Abstract
Three siderophore–drug conjugates (sideromycins) were synthesized by preparation of a maleimide linked derivative of the siderophore desferrioxamine B and reacting the corresponding Ga3+-complex with freshly prepared thiol-containing antibiotics: loracarbef, ciprofloxacin, and nadifloxacin. The conjugates and their synthetic precursors were tested against a broad panel of bacteria and were found to display Gram-positive selective, growth inhibitory activity (μM), indicating that this approach is suitable for the convergent syntheses and screening of novel sideromycins.
Keywords: Antibiotic, drug-conjugate, siderophore
Siderophores are high affinity, Fe3+-selective chelators produced by bacteria and fungi. Under iron deficient conditions, these molecules are secreted into the environment where they gather essential iron from diverse sources and deliver it to the producing organism.1−6 The biological importance of iron has prompted bacteria to develop means for the recognition of exogenous siderophores in order to gain a competitive advantage, which in turn has led to the biosyntheses of naturally occurring siderophore-drug conjugates (sideromycins) such as the albomycins,7,8 salmycins,9 and microcins.10,11 These conjugates are modified siderophores containing an antibiotic portion that is delivered into a microorganism upon acquisition of the siderophore–Fe3+ complex.17−19 The inability to handle the actively transported drug might lead to cell death, thus combating siderophore thievery.
Exploiting iron uptake as a biological target has been an exciting field for the study and development of new methods that allow the transport of antibacterial agents into pathogenic cells, and a variety of synthetic sideromycins have been reported in the literature.12−19 One of the advantages of using these chelators as carriers is the often selective recognition of ferri-siderophores by microorganisms. Drug-conjugates of these molecules have been demonstrated to be highly potent antibiotic agents, stressing the value of their syntheses and study.
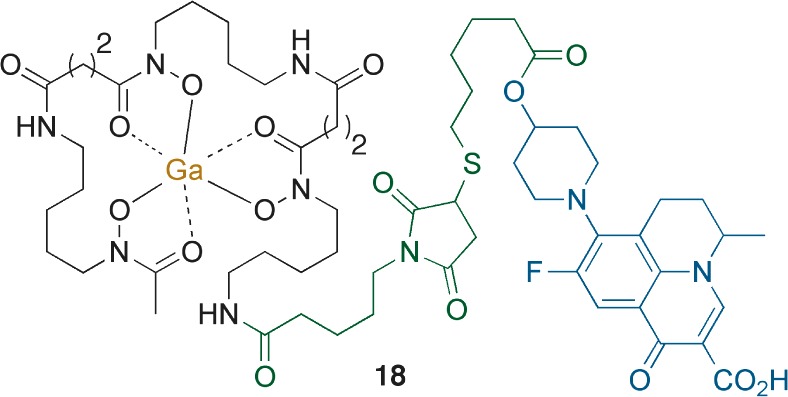
The assembly of these molecules, however, is a chemically challenging effort that typically requires manipulation of protecting groups and tailoring of conditions suitable to the drug or compound of interest. With the limited availability of some siderophores, it is highly desirable to minimize the number of steps needed to accomplish the conjugation, and thus, a convergent approach would provide a new tool toward these fascinating molecules. An example of versatile siderophore functionalization was the report by Koizumi and collaborators on the conjugation of desferrioxamine B (DfoB) with monoclonal antibody OST7 (IgG1) to deliver radiogallium 67Ga into tumors of mice,20 for scintigraphic imaging, by employing three different strategies: (1) using glutaraldehyde as a linker, (2) acylation of both building blocks with 2-pyridyl disulfide and subsequent condensation via a disulfide bond, and (3) linking a maleimide to DfoB and conjugation with the thiol-containing antibody (1, Figure 1). In order to optimize the properties of the conjugates and reduce radionuclide localization within nontargeted organs, a metabolizable maleimide linker (2) was later reported,21 indicating that specific characteristics of the conjugates could be modified by the linker of choice. Tinianow also reported the use of a maleimide-containing DfoB (3) to deliver 89Zr using the monoclonal antibody thiotrastuzumab, for positron emission tomography (PET) imaging.22
Figure 1.
