information on long-term trends can be collected); (ii) P. vivax trials are routinely conducted to track the emergence of chloroquine resistance in this species; (iii) blood smear examinations and data analysis undergo central quality control; (iv) routine genotyping is performed to separate post-treatment reinfections from any recrudescent infections resulting from treatment failures; (v) molecular markers of drug resistance are genotyped simultaneously; and (vi) in-vivo trials of drug efficacy are integrated with supplementary studies, such as the evaluation of plasma drug concentrations and other pharmacokinetic parameters. The focus of the present study was on the data collected, nationwide, during the first 2 years of the new surveillance system’s operation. These data were used to evaluate the efficacies of AS+SP against P. falciparum and of chloroquine against P. vivax , to determine the prevalences of several molecular markers of sulfadoxine-pyrimethamine resistance in P. falciparum (and so assess, independently, the probable efficacy of the “partner drug” in the artemisinin combination therapy) and to determine the clinical, demographic and/or parasite-related risk factors for treatment failure.
Methods
Study sites and subjects
Through its extensive surveillance system, which screens more than 100 million people in India for malaria each year, the NVBDCP identified 1.6 million malaria cases in 2009 and 2010.11 In 2008, during an annual review of data on antimalarial drug resistance, the NIMR and NVBDCP selected 25 sentinel sites for the routine monitoring of such resistance: 24 districts (each with a population of 1 to 4 million) and one city (Appendix A, available at: http://mrcindia.org/appendix_a.pdf). These sites were purposively selected to provide a representative “cross-section” of transmission intensities, malaria ecotypes and geographical regions. Open-label, single-arm prospective studies have since been conducted in each of the sites in accordance with the World Health Organization (WHO) 2009 protocol for a therapeutic efficacy trial with a target sample size of at least 50 patients per site.12 Patients with P. falciparum monoinfection (500–100 000 asexual parasites per μl of blood) or P. vivax monoinfection (> 250 asexual parasites per μl) and either current fever (i.e. an axillary temperature of ≥ 37.5 °C) or a history of fever over the preceding 24 hours were enrolled in these studies. Pregnant or lactating women, children weighing < 5 kg, patients with signs of severe malaria, adults who declined to give informed consent and children with legal guardians who declined to give consent were all excluded.
Data collection, treatment and follow-up
At enrolment (i.e. on “day 0”), a complete medical history (i.e. presenting symptoms, current medications and previous antimalarial drug use) was recorded for each patient. Female patients of child-bearing age were tested for pregnancy. A pre-treatment blood sample was collected from each eligible patient and used to make thick and thin smears and dried blood spots on filter paper. Patients were then given doses of an antimalarial drug according to their age, as recommended in the national drug policy.10 Patients infected with P. falciparum were given AS+SP (the recommended dose is 4 mg artesunate per kg of body weight, given daily for 3 days, plus a single dose of sulfadoxine-pyrimethamine that is equivalent to 25 mg sulfadoxine per kg and 1.25 mg pyrimethamine per kg). Patients infected with P. vivax received chloroquine for 3 days (the total recommended dose is 25 mg mg per kg, given over 3 days) and then, after completing the 28 days of follow-up, primaquine for 14 days (the recommended daily dose is 0.25 mg per kg). The drug doses administered, which were based on age rather than body weight, may not have matched the recommended doses. The drug tablets used were quality-assured and obtained via the relevant state government. The intake of each treatment dose was observed, and patients who vomited within 30 minutes of a dose were retreated with the same drug and dose. Patients who did not complete treatment were withdrawn from the study. Patients were followed up on days 1, 2, 3, 7, 14, 21 and 28 in the clinics where they were treated, their residences or places of work. Patients were recommended to return to a clinic at any time if they experienced fever or other symptoms. At each follow-up visit, each patient was given a physical examination and a fresh sample of blood was collected and used to make both dried blood spots on filter paper and smears on a microscope slide.
