Abstract
In the present study we tested the hypothesis that, in subjects with Asperger’s syndrome (ASP), the dynamics of language-related regions might be abnormal, so that repetitive transcranial magnetic stimulation (rTMS) over Broca’s area leads to differential behavioral effects as seen in neurotypical controls. We conducted a five-stimulation-site, double-blind, multiple crossover, pseudo-randomized, sham-controlled study in 10 individuals with ASP and 10 age- and gender-matched healthy subjects. Object naming was assessed before and after low-frequency rTMS of the left pars opercularis, left pars triangularis, right pars opercularis and right pars triangularis, and sham stimulation, as guided stereotaxically by each individual’s brain magnetic resonance imaging. In ASP participants, naming improved after rTMS of the left pars triangularis as compared with sham stimulation, whereas rTMS of the adjacent left opercularis lengthened naming latency. In healthy subjects, stimulation of parts of Broca’s area did not lead to significant changes in naming skills, consistent with published data. Overall, these findings support our hypothesis of abnormal language neural network dynamics in individuals with ASP. From a methodological point of view, this work illustrates the use of rTMS to study the dynamics of brain–behavior relations by revealing the differential behavioral impact of non-invasive brain stimulation in a neuropsychiatric disorder.
Keywords: autism, human, language, repetitive transcranial magnetic stimulation
Introduction
Functional imaging studies have shown that naming prominently enhances activity in Broca’s area of healthy subjects, consistent with its nodal role in the distributed, bihemispheric language network (see meta-analysis from Price et al., 2005). Non-invasive brain stimulation techniques enable the further study of dynamic brain networks and their relation to a given behavior (Robertson et al., 2003). They can disrupt or paradoxically facilitate a normal or abnormal cognitive function identifying causal links between a brain network and a cognitive function. Here, we aimed at investigating the integrity of the neural network associated with naming in individuals with Asperger’s syndrome (ASP) as compared with neurotypical controls using neuronavigated non-invasive brain stimulation.
Naming is an ability that has been extensively studied with repetitive transcranial magnetic stimulation (rTMS) in both clinical and healthy populations. Low-frequency rTMS (known to mainly suppress brain activity) over the right Broca’s area homologue or high-frequency rTMS (known to mainly enhance brain activity) over the perilesional areas in the left Broca’s area can improve naming skills in patients with non-fluent aphasia due to stroke (Naeser et al., 2005a,b; Monti et al., 2008).
Here, we aimed at investigating the integrity of the neural network associated with naming in ASP as compared with neurotypical controls using rTMS to right or left hemispheric frontal opercular regions. Although individuals with ASP are known to show normal or abnormally enhanced naming skills (Müller et al., 1999; Walenski et al., 2008), they also display differences in Broca’s area when compared with neurotypical subjects (Nishitani et al., 2004; McAlonan et al., 2009; but see Nordhal et al., 2007) and deficits in high-order language and communication abilities (Howlin, 2003; Macintosh & Dissanayake, 2004; Woodbury-Smith & Volkmar, 2009). Our hypothesis was that, even if the behavioral manifestation is normal, the underlying neural activity might be different. As a general conceptualization, the same performance in a given task (behavior) can be the result of different patterns of brain activity. This is not a novel idea; consider for example the growing literature on interindividual differences in brain activity for a given task, or the long-standing concept of cognitive strategies. Here we argue that related phenomena may apply to group differences in specific domains between healthy and certain pathological populations (in this case for naming between ASP and neurotypical individuals). Naming ability is a crucial milestone in language acquisition (Swan & Goswami, 1997) and, if its underlying neural network is different, this might contribute to the development of language differences at a higher order. Previous work on ASP has focused on impaired language skills and the related neural network. We believe that the study of neural networks supporting lower order language skills, even behaviorally normal, could shed light on the higher-order language deficits observed in ASP, which include pragmatics and language comprehension (Howlin, 2003; Macintosh & Dissanayake, 2004; Woodbury-Smith & Volkmar, 2009).
Materials and methods
We performed a five-stimulation-site, pseudo-randomized, double-blind, multiple crossover, sham-controlled trial in 10 adults with ASP and 10 healthy subjects. Language abilities were assessed using a standardized test of object picture naming before and after each stimulation session. Participants received sham stimulation or active stimulation to the right or left pars opercularis or pars triangularis. Stimulation was guided stereotaxically by each individual’s anatomical brain magnetic resonance imaging.