Use of maleimide linkers for the functionalization of DfoB.20−22.
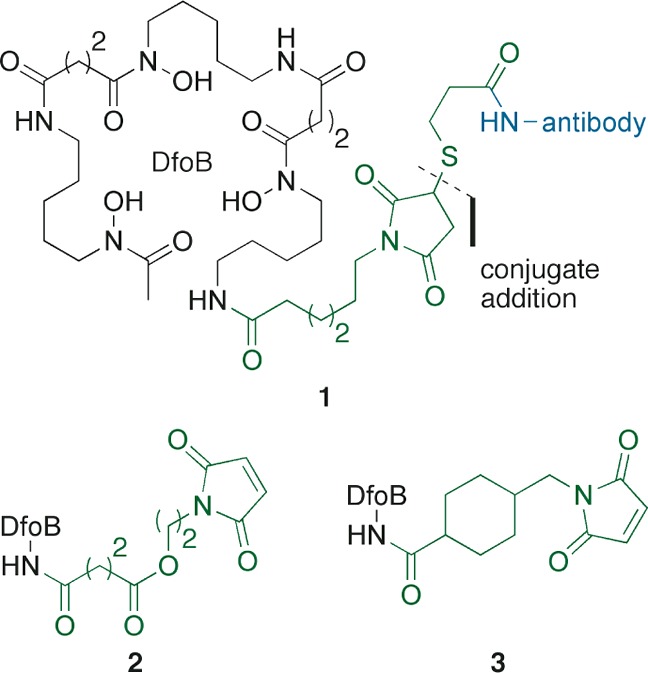
We are interested in the assembly of novel sideromycins through the previously described thiol-maleimide strategy, utilizing different siderophores. In this work, we explore the scope and limitations of this approach by conjugation of functionalized DfoB 12 (Figure 2) and thiol-containing antibiotics (e.g., 14) to afford siderophore–drug conjugates such as Ga-DfoB-ciprofloxacin 17.
Figure 2.
Use of maleimide–DfoB 12 and thiol–ciprofloxacin 14 in the synthesis of sideromycin 17.
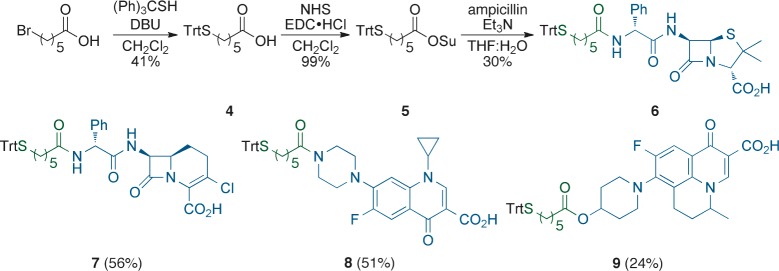
The attachment of a thiol-containing linker to the drugs of interest: ampicillin, loracarbef, ciprofloxacin, and nadifloxacin (representing drugs with different targets) was envisioned by the use of S-trityl protected acid 4(23,24) (Scheme 1), which was obtained from the condensation between 6-bromohexanoic acid and S-triphenylmethane thiol in the presence of DBU to afford the desired intermediate in 41% yield. Activated ester 5 was obtained from coupling fragment 4 with NHS using EDC·HCl. The resulting material could be purified by silica gel chromatography but was often found to be pure enough for further use after aqueous workup. Ampicillin was acylated by reaction with 5 in THF/H2O/Et3N to give the desired adduct, 6, in 30% yield. Optimized conditions allowed the isolation of drug-derivatives loracarbef 7 (56% yield), ciprofloxacin 8 (51% yield), and nadifloxacin 9 (24% yield). The last was obtained from the EDC·HCl-mediated condensation between nadifloxacin and synthetic precursor 4.