Laboratory procedures
Each blood sample was used to make a thick and a thin smear on a single slide. The smears were Giemsa-stained. The level of parasitaemia (i.e. the number of asexual parasites per μl of blood) was determined from the thick smears by counting the asexual parasites against 200 leukocytes and assuming that each patient had 8000 leukocytes per μl of blood. Slides were declared negative if no parasites were detected in 100 high-power fields. Technicians with more than 10 years’ experience rechecked the smears from all patients with treatment failure, plus a 10% random sample of all other smears from each sentinel site, at the NIMR’s central quality control unit in New Delhi. Whenever parasitaemia was detected in a patient who had previously been found to be aparasitaemic, the malaria parasites in that patient’s dried blood samples from day 0 and from the day of apparent recrudescence were genotyped by polymerase chain reaction (PCR) to enable post-treatment reinfection to be distinguished from treatment failure.13 The dried day-0 blood samples from patients who failed treatment and from 25% of other patients selected at random were also checked for mutations in dhfr (at codons 51, 59, 108 and 164) and dhps (at codons 436, 437 and 540), using the primers described earlier.14
Trial end-points
Primary study end-points were classified according to the standard criteria defined in WHO’s current guidelines.12 Parasite clearance intervals (categorized as ≤ 24, > 24 to 48, > 48 to 72 or > 72 hours) were used as a secondary end-point, with an interval of > 72 hours used as the indicator of risk in the analysis of risk factors.
Data analysis
Patient and demographic factors that could be associated with treatment failure were investigated. Using the body weights recorded on day 0, the doses of artesunate given to patients with P. falciparum were categorized as being ≥ 87.5%, < 87.5% to 75% and < 75% of the recommended dose per kg. Kaplan–Meier survival analyses of treatment failure and parasite clearance were conducted with and without including the results of the PCR-based identification of the parasitaemias that resulted from post-treatment reinfection. Log-risk models were used to evaluate the univariate and multivariate associations observed between risk factors and end-points. Multivariate analysis was based on full models that included age, geographical area, sex, fever at enrolment, level of parasitaemia at enrolment, infection with a parasite that harboured any of the investigated mutations in dhfr or dhps, and artesunate dose, as well as the interactions between age and artesunate dose, age and level of parasitaemia on enrolment, and between presence of mutations and level of parasitaemia on enrolment. A strategy of backward elimination was followed in which P-values of < 0.10 and < 0.15 were used, respectively, as the cut-offs in eliminating individual factors and interaction terms as statistically significant risk factors for treatment failure. To account for the clustering of data by study site, clustered robust standard errors were used in the multivariate models. All data analysis was performed using version 7.2 of a software package developed by WHO’s Global Malaria Programme for evaluating therapeutic efficacy (WHO, Geneva, Switzerland) or Stata version 10 (StataCorp. LP, College Station, United States of America).
Ethics
Informed, written consent was obtained from each adult enrolled and from a legal guardian of each child enrolled. The study protocol was approved by the Government of India’s Ministry of Health and Family Welfare and by the Ethics Committee of the National Institute of Malaria Research in New Delhi.
Results
Patient enrolment and characteristics
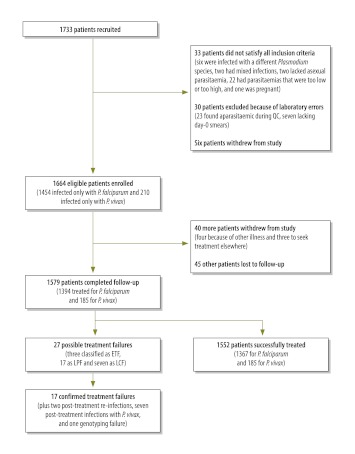
Overall, 1733 patients were enrolled: 895 patients were from 13 sentinel sites between June 2009 and January 2010; 838 were from the 12 other sites, between June 2010 and December 2010 (Fig. 1). Some of these patients subsequently withdrew their consent or were found not to satisfy the inclusion criteria. This left 1664 eligible patients, 1579 (94.8%) of whom completed the 28 days of follow-up (Fig. 1). Most of the patients investigated were men who were febrile at the time of enrolment (Table 1). A history of fever was nearly universal but the intake of antimalarial drugs in the previous week was rare. The geometric mean of the level of asexual parasitaemia on day 0 was 7975 parasites per μl (range: 560–99 707) for P. falciparum and 7133 parasites per μl (range: 378–123 317) for P. vivax .