Participants
We studied 10 native English-speaking adults diagnosed with ASP from an independent clinician using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria (American Psychiatric Association (APA), 2000) by a board-certified neurologist or psychiatrist and the Adult Asperger Assessment (Baron-Cohen et al., 2005). Half of the patients also completed the Autism Diagnostic Observation Schedule and the Autism Diagnostic Interview-Revised, which confirmed the diagnosis in all cases. In addition, we studied 10 age- and gender-matched healthy participants (mean ± SD age of subjects with ASP and healthy subjects, respectively: 36.6 ± 16.0 and 36.6 ± 16.4 years; three women with ASP and three healthy women). Patients were recruited through flyers posted in the Massachusetts area (e.g. at the Asperger’s Association of New England). IQ scores were obtained using the Wechsler Abbreviated Scale of Intelligence [mean ± SD IQ scores of subjects with ASP and healthy subjects, respectively: full IQ of 122.4 ± 7.2 and 111.2 ± 4.3, t(17) = −4.18, P = 0.001; verbal IQ of 123.8 ± 6.8 and 109.4 ± 5.6, t(17) = −5.06, P = 0.0001; non-verbal IQ of 115.7 ± 11.5 and 111.7 ± 9.0, t(17) = −0.84, P > 0.05]. Participants were right-handed as assessed with the Edinburgh Handedness Inventory, except one ASP who was ambidextrous. Participants were screened for rTMS (Keel et al., 2001) and magnetic resonance imaging contraindications. Inclusion and exclusion criteria strictly followed the recommendations and safety guidelines for transcranial magnetic stimulation (TMS) endorsed by the International Federation for Clinical Neurophysiology (Wasserman, 1998; Rossi et al., 2009). A medical and psychiatric history was obtained prior to each subject’s participation. All subjects underwent an evaluation by a neurologist and a psychiatrist. All underwent a neuropsychological assessment. No subjects were on psychotropic medications. Subjects had a negative personal and family history of epilepsy. In female subjects, a negative pregnancy test was confirmed prior to stimulation. Written informed consent was obtained from all participants prior to inclusion in the study, which was approved by the Institutional Review Board of Beth Israel Deaconess Medical Center. A signed Patient Consent-to-Disclose Form was obtained for the photograph displayed in Fig. 1.
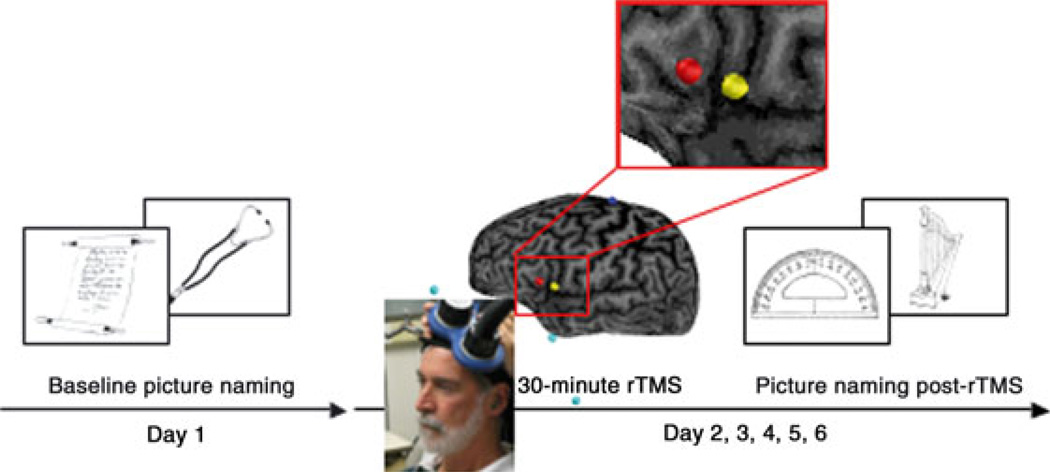
Fig. 1.
Design of the experiment. Participants first performed object naming (baseline condition) on Day 1. On the following days, they performed the naming task after receiving brain stimulation: sham rTMS, active rTMS to the pars opercularis and active rTMS to the pars triangularis.
Language assessment
Object naming abilities were assessed using the Boston Naming Test (Kaplan et al., 1976) by an experimenter blinded to stimulation conditions. We generated five subsets of 15 pictures counterbalanced by frequency and difficulty. At baseline, participants were tested on the full set of pictures. Then, after each stimulation session, participants were tested with one of the subsets of pictures. The order of the subsets was counterbalanced across participants and stimulation sessions. The pictures were presented on a computer using Presentation software (Neurobehavioral Systems Inc., CA, USA) and the order of pictures in each subset was randomized across presentations. Participants were instructed to name each picture as soon as possible after presentation. Verbal responses were recorded with a microphone attached to a headset (Logitech Inc., CA, USA). Participants with ASP accurately named objects at each condition: baseline (mean 98%, range 92–100%), sham rTMS (mean 100%, range 100–100%) and rTMS over the left pars opercularis (mean 99%, range 93–100%), left pars triangularis (mean 99%, range 93–100%), right pars opercularis (mean 98%, range 87–100%) and right pars triangularis (mean 99%, range 93–100%). The control group showed a similar accuracy of performance: baseline (mean 96%, range 88–100%), sham rTMS (mean 98%, range 93–100%) and rTMS over the left pars opercularis (mean 98%, range 93–100%), left pars triangularis (mean 98%, range 93–100%), right pars opercularis (mean 97%, range 93–100%) and right pars triangularis (mean 96%, range 87–100%).