Scheme 1. Syntheses of Thiol-Containing Antibiotic Precursors.
The maleimide-functionalized siderophore was obtained by reacting commercially available DfoB mesylate salt and freshly prepared NHS ester 10 under buffered conditions to give 11 in 50% yield (Scheme 2). Ga3+-complex 12 was isolated after refluxing the previous intermediate with Ga(acac)3 in MeOH in 84% yield. We decided to use gallium as a protecting group for the hydroxamic chelators in DfoB. We were also interested in the potential role of gallium within our conjugates, since growth inhibition of Pseudomonas aeruginosa has been reported in the literature with Ga(NO3)3,25 GaCl3, and Ga-DfoB.26
Scheme 2. Assembly of Ga-DfoB–Drug Conjugates.
The removal of the S-trityl protecting group from intermediates 7–9 was achieved with a mixture of TFA (20% v/v) and iPr3SiH (2% v/v) in deoxygenated CH2Cl2 (Scheme 2). Sulfur deprotection was monitored by TLC, and the formation of the corresponding thiol-containing drugs 13–15 was confirmed by LCMS. We were unable to prove the formation of a thiol-ampicillin derivative from the deprotection of 6 or to detect any adduct from the conjugation with maleimide-Ga-DfoB 12. Ga-DfoB-loracarbef sideromycin 16 was obtained in 56% yield from the reaction between freshly prepared thiol 13, maleimide 12, and DIPEA in deoxygenated THF. The corresponding Ga-DfoB-ciprofloxacin 17 (24% yield) and Ga-DfoB-nadifloxacin 18 (21% yield) were prepared in a similar fashion. The solvent used in the drug conjugation influenced product formation. When the assembly of 17 was attempted in CH2Cl2, the desired sideromycin was not detected even after 70 h.
The novel conjugates and their synthetic precursors were screened for antibacterial activity against a panel of Gram-positive and Gram-negative bacteria in an agar diffusion assay27,28 (Table S1 of the Supporting Information (SI)). The S-trityl protected antibiotics displayed broad growth inhibition of bacteria (2 mM), with the exception of ciprofloxacin derivative 8, where significant levels of precipitation were observed in aqueous media due to limited solubility. In general, Mycobacterium smegmatis and Escherichia coli were not affected by our compounds. To study the effect of gallium, we tested Ga(acac)3 and Ga(NO3)3 but no growth inhibition (at 0.2 mM) was observed under the assay conditions. Maleimide-functionalized DfoB 11 and the corresponding Ga-DfoB complex 12 did not display antibiotic properties. However, drug-conjugates 16–18 were potent growth inhibitors, particularly against Gram-positive bacteria and Mycobacterium vaccae.
After susceptible bacteria were identified, MIC values were determined in MHII media as well as iron-deficient conditions (MHII + 2,2′-bipyridine).29 In MHII (Table 1), both S-trityl protected β-lactams 6 and 7 displayed potent (MIC = 0.39–3.13 μM) antibiotic activity against Bacillus subtilis, Staphylococcus aureus, and Micrococcus luteus, comparable to that of loracarbef (MIC = 0.39–0.78 μM). In the case of M. vaccae, derivative 7 (6.25 μM) was more potent than the parent β-lactam (>25 μM), possibly due to increased lipophilicity.