Fig. 1.
Flowchart for patient recruitment and follow-up in the efficacy trials within India’s National Antimalarial Drug Resistance Monitoring System, 2009–2010
ETF, early treatment failure; LCF, late clinical failure; LPF, late parasitological failure; QC, quality control.
Table 1. Characteristics of malaria patients enrolled in studies conducted under India’s National Antimalarial Drug Resistance Monitoring System, 2009–2010.
| Characteristic | No. (%) of patients infected |
|
|---|---|---|
| Plasmodium falciparum | Plasmodium vivax | |
| Area | ||
| Central | 829 (57.0) | 0 |
| Western | 391 (26.9) | 55 (26.2) |
| North-eastern | 234 (16.1) | 0 |
| Southern | 0 | 155 (73.8) |
| Season | ||
| Monsoon | 415 (28.5) | 115 (54.8) |
| Post-monsoon | 645 (44.4) | 95 (45.2) |
| Winter | 394 (27.1) | 0 |
| Sex | ||
| Male | 835 (57.4) | 172 (81.9) |
| Female | 619 (42.6) | 38 (18.1) |
| Age (years) | ||
| < 1 | 2 (0.1) | 0 |
| 1–4 | 120 (8.3) | 2 (1.0) |
| 5–9 | 290 (19.9) | 14 (6.7) |
| 10–14 | 252 (17.3) | 18 (8.6) |
| 15–49 | 689 (47.4) | 154 (73.3) |
| ≥ 50 | 101 (7.0) | 22 (10.4) |
| Parasitaemia (asexual parasites/μl)a | ||
| < 1 000 | 14 (1.0) | 10 (4.8) |
| 1000–4999 | 548 (37.7) | 69 (32.9) |
| 5000–9999 | 251 (17.3) | 42 (20.0) |
| 10 000–49 999 | 526 (36.2) | 83 (39.5) |
| ≥ 50 000 | 115 (7.8) | 6 (2.8) |
| Febrile (≥ 37.5 °C) | ||
| No | 451 (31.0) | 63 (30.1) |
| Yes | 1003 (69.0) | 146 (69.9) |
| History of fever | ||
| No | 31 (2.1) | 4 (1.9) |
| Yes | 1423 (97.9) | 206 (98.1) |
| Previous antimalarial drug intakeb | ||
| No | 1428 (98.2) | 207 (98.6) |
| Yes | 9 (0.6) | 0 (0.0) |
| Unknown | 17 (1.2) | 3 (1.4) |
a At enrolment, before treatment.
b No data were available for 25 patients enrolled at one sentinel site.
Plasmodium falciparum
Primary and secondary end-points
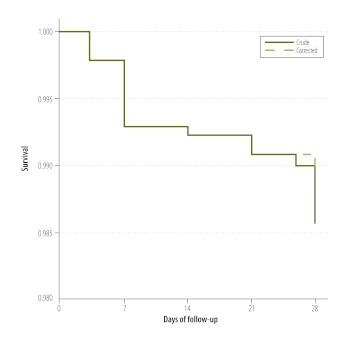
Of the 1394 patients infected with P. falciparum who completed follow-up (Table 2), 27 were initially categorized as treatment failures. However, when parasites in paired dried blood spots from these 27 patients were genotyped by PCR, only 17 (63%) of the patients were confirmed as treatment failures. Of the other 10 patients, 7 were found to have developed P. vivax parasitaemia during follow-up, 2 were found to have P. falciparum reinfection on day 28, and one had an untypeable P. falciparum parasitaemia on day 28. The 17 patients who were confirmed to have failed AS+SP treatment came from 10 different sentinel sites. Two early treatment failures occurred in Betul district (Madhya Pradesh); both patients had parasitaemia and fever on day 3. Another occurred in Angul (Odisha) in a patient whose level of parasitaemia on day 3 was more than 25% higher than on day 0. In Gadchiroli district (Maharashtra), five patients failed treatment on day 7. The crude and PCR-corrected Kaplan–Meier survival estimates were 98.6% (95% confidence interval, CI: 97.8–99.1) and 98.8% (95% CI: 98.1–99.3), respectively (Fig. 2). Among the 25 sentinel sites, the PCR-corrected survival estimates varied from 93.8% to 100% (Appendix A). In general, the P. falciparum patients treated with AS+SP cleared their parasitaemias rapidly; 62% were aparasitaemic within 24 hours of the first dose (Table 3). Overall, 0.7% (6 of 873), 1.3% (5 of 384) and 1.3% (2 of 154) of the patients who showed parasite clearance