Repetitive transcranial magnetic stimulation
Participants completed four sessions of 30-min 1 Hz rTMS to the left pars opercularis, left pars triangularis, right pars opercularis or right pars triangularis, and a fifth session of sham rTMS over the central lobe midline (Fig. 1). The sessions were completed at the same time of day on separate days over the course of approximately 4 weeks. The order of stimulation conditions was randomly assigned for each participant. The interval between sessions was at least 5 days to further control for any possible carry-over effects. Stimulation was performed at an intensity of 70% of the stimulator output, with a commercially available figure-of-eight coil (7 cm in diameter) and a Magstim Rapid Magnetic Stimulator unit (Magstim Corporation, UK). We localized each stimulation site on each participant’s individual anatomical brain magnetic resonance imaging. The pars opercularis of both hemispheres was identified as the area rostral to the precentral sulcus, caudal to the anterior vertical ramus of the lateral sulcus, dorsal to the anterior / posterior ramus of the lateral sulcus, and ventral to the inferior frontal sulcus. The pars triangularis of both hemispheres was identified as the area rostral to the anterior vertical ramus of the lateral sulcus, caudal and dorsal to the anterior horizontal ramus of the lateral sulcus, and ventral to the inferior frontal sulcus. In order to precisely target the stimulation site and keep the brain target constant throughout the stimulation session, we used a frameless stereotactic system (Brainsight; Rogue Inc.).
All stimulation sessions were conducted with strict adherence to current safety guidelines and recommendations (Wassermann, 1998; Hallett et al., 1999; Rossi et al., 2009) at the Harvard–Thorndike Clinical Research Center and the Berenson–Allen Center for Noninvasive Brain Stimulation (Beth Israel Deaconess Medical Center, Harvard Medical School). A standardized questionnaire on the side-effects of stimulation (modified after Keel et al., 2001) was administrated following each stimulation session upon completion of the naming task. All side-effects reported by participants who completed the protocol are listed in Table 1. One neurotypical subject did not complete the protocol. He withdrew during the second session due to discomfort (i.e. he did not like the facial twitching). No adverse effect occurred. This was reported to the Institutional Review Board committee.
Table 1.
Side effects reported by participants with Asperger’s syndrome after each brain stimulation session. All other participants reported no side effect
| Participant | Sham stimulation | Left pars opercularis | Left pars triangularis | Right pars opercularis | Right pars triangularis |
|---|---|---|---|---|---|
| P1 | Stiff neck Improved mood |
Sleepy | Trouble concentrating | Trouble concentrating | |
| P2 | Improved mood | ||||
| P5 | Improved mood | ||||
| P6 | Improved concentration Subtle disorientation |
Dizziness | Trouble concentrating | ||
| P7 | More emotional | Sleepy | More emotional Headache |
||
| P10 | Stiff neck | Stiff neck Sleepy |
Stiff neck Sleepy |
Sleepy Discomfort at the stimulation site |
Sleepy |
Data analyses
The outcome measure was the response latency for correct answers. Vocal responses were transformed into spectrograms in order to identify the latency from the onset of the picture presentation to the onset of the subject’s correct answer. The response latency was measured in milliseconds using Praat (http://www.praat.org). Verbal responses were coded so that the investigator was blind to the stimulation conditions. The mean of response latency was calculated for each participant and for each stimulation condition. Single trials with response times more than 2 SDs above or below the mean response for each condition and participant were considered outliers and excluded. Excluded outliers represented 4.6% of the overall data. All statistical analyses were performed using spss software (version 16.0, SPSS Inc., IL, USA). Results with a P-value ≤ 0.05 were considered significant for all statistical analyses.
Results
We first tested for group differences in response latency at baseline and sham conditions. There was no significant difference (repeated-measurements anova; F1,17 = 3.90, P > 0.05), confirming similar performance between the ASP and neurotypical matched control group at baseline or after receiving sham rTMS.