Table 1. Minimum Inhibitory Concentration of Compounds (MIC, μM) Determined in MHII Mediaa–d.
| B. subtilis | S. aureus | M. luteus | M. vaccae | P. aeruginosa | |
|---|---|---|---|---|---|
| compd | ATCC 6633 | SG511 | ATCC 10240 | IMET 10670 | K799/wt |
| 6c | 2.15b | 0.39b | 1.19 | >25 | >25b |
| 7c | 3.13b | 2.35b | 1.71 | 6.25 | >25b |
| 8c | >25bP | >25b | >25b | >25 P | >25b |
| 9c | 3.13 | 25 | >25 | 10.4 | >25 |
| Ga(NO3)3c | >25 | >25 | >25 | >25 | >25 |
| 12c | >50b | >50b | >50b | >50 | >50b |
| 16c | 27 | 9.38b | 0.001 | >50 | >50b |
| 17c | >50b | 31.25b | >50b | >50 | >50b |
| 18c | <0.1 | 12.5 | 50 | 3.13 | >50 |
| Lorac | 0.46 | 0.78 | 0.39 | >25 | >25 |
| Nadic | 0.008 | 0.06 | 0.20 | 0.06 | >2 |
| Cipro | 0.06 | 0.47 | 7.54 | 0.24 | 0.94 |
MICs were determined by the visual end point broth microdilution method following CLSI guidelines.29
Reported values are the average of two sets of duplicates (N = 4).
Reported values are the average of triplicates (N = 3).
P, observed precipitation.
The MIC values of ciprofloxacin analog 8 were not adequately determined because of its limited solubility in aqueous solution. However, nadifloxacin (Nadi) derivative 9 displayed improved solubility and a range of inhibitory activity (MIC = 3.13 → 25 μM) without enhancing the potency of Nadi. Consistent with our findings in the agar diffusion assay, Ga(NO3)3 and Ga-DfoB 12 did not display growth inhibition (MIC >50 μM). P. aeruginosa (K799/wt) was not inhibited at the highest concentrations tested, in either MHII or iron-depleted media (MIC >50 μM, Table S2 of the SI).
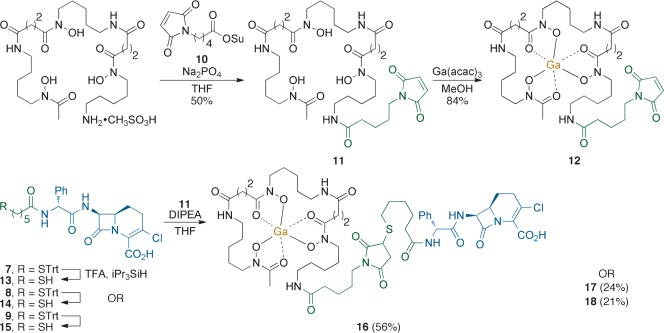
Ga-DfoB-loracarbef conjugate 16 retained the antibiotic activity of the β-lactam drug, while displaying remarkable inhibition of M. luteus (MIC = 0.001 μM) enhancing the activity of loracarbef (MIC = 0.39 μM). While this result merits further study, the finding that M. luteus was strongly inhibited by 16 is consistent with the fact that it possesses a limited set of penicillin-binding proteins (PBPs) that render this Gram-positive bacteria particularly sensitive to β-lactam antibiotics.30 Ga-DfoB-ciprofloxacin 17 displayed reduced inhibition when compared to 16 (MIC = 31.25 → 50 μM), but enhanced solubility and therefore improved antibiotic activity over the S-trityl protected precursor 8. Ga-DfoB-nadifloxacin conjugate 18 (Figure 3) was a strong inhibitor of B. subtilis (MIC = <0.1 μM) and M. vaccae (MIC = 3.13 μM), consistent with the increased potency of Nadi when compared to Cipro.
Figure 3.
