Abstract
Although secondary ion mass spectrometry (SIMS) has been successfully employed for mapping lipid distributions at the cellular level, the identification of intact lipid species in situ is often complicated by isobaric interference. The high mass resolution and tandem MS capabilities of a C60-QSTAR hybrid instrument has been utilized to identify over 50 lipid species from mouse macrophages (RAW 264.7). In this investigation, lipid assignments made based on mass accuracy were confirmed with tandem MS analyses. Data obtained from C60-SIMS was compared to LC-MS data obtained by the LIPID MAPS consortium. A majority of the lipids detected with LC-MS, but not detected with C60-SIMS were present at concentrations below 2.0 pmol/µg of DNA. Matrix related effects prevented the detection of lipids with the glycerophosphoethanolamine (PE) headgroup, glycerophosphoserine (PS) headgroup and lipids with polyunsaturated fatty acyl (PUFA) chains in the C60-SIMS analyses. Lipid distributions obtained from a lawn of RAW 264.7 cells stimulated with the endotoxin KDO2-Lipid A were also studied. The results obtained with C60-SIMS agreed with the established LC-MS data for the glycerophosphoinositol lipid class (PI) with adequate molecular sensitivity achieved with as few as 500 cells.
Introduction
The normal function of a cell depends upon an elaborate series of interconnected biochemical interactions and reactions. In the past, lipids have been dismissed as mere structural components for cell membranes; their role in complex cellular chemistry is just being realized. As a result, analytical techniques, such as SIMS, capable of providing information on the chemical composition and spatial distribution of these molecules are potential tools for elucidating complex biochemical processes.
ToF-SIMS is an established technique in the field of lipid imaging and is routinely used to map lipid distributions in both cellular and tissue systems.[1] The ability of the technique to provide chemical and spatial information at sub-micron resolution contributes to its success. The emergence of cluster ion sources has greatly improved the analytical power of SIMS in the field of lipid analysis by allowing for the detection of intact lipid molecular ion species.[2] Now with the abundant molecular ion sensitivity, in situ lipid characterization is a new challenge. Extraction, separation and derivatization efforts typically employed to identify and quantify lipids are not compatible with this in situ technique and, as a result, new protocols are needed to elucidate the lipid biochemistry of the sample.
Isobaric interferences, matrix effects and biological salt adducts contribute to spectral complexity and convolute in situ lipid characterization. For glycerophospholipids, the sheer number of species with different structural components, but similar mass-to-charge ratios, quickly congests the 600–900 Dalton range of a ToF mass spectrum. With several possible lipid species in a single mass unit, identification can be difficult. In the past, prior knowledge of the sample biochemistry and mass accuracy was used to identify the most abundant peaks. This method has led to numerous false identifications and is unrealistic for probing increasingly complex and unknown lipid chemistry. Recently developed SIMS instruments, such as the C60-QSTAR[3] and the J105 Chemical Imager[4], equipped with tandem MS capabilities can identify lipids based on their characteristic decomposition patterns. In this report tandem MS using C60-SIMS is utilized to identify the glycerophospholipids and to determine the lipid profile detectable in situ in RAW 264.7 cells, a mouse leukemic monocyte macrophage cell line.
The report also describes the use of SIMS to elucidate changes in lipid biochemistry associated with the stimulation of mouse macrophages with the endotoxin KDO2-Lipid A, a model system for investigating the role of lipids in chronic inflammatory based diseases such as atherosclerosis, diabetes, and arthritis.[5–7] KDO2-Lipid A, an E. coli derived endotoxin binds to the MD-2 subunit of the TLR-4 receptor on the surface of the macrophage and initiates innate immune response of the cell. In this process, the lipid biochemistry of the RAW 264.7 cells is altered. LC-MS analyses performed by the LIPID MAPS consortium have thoroughly mapped this dynamic and are compared with C60-SIMS data.[5]
Experimental
Cell culturing
Frozen RAW 264.7 cells were obtained from a LIPID MAPS associated laboratory (The Glass Lab at the University of California, San Diego) and stored at −80 °C. Protocols established by the LIPID MAPS consortium for the thawing, passage, KDO2 Lipid A stimulation and lipid extraction were obtained at the following website, http://www.lipidmaps.org/protocols/index.html. Briefly, the cells were grown in a solution of Dulbecco’s Modified Eagles Media (DMEM) from Cellgro, Inc. with 10% fetal bovine serum (FBS) from Cellgro, Inc. and 1% streptomycin penicillin. The cells were maintained at 37° C, in a humidified atmosphere, with 5% CO2. The cells were grown to 80% confluence between passages.