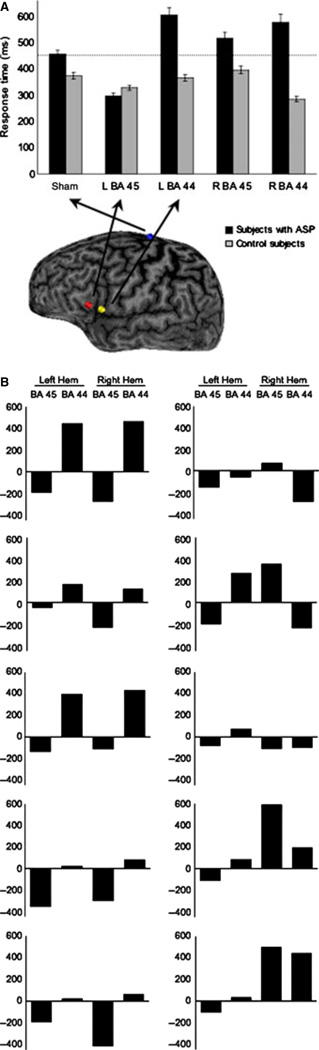
In order to identify a significant modulation of performance after brain stimulation, response latencies were submitted to a repeated-measures anova with group (ASP, neurotypical) and stimulation site (sham, left pars opercularis, left pars triangularis, right pars opercularis, right pars triangularis) as independent factors. There was a main group effect (F1,7 = 8.42, P = 0.023) and a significant group × stimulation site interaction (F4,28 = 3.73, P = 0.045), but no effect of stimulation site (F4,28 = 1.18, P > 0.05). Compared with controls, ASP participants showed worse performance following stimulation of the left pars opercularis (F1,7 = 9.12, P = 0.019), but better performance after stimulation of the left pars triangularis (F1,7 = 8.53, P = 0.022) than after sham stimulation (Fig. 2A). No other comparisons yielded to statistical significance. As illustrated in Fig. 2B, a faster response time after stimulation over the left pars triangularis was observed in each individual participant with ASP.
Fig. 2.
(A) Mean of response latency for each condition for both the ASP and healthy groups. Error bars represent SEM. (B) Mean of response latency (in milliseconds) for each condition as compared with sham stimulation for each subject with ASP. It appears that, with stimulation of the left hemisphere, the effects of rTMS are consistent across ASP patients (10 / 10 showed a reduced latency with rTMS of the left pars triangularis; 9 / 10 showed a lengthened latency with stimulation of the left pars opercularis). This contrasts with stimulation of the right hemisphere. In the ASP group, for right stimulation to the pars triangularis, when response latencies were calculated only for the participants who improved, they were faster by 239 ms (SD 116.7 ms) as compared with sham stimulation. When the same calculation was performed with the four participants who showed worsening, they were slower by 381.2 ms (SD 226.6 ms; stimulation to the left pars triangularis: faster by 156.0 ms; SD 85.6). As an exploratory investigation, Pearson correlations performed with latency changes (sham minus stimulation to right pars triangularis) and full IQ, verbal IQ, non-verbal IQ, Autism Spectrum Quotient, age, handedness indices and accuracy variables did not reach statistical significance (all P > 0.05). With regards to gender, the three women showed improvement with stimulation to the right pars triangularis. BA 45, Broadman area 45 (presumably pars triangularis); BA 44, Broadman area 44 (presumably pars opercularis).
Discussion
Our results show that naming performance at baseline did not differ between ASP and healthy groups. However, rTMS had a significantly different impact on performance between the two groups. We found that rTMS to the left pars triangularis significantly improved the naming performance of ASP volunteers.We also observed in the ASP group that rTMS to the left pars opercularis significantly worsened performance. These effects contrast with those of the healthy matched subjects, in whom, consistent with previous studies (Mottaghy et al., 1999, 2006; Sparing et al., 2001), stimulation of the left or right pars triangularis or pars opercularis failed to significantly modulate naming skills. These findings suggest that the language-related neural network is different in individuals with ASP than in neurotypical individuals. We discuss two potential, not mutually exclusive, factors that may account for the differential effects of rTMS on naming in ASP vs. healthy individuals.
Potential difference of the local impact of repetitive transcranial magnetic stimulation between the Asperger’s syndrome and healthy groups
The impact of rTMS on the targeted region could be different in participants with ASP than in healthy subjects. Low-frequency rTMS is thought to suppress activity in the targeted area, but we cannot affirm that rTMS had the same suppressive effect in ASP and neurotypical participants, as we did not measure brain activity changes related to stimulation. Modeling work suggests that the current density distribution is critically influenced by brain morphology and tissue characteristics (Wagner et al., 2007, 2008), and morphometric abnormalities have been reported in adults with ASP, including smaller frontostriatal white-matter tracts (Toal et al., 2009) and reduced gray matter in frontostriatal regions (McAlonan et al., 2002). Model work also indicates that the cerebrospinal fluid in sulci can direct current flow in ways that are difficult to predict a priori (Wagner et al., 2007). Therefore, the opposite behavioral differences observed in ASP when applying the TMS coil over these two neighboring cortical areas (improved performance with the left pars triangularis condition and worsened performance with the left pars opercularis condition) may not be interpreted, as the effects of the stimulation were exactly and exclusively where the TMS coil was placed.