intervals of ≤ 24 hours, > 24 to 48 hours and > 48 to 72 hours, respectively, were identified as treatment failures. Six patients infected with P. falciparum (including four from Betul) did not clear their parasitaemias within 72 hours. Four (67%) of them were identified as treatment failures (including three early treatment failures), whereas the other two achieved cure. In general, the AS+SP treatment was well tolerated. The most common adverse events reported during follow-up were vomiting, fever and jaundice, which were seen in 46 patients, three patients and one patient, respectively
Table 2. Treatment outcomes among eligible malaria patients in studies conducted under India’s National Antimalarial Drug Resistance Monitoring System, 2009–2010.
| Outcome after 28 days | No. (%) of patients infected |
|
|---|---|---|
| Plasmodium falciparum | Plasmodium vivax | |
| Primary classification | ||
| Adequate clinical and parasitological responsea | 1367 (94.0) | 185 (88.1) |
| Early treatment failureb | 3 (0.2) | 0 (0.0) |
| Late clinical failurec | 7 (0.5) | 0 (0.0) |
| Late parasitological failured | 17 (1.2) | 0 (0.0) |
| Lost to follow-up | 33 (2.3) | 12 (5.7) |
| Withdrawal after day 0 | 27 (1.8) | 13 (6.2) |
| PCR-corrected results | ||
| Recrudescence | 17 (63.0) | – |
| Reinfection | 2 (7.4) | – |
| Infection with other species | 7 (25.9) | – |
| Unknown | 1 (3.7) | – |
| Parasite clearance interval (h) | ||
| ≤ 24 | 873 (61.6) | 20 (10.5) |
| > 24 to 48 | 384 (27.1) | 88 (45.8) |
| > 48 to 72 | 154 (10.9) | 78 (40.6) |
| > 72 | 6 (0.4) | 6 (3.1) |
PCR, polymerase chain reaction.
a Absence of parasitaemia on day 28 without previous criteria for failure.
b Marked by the following, alone or in combination: danger signs for severe malaria on days 1, 2 or 3 in the presence of parasitaemia; parasitaemia level on day 2 higher than on day 0; parasitaemia level on day 3 more than 25% higher than on day 0; parasitaemia on day 3 plus fever.
c Danger signs for severe malaria and/or fever plus parasitaemia from day 4 to day 28.
d Parasitaemia from day 7 to day 28 even if patient afebrile.
Fig. 2.
Crude and corrected Kaplan–Meier survival curves, India, 2009–2010
Note: The data used to create these curves came from patients with Plasmodium falciparum infection who had been treated with a combination of artesunate and sulfadoxine-pyrimethamine. The corrected data reflect the results of using a polymerase chain reaction to separate recrudescences resulting from treatment failure from post-treatment reinfections with P. falciparum.
Table 3. Predictors of treatment failure among patients in studies conducted under India’s National Antimalarial Drug Resistance Monitoring System, India, 2009–2010.
| Predictor | RRa (95% CI) |
|---|---|
| Artesunate dose (mg/kg)b | |
| ≥ 3.5 | – |
| 3.0 to < 3.5 | 2.45 (0.58–10.3) |
| < 3.0 | 6.10 (1.04–35.8) |
| Age (years) | |
| < 5 | 4.46 (1.35–14.7) |
| ≥ 5 | – |
| Parasitaemia (asexual parasites/μl) | |
| ≥ 5 000 | 0.47 (0.22–1.00) |
| < 5 000 | – |
| Fever at enrolment (≥ 37.5 °C) | |
| Yes | 3.94 (1.40–11.1) |
| No | – |
RR, relative risk; CI, confidence interval.
a Relative risk for the failure of treatment of Plasmodium falciparum infection with a combination of artesunate and sulfadoxine-pyrimethamine, as estimated in a multivariate analysis.
b The dose given per day.