LPS induction procedures
KDO2- Lipid A was obtained from Avanti Polar Lipids, Inc. A 100 µg/mL working solution of KDO2-Lipid A was prepared in Dulbecco’s phosphate buffered saline (DPBS). Congruently, RAW 264.7 cells were grown in a six-well plate (3 mL) on conductive ITO (indium tin oxide) coated slides. After thirty hours of incubation, the cells were washed with DPBS and fresh media was added. The cells were incubated for an additional 28 h, before 100 µL of the KDO2-Lipid A working solution (100µg/mL) was added. An equivalent amount of DPBS was added to the control samples. The cells were incubated for an additional 24 h.
The cells in the culture flasks were used for lipid extracts. For this procedure, the cells were washed with DPBS twice before being scraped into a solution of fresh cold DPBS. The cell suspension was centrifuged (relative centrifugal force (RCF) = 600 × g) for 10 min. The supernatant was aspired and a solution of 0.8 mL cold MeOH: 0.1 N HCl (1:1) plus 0.4 mL of cold chloroform was added. The solution was vortexed for 1 min and centrifuged (RCF = 18000 × g) for 5 min. The lower phase containing the glycerophospholipids was isolated and dry dropped on a glycerol droplet before analysis.[8]
Cells grown on the ITO slides were washed with 0.15 mM ammonium formate (pH 7.4). Excess solution was wicked off with a Kimwipe. The cells were flash frozen in liquid propane, temporarily stored in liquid nitrogen, and brought to room temperature in a freeze-dryer. To improve secondary ion yields, a 5-µL aliquot of glycerol[9] was spotted on top of the lawn of cells, covering approximately 500 cells.
Instrumentation
All SIMS acquisitions were obtained using the C60-QSTAR mass spectrometer.[3] Mass spectra were acquired for 60 s with a 250 pA beam, approximately 1 × 10 11 C60 per spectra, in both the positive and negative ion mode. The mass resolution in the lipid region was routinely 8000 and the mass accuracy based on the ToF calibration was 6 ppm. The tandem MS spectra were accumulated for 60 s; the molecular ion was selected with unit resolution in the quadrupole mass filter and fragmented with 40 eV of collision energy.
Results and Discussion
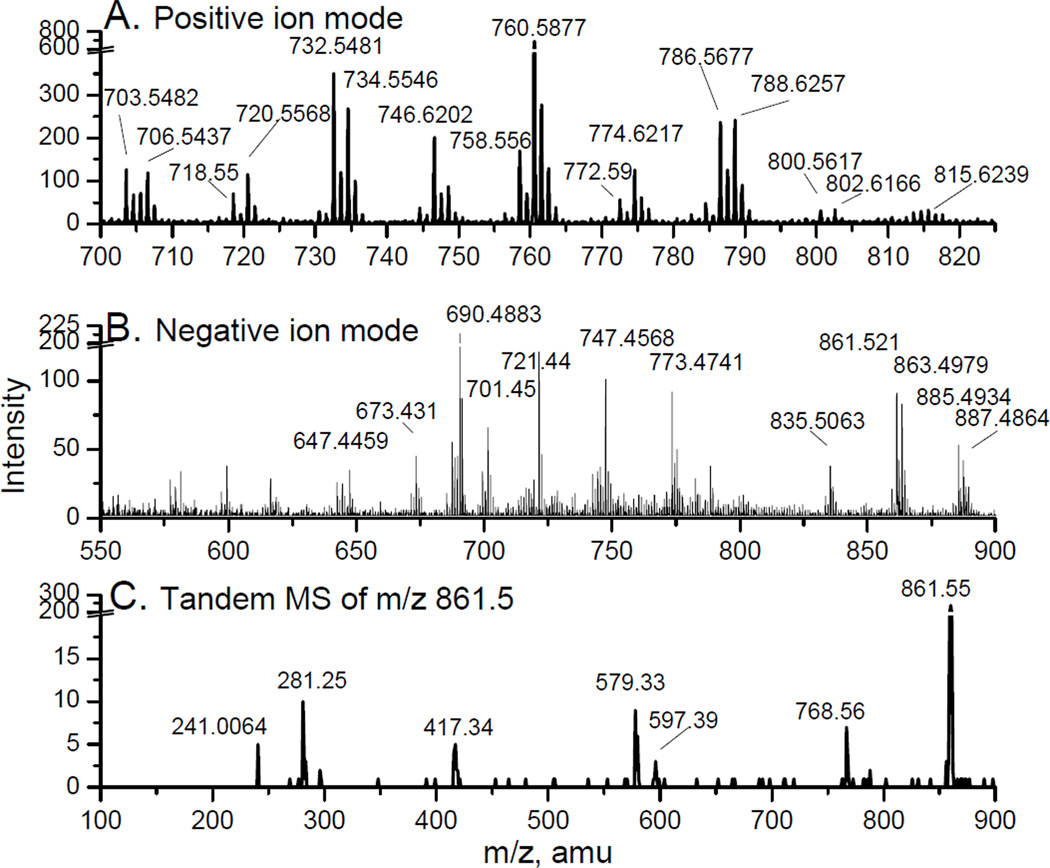
Lipid extracts from RAW 264.7 cells were characterized with C60-SIMS and tandem MS. The positive ion mode mass spectrum shows m/z values consistent with glycerophosphocholine and sphingomyelin lipid species (see Figure 1a). Peaks above 1000 integral counts were subjected to tandem MS analyses, not shown. The results of which show that the majority of the lipids detected in the positive ion mode contained the phosphocholine headgroups, based on the fragment ion at m/z 184.07. Due to the mass parity effect, the sphingomyelin and glycerophosphocholine species in the ToF-MS spectrum are represented by the nominal mass peaks with odd values and even nominal mass peaks, respectively.