Potential difference of the language neural network between the Asperger’s syndrome and healthy groups
Another possible explanation for the differential effect of rTMS on naming in ASP and healthy subjects is differences in the language neural network. In the light of our findings, we propose that, in the ASP brain, the left pars triangularis exerts an abnormal inhibitory effect on the left pars opercularis. We suggest that the behavioral facilitation observed with stimulation to the left pars triangularis is due to a TMS-induced inhibitory effect on the left pars triangularis, which in turn had released its suppressive effect on the left pars opercularis and enhanced its functional role. This would also explain the observed significant worsening of naming when targeting the left pars opercularis with 1-Hz rTMS (presumably suppressing its activity). This will be tested in future work.
Indeed, the behavioral, functional impact of rTMS is ultimately not solely the consequence of the stimulation’s direct impact on the targeted region, but rather a manifestation of the capacity of the brain to adjust and compensate for the disruption (e.g. Li et al., 2004). It is possible that the capacity to compensate for the disruption of rTMS is less in ASP than in healthy subjects. Clear abnormal brain connectivity in ASP has not yet been reported. However, abnormal connectivity in Autism Spectrum Disorders (ASD) was reported in 1988 by Horwitz et al. and has since received compelling support (Brock et al., 2002; Belmonte et al., 2004; Courchesne & Pierce, 2005; Courchesne et al., 2005; Herbert, 2005; Lainhart et al., 2006; Hughes, 2007; Minshew & Williams, 2007; Williams & Minshew, 2007; Groen et al., 2008; Hadders-Algra, 2008; Müller et al., 2008) [aberrant connectivity in ASD was also discussed in the review papers by Hill & Frith (2003), Frith (2004) and Paul et al. (2007)]. Relevant to this work, abnormalities include reduced intra- and interhemispheric functional connectivity involving Broca’s area and its right hemispheric homolog. For instance, underconnectivity was reported between the left pars triangularis and left inferior extrastriate and between the left pars triangularis and left frontal eye field during sentence comprehension (Just et al., 2004), between the bilateral pars opercularis and visual area 17 during visuomotor coordination (Villalobos et al., 2005), and between the bilateral prefrontal cortex (extending to the right pars triangularis) and left parietal region during working memory with letters (Koshino et al., 2005). Finally, the correlation between the frontal and temporal activations observed in healthy subjects during semantic processing was not detected in ASD subjects (Knaus et al., 2008). Even in the resting state, atypical connectivity has been observed between the ASD frontal and temporal region of the left hemisphere (Murias et al., 2007).
Just et al. (2004) have argued that the ASD brain is characterized by underfunctioning integrative circuitry that results in a deficit of integration of information at the neural and cognitive levels (underconnectivity theory). The neural basis of impaired language in ASD entails a lower degree of information integration and synchronization across the language cortical network (Groen et al., 2008). Thus, ASD individuals may possess abnormally weak functional connectivity, which could increase the likelihood of behavioral deficits following a focal disruption, for example by rTMS.
State dependency, which can lead to differential effects of rTMS on cognitive tasks (Silvanto et al., 2008), might also have contributed to a differential effect of stimulation on naming between ASP and healthy subjects. We are not aware of any studies specifically characterizing naming-related activity in ASP, but there are some examples of group differences on Broca’s activity. In ASD, as compared with neurotypicals, weaker activity was found in the left pars triangularis during semantic processing (Harris et al., 2006; Gaffrey et al., 2007) and processing high-imagery sentences (Kana et al., 2006), in the left pars opercularis during semantic processing (Gaffrey et al., 2007), and in both the left pars opercularis and pars triangularis during sentence comprehension (Just et al., 2004). Knaus et al. (2008) reported greater overall activity in Broca’s area in ASD during semantic processing. Studies combining TMS with neuroimaging may provide valuable insights into such notions of connectivity and state dependency.
The effects of Broca’s stimulation on naming in healthy subjects
With regard to healthy subjects, the neural basis of naming has been extensively studied. Semantic and phonological processes during naming have both been associated with activations in the bilateral inferior frontal cortex, with left dominance. In their meta-analysis, Price et al. (2005) reported that activity in the left pars triangularis is especially involved in the linguistic aspects of naming, i.e. semantic and phonology (as compared with perceptual and motor components of speech). These imaging findings seem to contrast with neuromodulation findings. Broca’s activity is enhanced during naming but Broca’s stimulation does not seem to significantly change naming processes in healthy subjects. None of the previous neuromodulation studies have distinctively targeted regions within Broca’s area (pars triangularis vs. pars opercularis) using a stereotactic system, which could have explained the negative findings. Here, although we have differentiated the pars opercularis and pars triangularis, there were no significant effects on naming. This certainly needs further investigation, which is however out of the scope of this work. The present findings highlight the importance of distinguishing discrete stimulation sites, in line with Gough et al. (2005) who reported a functional difference between two neighboring regions, the rostral part of the inferior frontal gyrus interfered with semantic processing, whereas the caudal part, < 3 cm apart, impacted phonological processing.