Molecular markers of drug resistance
All but 31 of the 373 P. falciparum isolates genotyped for dhfr were successfully amplified. Single (n = 65) and double mutations (n = 234) in this gene were common. The most frequent haplotype was the double mutant 108/59, followed by single mutant 108 (Appendix A). In terms of the dhfr codons investigated, only 10 isolates were identified as triple mutants and only one was found to be a quadruple mutant. The typing of dhps proved more difficult and was only successful for 261 of the 373 isolates investigated. Mutations in dhps were only detected in six (2.3%) of the isolates that were successfully investigated; five isolates carried single mutations and one carried a double mutation, with a predominance of mutations in codon 437. When the day-0 samples from the 17 confirmed treatment failures were investigated, one sample did not amplify, 12 samples were found to be dhfr double mutants, four were found to be dhfr single mutants and 16 were found to be wild-type for dhps.
Predictors of treatment failure and parasite clearance interval
In both the univariate (Appendix A) and multivariate analyses (Table 3), an age of < 5 years (relative to an age of ≥ 15 years) and a daily dose of artesunate of either < 3 or 3 to < 3.5 mg per kg (relative to higher doses) were each significantly associated with treatment failure. In the multivariate analysis, fever at enrolment was also positively and significantly associated with treatment failure (Table 3). The risk of failure increased as the dose of artesunate decreased. A day-0 level of parasitaemia ≥ 5000 asexual parasites per µl was associated with a reduced risk of treatment failure. No association was detected between infection with parasites harbouring three or more mutations in the genes investigated (i.e. dhfr and dhps) and failure of AS+SP treatment. There were insufficient observations to determine the risk factors for a parasite clearance interval of > 72 hours.
Plasmodium vivax
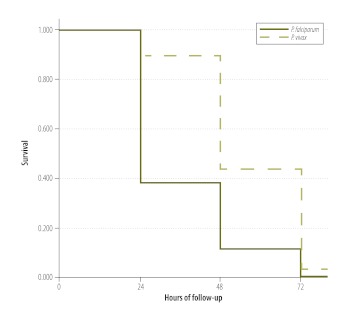
In three trials involving a total of 185 patients infected with P. vivax , chloroquine had 100% efficacy, although, compared with the patients infected with P. falciparum, larger percentages of the patients infected with P. vivax withdrew from the study or were lost to follow-up (Table 1). Most (56.2%) of the patients infected with P. vivax cleared their parasitaemias within 48 hours of the first dose of chloroquine (Fig. 3). The parasitaemias in six patients persisted for > 72 hours, without any concurrent symptoms, but cleared by day 7 of follow-up. Chloroquine treatment was well tolerated, with just one case each of gastritis, stomatitis and vomiting.
Fig. 3.
Kaplan–Meier survival curves for Plasmodium falciparum and P. vivax parasitaemias, India, 2009–2010
Note: The data used to create these curves came either from patients with Plasmodium falciparum infection who had been treated with a combination of artesunate with sulfadoxine-pyrimethamine or from patients with P. vivax infection who had been treated with chloroquine.