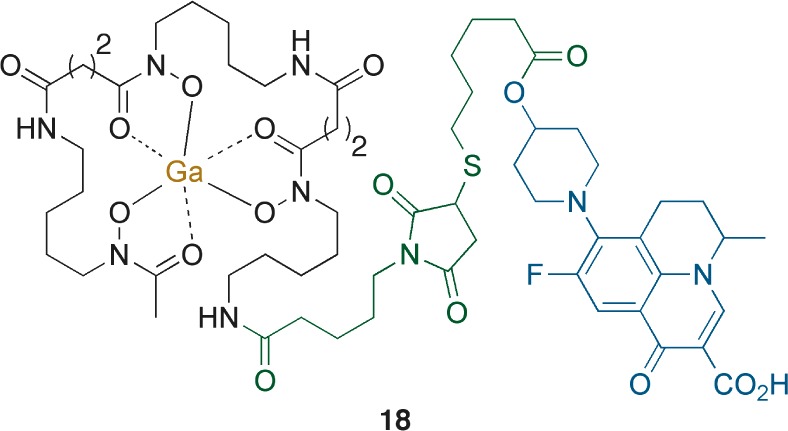
Ga-DfoB-nadifloxacin conjugate 18.
Considering that Ga(NO3)3, Ga(acac)3, and Ga-DfoB 12 did not display growth inhibition under the different conditions tested, the observed antibiotic activity of sideromycins 16–18 can be attributed to a functional drug moiety. Our results are also consistent with bacterial internalization of the conjugates, as reflected by the growth inhibition of cytoplasmic targets with fluoroquinolone-based sideromycins (17 and 18). Recently, Rzhepishevska and collaborators reported the antibacterial properties of gallium citrate (Ga-Cit) and Ga-DfoB against Gram-positive and Gram-negative bacteria.31 Growth inhibition by gallium was affected by the metal–ligand and the media composition. IC90 values for Ga-DfoB against several bacteria were higher (mM) than the concentrations screened in this work. To explore the effect of further iron-restricted conditions, we determined the MIC values in MHII media with the addition of metal-chelator 2,2′-bipyridine (Table S2 of the SI). In general, we observed comparable values between both assays, corroborating the displayed antibiotic activity of our synthetic compounds. Moderate enhancement (3-fold) was observed with loracarbef-conjugate 16 against B. subtilis and S. aureus (MIC = 3.9–7.8 μM). The iron-depleted media was not conducive for the culturing of M. vaccae, which failed to grow after two attempts.
In conclusion, we have developed a convergent approach for the assembly of siderophore–drug conjugates using a thiol–maleimide strategy. As anticipated, the novel sideromycins displayed selective antibacterial properties and provided us with valuable information suitable for the study of these molecules. Because the siderophore of choice often determines the spectrum of activity of these conjugates, the described methodology could be expanded to the design of Gram-negative antibacterials through the use of an appropriate chelator.
Acknowledgments
We acknowledge the University of Notre Dame and the NIH (AI 054193) for supporting this work. We appreciate constructive comments and advice regarding antibacterial studies from Dr. Ute Möllman, Jena, Germany. We gratefully acknowledge the use of the NMR facilities provided by the Lizzadro Magnetic Resonance Research Center at The University of Notre Dame (UND) and the mass spectrometry services provided by The UND Mass Spectrometry & Proteomics Facility (Mrs. N. Sevova, Dr. W. Boggess, and Dr. M. V. Joyce; supported by the National Science Foundation under CHE-0741793).