Limitations of the present study
We acknowledge that, although we targeted two adjacent anatomical regions as guided by a stereotaxic system, the reported behavioral changes are functional differences. We are limited to the functional resolution of the device. Moreover, we did not measure rTMS-induced brain activity changes Therefore, we do not know whether the induced current distribution is such that we are indeed targeting brain areas separated by only 1 cm or instead reaching different brain areas. To address this, future studies should combine brain stimulation with neuroimaging techniques such as TMS-position emission tomography and TMS-functional magnetic resonance imaging. It is also important to note that the observed behavioral changes were reported in a group of individuals with ASP and these findings might not be replicated in individuals with other autism spectrum disorders, or those with lower functional or IQ levels. Further, the sample size was modest and we cannot rule out that a larger sample size could have led to significant effects within the control group. Finally, another limitation is that the groups differed in their full and verbal IQ scores. Results could be different in perfectly IQ-matched groups.
Translational relevance
Our findings do not establish a clinical utility of rTMS in ASP; however, the potential clinical relevance is worth discussing. Improved naming was associated with stimulation to the pars triangularis of the left hemisphere in adults with ASP. It has been shown that ASD individuals trained for the identification of pictures and production of spoken words can not only improve these skills, but learn new language and can transfer it in a natural, untrained environment (Bosseler & Massaro, 2003). Naming ability, one of the earliest milestones in linguistic development, is a signature of future reading ability (Swan & Goswami, 1997). Further studies seem warranted. Improvements in naming associated with brain stimulation could ultimately lead to sustained improved communication skills, such as propositional speech, as well as social interactions in individuals with ASD, especially with more severely affected and younger patients. In aphasic patients, the beneficial effects on language following stimulation were not only sustained, but seemed to increase with time (Naeser et al., 2005a,b; Chastan et al., 2009), suggesting the induction of a behaviorally beneficial plastic process.
The cause of the communication and language problems, as with the etiology and pathophysiology of ASP and ASD in general, remains uncertain. However, individuals with ASP and ASD display definite abnormalities in the language-related brain regions [and the degree of abnormalities in the left pars opercularis of the inferior frontal gyrus in ASD has been correlated with the severity of impairments in social interaction and communication (Dapretto et al., 2005; Hadjikani et al., 2006) and the age of acquisition of phrase speech (McAlonan et al., 2008)]. New strategies should particularly address language and communication deficits, which critically contribute to deficits in social integration and cognitive abilities (Hale and Tager-Flusberg, 2005; Joseph et al., 2005).
Acknowledgements
We would like to thank Erica Bae for help with collecting the data. We would also like to thank all subjects for their participation. This work was supported by the Harvard–Thorndike Clinical Research Center at Beth Israel Deaconess Medical Center integrated in the Harvard Clinical and Translational Science Center, and supported by grants M01-RR-01066 and UL1 RR025758 from the National Center for Research Resources, National Institutes of Health, by grants from the National Institutes of Health (K24RR018875), Autism Speaks and the Nancy Lurie Mark Family Foundation to A.P.-L., and by grants from the Fonds de Recherche en Santé du Québec and the Canadian Institutes of Health Research to S.F.