Discussion
India’s National Antimalarial Drug Resistance Monitoring System recruited 1733 patients and completed therapeutic efficacy trials in 25 sites across India during its first 2 years. The results indicate that the first-line therapies for P. falciparum malaria and P. vivax malaria recommended by the national antimalarial drug policy (i.e. AS+SP and chloroquine, respectively) remain efficacious. The 28-day efficacy of AS+SP for the treatment of P. falciparum infection, for example, was found to be > 98%. Although AS+SP treatment failures and parasitaemias showing prolonged clearance intervals after AS+SP treatment were rare, those identified were clustered in just a few sentinel sites. This clustering validates the design of the new monitoring system, which uses wide geographical coverage to increase the chances of detecting hotspots for resistance (as well as longitudinal studies to track emerging trends). No evidence of resistance to AS+SP was collected in the sentinel sites in north-eastern India, even though this is the region of the country where the highest frequencies of sulfadoxine-pyrimethamine treatment failure have been reported.15 The observation that four of the six patients who showed parasite clearance intervals of > 72 hours were confirmed to be treatment failures indicates the potential usefulness of measuring clearance intervals as a predictor of AS+SP treatment failure. Post-treatment reinfection with P. falciparum was rarely detected in the present study. While the frequency of reinfection recorded is likely to be correlated with the length of the follow-up period, it also depends on the intensity of transmission in the study sites. The intensity of malarial transmission in India is generally lower than in many other parts of the world.16
Most (87.1%) of the isolates of P. falciparum that were successfully typed showed genotypic evidence of partial resistance to pyrimethamine, with either single (S108N) or double mutations (S108N/C59R) in the relevant dhfr codons. Such mutations have been found to increase the median inhibitory concentration (IC50) of pyrimethamine 10-fold.14 While seven isolates possessed the I164L mutation that has been associated with high-level resistance,17 the prevalence of triple or quadruple mutants among the genotyped isolates was low (3.2%). As seen in a previous study based on the same methodos,9 the typing of dhps appeared to be relatively difficult; it was unsuccessful for 27.6% of the isolates for which typing was attempted. The prevalence of single or double dhps mutations among the isolates that were successfully genotyped was low (2.3%), although the possibility that dhps mutations caused non-amplification cannot be excluded. By monitoring trends in the prevalences of resistance-related mutations in dhfr and dhps, the threat to treatment with the AS+SP combination posed by resistance to sulfadoxine-pyrimethamine in P. falciparum could be evaluated, independent of any observations of the clinical response.
Treatment failure reflects a combination of drug resistance, host immunity and pharmacokinetics.18 In the present study, younger age, fever at enrolment and a low level of parasitaemia at enrolment – all potential markers of relatively low immunity to parasite antigens – were associated with recrudescence following AS+SP treatment. Another association observed, the negative correlation between the dose of artesunate (in mg per kg body weight) and the probability of treatment failure, was not surprising. Although the recommended daily dose of artesunate is 4 mg per kg, 8.8% of the subjects of the present study who were given AS+SP received 3.0 to < 3.5 mg of artesunate per kg, and 1.9% received < 3.0 mg per kg. The routine use of age, rather than body weight, as a guide for determining the dose of antimalarial drug needed by a patient is probably a cause of suboptimal dosing worldwide. The relationship between the administered dose and pharmacodynamic response is complex, however, and therapeutic levels may still be achieved when the dose is lower than recommended in standard guidelines.19
In spite of sporadic case reports of chloroquine-resistant P. vivax in India,20 all of the P. vivax -infected patients investigated in the present study appeared to be cured by chloroquine treatment. Although many of the patients in sentinel sites in southern and western India who were given chloroquine in the present study were migrant workers from elsewhere in India, more trials to investigate the therapeutic efficacy of chloroquine against P. vivax infections are needed in the north and east of India.
Primaquine treatment to prevent relapses forms a critical component in the effective treatment of P. vivax infections. Unfortunately, no standard protocols for evaluating the therapeutic efficacy of primaquine, alone or in combination with chloroquine, exist.2
Another remaining challenge is the treatment of mixed infections. In the present study, no data on the efficacy of AS+SP against P. vivax malaria were collected, although, according to India’s national drug policy, AS+SP is the recommended treatment for a patient found to be co-infected with P. falciparum and P. vivax . Recent reports across south-eastern Asia have described a high incidence of P. vivax malaria following the treatment of P. falciparum infection, presumably the result of the reactivation of the liver stages of P. vivax .21 In the present study, P. vivax infections were detected during the follow-up of seven cases of P. falciparum malaria who had been treated with AS+SP. Six of these seven cases were observed at Angul and Dhenkenal (both in the state of Odisha) and represented about one in every 20 patients treated with AS+SP at these two sentinel sites.