Glossary
Abbreviations
- DBU
1,8-diazabicycloundec-7-ene
- DfoB
desferrioxamine B
- Ga-DfoB
Ga3+-complex of DfoB
- EDC·HCl
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide·HCl
- LCMS
liquid chromatography mass spectrometry
- MHII
Mueller–Hinton agar
- MIC
minimum inhibitory concentration
- NHS
N-hydroxysuccinimide
- THF
tetrahydrofuran
- TFA
trifluoroacetic acid
- TLC
thin layer chromatography
Supporting Information Available
Experimental procedures of chemistry and microbiology, and 1H and 13C NMR spectra of reported compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Miethke M.; Marahiel M. A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhalfa H.; Crumbliss A. L. Chemical aspects of siderophore mediated iron transport. Biometals 2002, 15, 325–339. [DOI] [PubMed] [Google Scholar]
- Andrews S. C.; Robinson A. K.; Rodríguez-Quiñones F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [DOI] [PubMed] [Google Scholar]
- Sandy M.; Butler A. A Microbial Iron Acquisition: Marine and Terrestrial Siderophores. Chem. Rev. 2009, 109, 4580–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hider R. C.; Kong X. L. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [DOI] [PubMed] [Google Scholar]
- Braun V.; Hantke K. Recent insights into iron import by bacteria. Curr. Opin. Chem. Biol. 2011, 15, 328–334. [DOI] [PubMed] [Google Scholar]
- Benz G.; Schröder T.; Kurz J.; Wünsche C.; Karl W.; Steffen G. J.; Pfitzner J.; Schmidt D. Konstitution der Deferriform der Albomycine δ1, δ2 und ε. Angew. Chem., Int. Ed. 1982, 94, 552–553. [Google Scholar]
- Braun V.; Günthner K.; Hantke K.; Zimmerman L. Intracellular Activation of Albomycin in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 1983, 156, 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vértesy L.; Aretz W.; Fehlhaber H.-W.; Kogler H.; Salmycin A.-D. Antibiotics from Streptomyces violaceus, DSM 8286, Having a Siderophore-Aminoglycoside Structure. Helv. Chim. Acta 1995, 78, 46–60. [Google Scholar]
- Duquesne S.; Destoumieux-Garzón D.; Peduzzi J.; Rebuffat S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat. Prod. Rep. 2007, 24, 708–734. [DOI] [PubMed] [Google Scholar]
- Nolan E. M.; Fischbach M. A.; Koglin A.; Walsh C. T. Biosynthethic Tailoring of Microcin E492m: Post-translational Modification Affords an Antibacterial Siderophore-Peptide Conjugate. J. Am. Chem. Soc. 2007, 129, 14336–14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L.; Roosenberg J. M. II; Miller M. J. Total Synthesis of Desferrisalmycin B. J. Am. Chem. Soc. 2002, 124, 15001–15005. [DOI] [PubMed] [Google Scholar]
- Bernier G.; Girijavallabhan V.; Murray A.; Niyaz N.; Ding P.; Miller M. J.; Malouin F. Desketoneoenactin-Siderophore Conjugates for Candida: Evidence of Iron Transport-Dependent Species Selectivity. Antimicrob. Agents Chemother. 2005, 49, 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoganathan S.; Sit C. S.; Vederas J. C. Chemical synthesis and biological evaluation of gallidermin-siderophore conjugates. Org. Biomol. Chem. 2011, 9, 2133–2141. [DOI] [PubMed] [Google Scholar]
- Miller M. J.; Walz A. J.; Zhu H.; Wu C.; Moraski G.; Möllmann U.; Tristani E. M.; Crumbliss A. L.; Ferdig M. T.; Checkley L.; Edwards R. L.; Boshoff H. I. Design, Synthesis, and Study of a Mycobactin-Artemisinin Conjugate That Has Selective and Potent Activity against Tuberculosis and Malaria. J. Am. Chem. Soc. 2011, 133, 2076–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosenberg J. M. II; Lin Y. M.; Lu Y.; Miller M. J. Studies and Syntheses of Siderophores, Microbial Iron Chelators, and Analogs as Potential Drug Delivery Agents. Curr. Med. Chem. 2000, 7, 159–197. [DOI] [PubMed] [Google Scholar]