Abbreviations
- ASD
Autism spectrum disorders
- ASP
Asperger’s syndrome
- rTMS
repetitive transcranial magnetic stimulation
- TMS
transcranial magnetic stimulation
References
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 4th-TR edn. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Robinson J, Woodbury-Smith M. The Adult Asperger Assessment (AAA): a diagnostic method. J. Autism Dev. Disord. 2005;35:807–819. doi: 10.1007/s10803-005-0026-5. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J. Neurosci. 2004;20:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosseler A, Massaro DW. Development and evaluation of a computer-animated tutor for vocabulary and language learning in children with autism. J. Autism Dev. Disord. 2003;33:653–672. doi: 10.1023/b:jadd.0000006002.82367.4f. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Dev. Psychopathol. 2002;14:209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Chastan N, Parain D, Vérin E, Weber J, Faure MA, Marie J-P. Psychogenic aphonia: spectacular recovery after motor cortex transcranial magnetic stimulation. J. Neurol. Neurosurg. Psychiatry. 2009;80:94. doi: 10.1136/jnnp.2008.154302. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr. Opin. Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Redcay E, Morgan JT, Kennedy DP. Autism at the beginning: microstructural and growth abnormalities underlying the cognitive and behavioral phenotype of autism. Dev. Psychopathol. 2005;17:577–597. doi: 10.1017/S0954579405050285. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2005;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U. Emanuel Miller lecture: confusions and controversies about Asperger syndrome. J. Child Psychol. Psychiatry. 2004;45:672–686. doi: 10.1111/j.1469-7610.2004.00262.x. [DOI] [PubMed] [Google Scholar]
- Gaffrey MS, Kleinhans NM, Haist F, Akshoomoff N, Campbell A, Courchesne E, Muller RA. A typical participation of visual cortex during word processing in autism: an fMRI study of semantic decision. Neuropsychologia. 2007;45:1672–1684. doi: 10.1016/j.neuropsychologia.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J. Neurosci. 2005;25:8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen WB, Zwiers MP, van der Gaag RJ, Buitelaar JK. The phenotype and neural correlates of language in autism: an integrative review. Neurosci. Biobehav. Rev. 2008;32:1416–1425. doi: 10.1016/j.neubiorev.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Hadders-Algra M. Reduced variability in motor behaviour: an indicator of impaired cerebral connectivity? Early Hum. Dev. 2008;84:787–789. doi: 10.1016/j.earlhumdev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb. Cortex. 2006;16:1276–1282. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Hale CM, Tager-Flusberg H. The influence of language on theory of mind: a training study. Dev. Sci. 2006;6:346–359. doi: 10.1111/1467-7687.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M, Wassermann EM, Pascual-Leone A, Valls-Sole J. Repetitive transcranial magnetic stimulation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999;52:105–113. [PubMed] [Google Scholar]
- Harris GH, Chabris CF, Clark J, Urban T, Aharon I, Steele S, McGrath L, Condouris K, Tager-Flusberg H. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Large brains in autism: the challenge of pervasive abnormality. Neuroscientist. 2005;11:417–440. doi: 10.1177/0091270005278866. [DOI] [PubMed] [Google Scholar]
- Hill EL, Frith U. Understanding autism: insights from mind and brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358:281–289. doi: 10.1098/rstb.2002.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Grady CL, Rapoport SI. The cerebral metabolic landscape in autism. Intercorrelations of regional glucose utilization. Arch. Neurol. 1988;45:749–755. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- Howlin P. Outcome in high-functioning adults with autism with and without early language delays: implications for the differentiation between autism and Asperger syndrome. J. Autism Dev. Disord. 2003;33:3–13. doi: 10.1023/a:1022270118899. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Autism: the first firm finding = underconnectivity? Epilepsy Behav. 2007;11:20–24. doi: 10.1016/j.yebeh.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Joseph RM, McGrath LM, Tager-Flusberg H. Executive dysfunction and its relation to language ability in verbal school-age children with autism. Dev. Neuropsychol. 2005;27:361–378. doi: 10.1207/s15326942dn2703_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test, Experimental Edition. Boston: Aphasia Research Center, Boston University; 1976. [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin. Neurophysiol. 2001;112:720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Lindgren KA, Hadjikhani N, Tager-Flusberg H. fMRI activation during a language task in adolescents with ASD. J. Int. Neuropsychol. Soc. 2008;14:967–979. doi: 10.1017/S1355617708081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, Deutsch CK, Dunn M, Estes A, Tager-Flusberg H, Folstein S, Hepburn S, Hyman S, McMahon W, Minshew N, Munson J, Osann K, Ozonoff S, Rodier P, Rogers S, Sigman M, Spence MA, Stodgell CJ, Volkmar F. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. Am. J. Med. Genet. A. 2006;140:2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Tenebäck CC, Nahas Z, Kozel FA, Large C, Cohn J, Bohning DE, George MS. Interleaved transcranial magnetic stimulation/functional MRI confirms that lamotrigine inhibits cortical excitability in healthy young men. Neuropsychopharmacology. 2004;29:1395–1407. doi: 10.1038/sj.npp.1300452. [DOI] [PubMed] [Google Scholar]