India’s National Antimalarial Drug Resistance Monitoring System was designed to give high data quality, national representation of different malaria ecotypes, coordinated use of resources and pooled data analysis. While the number of sentinel sites in the current system exceeds the minimum recommended by WHO, more may be required in a country as large as India. The alternation of sentinel sites will help to provide information on long-term trends. Those employed to design and implement the national system faced several challenges. While overall coordination in the joint NVBDCP-NIMR system was satisfactory, a few administrative issues delayed the recruitment of patients at some sentinel sites. Other difficulties encountered involved the central procurement of reagents, sample transport to New Delhi (for quality control and molecular studies) and the local recording of data in a digital format. Each of the problems was reviewed and addressed. Furthermore, NIMR investigators are currently using data and biological samples collected through the system to investigate the blood levels of residual antimalarial drugs at enrolment and their association with treatment outcome, the role of the pharmacokinetics of sulfadoxine-pyrimethamine in treatment failure, and pharmacovigilance for the antimalarial drugs in use.
The present study had several limitations. First, follow-up was confined to 28 days (the minimum period recommended by WHO). If drug efficacy is high, treatment failures are likely to occur relatively late, especially if the drugs used have long half-lives (as is the case with sulfadoxine-pyrimethamine). Follow-up in more recent studies within the drug resistance monitoring system has therefore been extended to 42 days. Second, the rarity of treatment failure limited our ability to identify the risk factors for, or predictors of, such failure. Also, once-a-day monitoring of the level of parasitaemia is not enough to accurately estimate mean clearance times or stage-specific clearance.22 However, by determining the proportion of aparasitaemic patients once each day, useful data on trends can still be collected. Third, the resources and time needed to check P. falciparum isolates for the molecular markers of resistance limited the number of isolates that could be typed in this manner. Finally, no attempt was made to determine the costs or cost-effectiveness of the national system. This would be a major challenge as the system uses resources from two different institutions and is supported by funding at both state and central levels.
Based on the data collected in the first two years of the existence of the National Antimalarial Drug Resistance Monitoring System, we recommend that: (i) the sentinel-site-based monitoring of antimalarial drug efficacy is maintained; (ii) the use of a parasite clearance interval of > 72 hours as a predictor of subsequent treatment failure be explored further, particularly in Betul; (iii) the nested PCR currently used for genotyping be replaced by a newer technology, such as real-time PCR, to enable the genotyping of more isolates; (iv) supplementary studies be undertaken on young children (because of their relatively high risk of AS+SP treatment failure); and (v) the relative benefits and disadvantages of age- and weight-based dosing be assessed. As part of the latter assessment, an increase in the doses of AS+SP in blister packs, to compensate for higher-than-average body weights, should be considered.
Conclusion
In India, AS+SP remains safe and effective for the treatment of uncomplicated P. falciparum malaria and chloroquine remains safe and effective for the treatment of uncomplicated P. vivax malaria.
Acknowledgements
We thank Pascal Ringwald and Nick White for their valuable inputs during the preparation of this manuscript. We also thank the NIMR field units and NVBDCP regional teams for their hard work. This paper was cleared by the NIMR’s Publication Screening Committee (004/2012).
Funding:
The financial support of the National Vector Borne Disease Control Programme (Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India) is gratefully acknowledged.
Competing interests:
None declared.
References
- 1.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–45. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 3.Global report on antimalarial drug efficacy and drug resistance: 2000–2010 Geneva: World Health Organization; 2010. Available from: http://www.who.int/entity/malaria/publications/atoz/9789241500470/en/index.html [accessed 11 October 2012].