Figure 1.
The positive (a) and negative (b) ion mode ToF-MS spectra obtained with C60-SIMS of lipid extract taken from RAW 264.7 cells shows the abundant number of detectable glycerophospholipid species. Tandem MS spectrum of m/z 861.5 in the negative ion mode (c) is similar to the LIPID MAPS reference spectrum for PI (18:1/18:1).
The mass values in the negative ion mode mass spectrum suggest a mixture of various glycerophosphoinsitol (PI), glycerophosphates (PA), glycerophosphoglycerols (PG) and one glycerophosphoethanolamine (PE) (see Figure 1b). Tandem MS of the dominant peaks in the negative ion mode spectrum reveals valuable fatty acid structural information that helps to make unequivocal assignments. In tandem MS mode, since the desired molecular ion is isolated from reactive secondary ion species before the CID chamber; the resulting tandem MS spectrum is independent of the ionization method. As a result, SIMS-based tandem MS spectra are the same as spectra obtained using ESI and MALDI ionization methods. This is advantageous to the SIMS community; since extensive spectral libraries already exist. For example, the tandem MS spectra of a lipid at m/z 861.5 obtained using SIMS (Figure 1c) matches the LIPID MAPS lipid reference spectrum for 1,2-di-octadecenoyl-sn-glycero-3-phospho-1'-myo-inositol [PI(18:1/18:1)] acquired with ESI. The major fragments at m/z 240.96, 281.2, 417.4, 579.5 and 597.6, correspond to inositol-phosphate headgroup, the 18:1 fatty acid, [18:1 LPA-H20-H]−, [18:1 LPI-H20-H]−, and [18:1 LPI-H]- fragments, respectively. The unequivocal identification of each lipid can be made based on its signature unimolecular fragmentation pattern observed in the tandem MS spectrum.
It is believed that thousands of individual lipid species contribute to the “lipidome” of a cell. In the LC-MS based experiments, over 400 major lipid species were detected and monitored during activation. In the study presented here, over 50 glycerophospholipid species have been identified from the lipid mixture, including 22 PC, 9 PG, 12 PI and 9 PA species. Matrix effects and molecular ion stability issues are possible reasons for the scarcity of PE species, lipids with polyunsaturated fatty acid groups, and absence of PS species.