- Macintosh KE, Dissanayake C. Annotation: the similarities and differences between autistic disorder and Asperger’s disorder: a review of the empirical evidence. J. Child Psychol. Psychiatry. 2004;45:421–434. doi: 10.1111/j.1469-7610.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, Simmons A, Sigmundsson T, Greenwood K, Russell A, Schmitz N, Happe F, Howlin P, Murphy DG. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;127:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Suckling J, Wong N, Cheung V, Lienenkaepemer N, Cheung C, Chua SE. Distinct patterns of grey matter abnormality in high-functioning autism and Asperger’s syndrome. J. Child Psychol. Psychiatry. 2008;49:1287–1295. doi: 10.1111/j.1469-7610.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung C, Cheung V, Wong N, Suckling J, Chua SE. Differential effects on white-matter systems in high-functioning autism and Asperger’s syndrome. Psychol. Med. 2009;39:1885–1893. doi: 10.1017/S0033291709005728. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch. Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, Vergari M, Zago S, Priori A. Improved naming after transcranial direct current stimulation in aphasia. J. Neurol. Neurosurg. Psychiatry. 2008;79:451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Hungs M, Brugmann M, Sparing R, Boroojerdi B, Foltys H, Huber W, Töpper R. Facilitation of picture naming after repetitive transcranial magnetic stimulation. Neurology. 1999;53:1806–1812. doi: 10.1212/wnl.53.8.1806. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Sparing R, Töpper R. Enhancing picture naming with transcranial magnetic stimulation. Behav. Neurol. 2006;17:177–186. doi: 10.1155/2006/768413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R-A, Behen ME, Rothermel RD, Chugani DC, Muzik O, Mangner TJ, Chugani HT. Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. J. Autism Dev. Disord. 1999;29:19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- Müller E, Schuler A, Yates GB. Social challenges and supports from the perspective of individuals with Asperger syndrome and other autism spectrum disabilities. Autism. 2008;12:173–190. doi: 10.1177/1362361307086664. [DOI] [PubMed] [Google Scholar]
- Murias M, Swanson JM, Srinivasan R. Functional connectivity of frontal cortex in healthy and ADHD children reflected in EEG coherence. Cereb. Cortex. 2007;17:1788–1799. doi: 10.1093/cercor/bhl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser M, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, Cayer-Meade C, Kobayashi M, Theoret H, Fregni F, Tormos JM, Kurland J, Doron KW, Pascual-Leone A. Improved naming after TMS treatments in a chronic, global aphasia patient – case report. Neurocase. 2005a;11:182–193. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser M, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Tormos JM, Kurland J, Doron KW, Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area, an open-protocol study. Brain Lang. 2005b;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Avikainen S, Hari R. Abnormal imitation-related cortical activation sequences in Asperger’s syndrome. Ann. Neurol. 2004;55:558–562. doi: 10.1002/ana.20031. [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Dierker D, Mostafavi I, Schumann CM, Rivera SM, Amaral DG, Van Essen DC. Cortical folding abnormalities in autism revealed by surface-based morphometry. J. Neurosci. 2007;27:11725–11735. doi: 10.1523/JNEUROSCI.0777-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat. Rev. Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, Moore CJ, Morton C, Laird AR. Meta-analyses of object naming: effect of baseline. Hum. Brain Mapp. 2005;25:70–82. doi: 10.1002/hbm.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Théoret H, Pascual-Leone A. Studies in cognition: the problems solved and created by transcranial magnetic stimulation. J. Cogn. Neurosci. 2003;15:948–960. doi: 10.1162/089892903770007344. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn. Sci. 2008;12:447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Sparing R, Mottaghy FM, Hungs M, Brugmann M, Foltys H, Huber W, Töpper R. Repetitive transcranial magnetic stimulation effects on language function depend on the stimulation parameters. J. Clin. Neurophysiol. 2001;18:326–330. doi: 10.1097/00004691-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Swan D, Goswami U. Picture naming deficits in developmental dyslexia: the phonological representations hypothesis. Brain Lang. 1997;56:334–353. doi: 10.1006/brln.1997.1855. [DOI] [PubMed] [Google Scholar]
- Toal F, Daly EM, Page L, Deeley Q, Hallahan B, Bloemen O, Cutter WJ, Brammer MJ, Curran S, Robertson D, Murphy C, Murphy KC, Murphy DG. Clinical and anatomical heterogeneity in autistic spectrum disorder: a structural MRI study. Psychol. Med. 2009;40:1171–1181. doi: 10.1017/S0033291709991541. [DOI] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Müller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25:916–925. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: a computer-based human model study. Neuroimage. 2007;35:1113–1124. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Wagner T, Eden U, Fregni F, Valero-Cabre A, Ramos-Estebanez C, Pronio-Stelluto V, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial magnetic stimulation and brain atrophy: a computer-based human brain model study. Exp. Brain Res. 2008;186:539–550. doi: 10.1007/s00221-007-1258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenski M, Mostofsky SH, Gidley-Larson JC, Ullman MT. Brief report: enhanced picture naming in autism. J. Autism Dev. Disord. 2008;38:1395–1399. doi: 10.1007/s10803-007-0513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr. Clin. Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Williams DL, Minshew NJ. Understanding autism and related disorders: what has imaging taught us? Neuroimaging Clin. N. Am. 2007;17:495–509. doi: 10.1016/j.nic.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury-Smith MR, Volkmar FR. Asperger syndrome. Eur. Child Adolesc. Psychiatry. 2009;18:2–11. doi: 10.1007/s00787-008-0701-0. [DOI] [PubMed] [Google Scholar]