- 4.Seghal P, Sharma M, Sharma S. Resistance to chloroquine in falciparum malaria in Assam state, India. J Commun Dis. 1973;5:175–80. [Google Scholar]
- 5.Garg M, Gopinathan N, Bodhe P, Kshirsagar NA. Vivax malaria resistant to chloroquine: case reports from Bombay. Trans R Soc Trop Med Hyg. 1995;89:656–7. doi: 10.1016/0035-9203(95)90432-8. [DOI] [PubMed] [Google Scholar]
- 6.Pattanayak S, Roy RG. Malaria in India and the modified plan of operations for its control. J Commun Dis. 1980;12:1–13. [PubMed] [Google Scholar]
- 7.Wijeyaratne PM, Chand PB, Valecha N, Shahi B, Adak T, Ansari MA, et al. Therapeutic efficacy of antimalarial drugs along the eastern Indo–Nepal border: a cross-border collaborative study. Trans R Soc Trop Med Hyg. 2005;99:423–9. doi: 10.1016/j.trstmh.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Shah NK, Dhillon GP, Dash AP, Arora U, Meshnick SR, Valecha N. Antimalarial drug resistance of Plasmodium falciparum in India: changes over time and space. Lancet Infect Dis. 2011;11:57–64. doi: 10.1016/S1473-3099(10)70214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed A, Bararia D, Vinayak S, Yameen M, Biswas S, Dev V, et al. Plasmodium falciparum isolates in India exhibit a progressive increase in mutations associated with sulfadoxine-pyrimethamine resistance. Antimicrob Agents Chemother. 2004;48:879–89. doi: 10.1128/AAC.48.3.879-889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Revised National Drug Policy (2010) for treatment of malaria. J Indian Med Assoc. 2010;108:844–5. [PubMed] [Google Scholar]
- 11.National Vector Borne Disease Control Programme [Internet]. Malaria. Delhi: NVBDCP; 2010. Available from: http://nvbdcp.gov.in/malaria2.html [accessed 18 October 2012].
- 12.Methods for surveillance of antimalarial drug efficacy Geneva: World Health Organization; 2009. [Google Scholar]
- 13.Recommended genotyping procedures (RGPs) to identify parasite populations Geneva: World Health Organization; 2008. Available from: http://www.who.int/malaria/publications/atoz/rgptext_sti/en/index.html [accessed 18 October 2012].
- 14.Ahmed A, Lumb V, Das MK, Dev V, Wajihullah, Sharma YD. Prevalence of mutations associated with higher levels of sulfadoxine-pyrimethamine resistance in Plasmodium falciparum isolates from Car Nicobar Island and Assam, India. Antimicrob Agents Chemother. 2006;50:3934–8. doi: 10.1128/AAC.00732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dev V, Phookan S, Barman K. Therapeutic efficacies of antimalarial drugs in the treatment of uncomplicated, Plasmodium falciparum malaria in Assam, north–eastern India. Ann Trop Med Parasitol. 2003;97:783–91. doi: 10.1179/000349803225002660. [DOI] [PubMed] [Google Scholar]
- 16.Sharma R, Sharma G, Dhillon G. Epidemiology and control of malaria in India Delhi: National Malaria Control Programme; 1996. [Google Scholar]
- 17.Andriantsoanirina V, Durand R, Pradines B, Baret E, Bouchier C, Ratsimbasoa A, et al. In vitro susceptibility to pyrimethamine of DHFR I164L single mutant Plasmodium falciparum. Malar J. 2011;10:283. doi: 10.1186/1475-2875-10-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidelines for the treatment of malaria Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 19.White NJ. Antimalarial pharmacokinetics and treatment regimens. Br J Clin Pharmacol. 1992;34:1–10. doi: 10.1111/j.1365-2125.1992.tb04100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valecha N, Joshi H, Eapen A, Ravinderan J, Kumar A, Prajapati SK, et al. Therapeutic efficacy of chloroquine in Plasmodium vivax from areas with different epidemiological patterns in India and their Pvdhfr gene mutation pattern. Trans R Soc Trop Med Hyg. 2006;100:831–7. doi: 10.1016/j.trstmh.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Lin JT, Bethell D, Tyner SD, Lon C, Shah NK, Saunders DL, et al. Plasmodium falciparum gametocyte carriage is associated with subsequent Plasmodium vivax relapse after treatment. PLoS One. 2011;6:e18716. doi: 10.1371/journal.pone.0018716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White NJ. The parasite clearance curve. Malar J. 2011;10:278. doi: 10.1186/1475-2875-10-278. [DOI] [PMC free article] [PubMed] [Google Scholar]