- Braun V.; Pramanik A.; Gwinner T.; Koeberle M.; Bohn E. Sideromycins: tools and antibiotics. Biometals 2009, 22, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C.; Garcia-Herrero A.; Johanson T. H.; Krewulak K. D.; Lau C. K.; Peacock R. S.; Slavinskaya Z.; Vogel H. J. Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals 2010, 23, 601–611. [DOI] [PubMed] [Google Scholar]
- Ji C.; Juárez-Hernández R. E.; Miller M. J. Exploiting bacterial iron acquisition: siderophore conjugates. Future Med. Chem. 2012, 4, 297–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi M.; Endo K.; Kunimatsu M.; Sakahara H.; Nakashima T.; Kawamura Y.; Watanabe Y.; Saga T.; Konishi J.; Yamamuro T.; Hosoi S.; Toyama S.; Arano Y.; Yokohama A. 67Ga-labeled Antibodies for Immunoscintigraphy and Evaluation of Tumor Targeting and Drug-Antibody Conjugates in Mice. Cancer Res. 1988, 48, 1189–1194. [PubMed] [Google Scholar]
- Arano Y.; Matsushima H.; Tagawa M.; Koizumi M.; Endo K.; Konishi J.; Yokoyama A. A Novel Bifunctional Metabolizable Linker for the Conjugation of Antibodies with Radionuclides. Bioconjugate Chem. 1991, 2, 71–76. [DOI] [PubMed] [Google Scholar]
- Tinianow J. N.; Gill H. S.; Ogasawara A.; Flores J. E.; Vanderbilt A. N.; Luis E.; Vandlen R.; Darwish M.; Junutula J. R.; Williams S.-P.; Marik J. Site-specifically 89Zr-labeled monoclonal antiodies for ImmunoPET. Nucl. Med. Biol. 2010, 37, 289–297. [DOI] [PubMed] [Google Scholar]
- Collman J. P.; Groh S. E. “Mercaptan-Tail” Porphyrins: Synthetic Analogues for the Active Site of Cytochrome P-450. J. Am. Chem. Soc. 1982, 104, 1391–1403. [Google Scholar]
- Moreno A. Y.; Mayorov A. V.; Janda K. D. Impact of Distinct Chemical Structures for the Development of a Methamphetamine Vaccine. J. Am. Chem. Soc. 2011, 133, 6587–6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y.; Thoendel M.; Olakanmi O.; Britigan B. E.; Singh P. K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Invest. 2007, 117, 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banin E.; Lozinski A.; Brady K. M.; Berenshtein E.; Butterfield P. W.; Moshe M.; Chevion M.; Greenberg E. P.; Banin E. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 16761–16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- General description of the modified Kirby-Bauer agar diffusion assay used in this work.Afonin S.; Glaser R. W.; Berditchevskaia M.; Wadhwani P.; Gührs K.-H.; Möllmann U.; Perner A.; Ulrich A. S. 4-Fluoro-phenylglycine as a Label for 19F-NMR Structure Analysis of Membrane Associated Peptides. ChemBioChem 2003, 4, 1151–1163. [DOI] [PubMed] [Google Scholar]
- Murray P. R.; Baron E. J.; Pfaller M. A.; Tenover F. C.; Yolken R. H.. Manual of Clinical Microbiology, 7th ed.; American Society for Microbiology: Washington, DC, 1999. [Google Scholar]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 8th ed.; Clinical and Laboratory Standards Institute (CLSI): Villanova, PA, 2009; approved standard document M07-A7.
- Young M.; Artsatbanov V.; Beller H. R.; Chandra G.; Chater K. F.; Dover L. G.; Goh E.-B.; Kahan T.; Kaprelyants A. S.; Kyrpides N.; Lapidus A.; Lowry S. R.; Lykidis A.; Mahillon J.; Markowitz V.; Mavromatis K.; Mukamolova G. V.; Oren A.; Rokem J. S.; Smith M. C. M.; Young D. I.; Greenblatt C. L. Genome Sequence of the Fleming Strain of Micrococcus luteus, a Simple Free-Living Actinobacterium. J. Bacteriol. 2010, 192, 841–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzhepishevska O.; Ekstrand-Hammarström B.; Popp M.; Björn E.; Buch A.; Sjöstedt A.; Antti H.; Ramstedt M. The Antibacterial Activity of Ga3+ Is Influenced by Ligand Complexation as Well as the Bacterial Carbon Source. Antimicrob. Agents Chemother. 2011, 55, 5568–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.