The stimulation of RAW 264.7 cells with KDO2-Lipid A
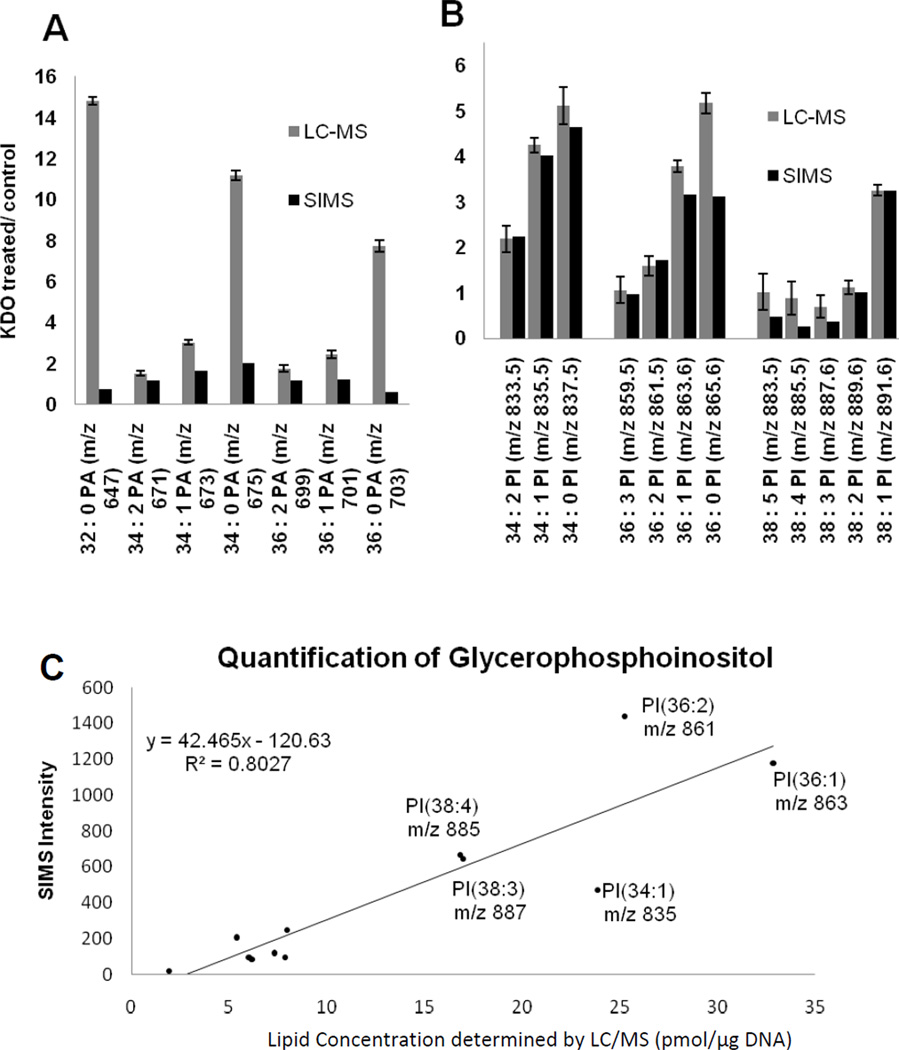
We used SIMS to examine the lipid biochemistry associated with the stimulation of mouse macrophages with the endotoxin KDO2-Lipid A. From the collection of detected lipid species, the majority did not exhibit a significant change as a result of KDO2-Lipid A stimulation with the exception of two classes of lipids, the PA and PI. The SIMS and LC-MS data for these two groups are shown in Figure 2. For PI, the SIMS data agree with the data obtained from LC-MS. In both studies, the concentration of PI lipids with saturated and monosaturated fatty acid functional groups were higher in activated macrophage cells compared to the inactive control cells. In addition, the down regulation of polyunsaturated fatty acids (PUFA) containing PI lipids was also observed in both studies. Due to the agreement between the two datasets, the LC-MS data were used to make a calibration curve (see Figure 2c). To evaluate the ability to obtain quantifiable data from SIMS, the integral counts obtained from the control lipid extract have been plotted as a function of concentration determined by the LC-MS data. The strong agreement between integral SIMS counts and concentration shows, in fact, that quasi-quantitative information can be obtained with SIMS analyses.
Figure 2.
Changes in the PA (a) and PI (b) chemistry associated with RAW 264.7 cell activation by KDO2-Lipid A and compared between SIMS of cell population surfaces and LC-MS of whole cell extracts. The SIMS data is compared to ratios obtained with LC-MS comparing the ratio of integral peak intensities for a given lipid in stimulated and inactive RAW 264.7 cells(c). The analysis of lipids from the ToF- SIMS spectrum is semi-quantitative within the PI lipid class.
In the case of PA lipids, the LC-MS studies revealed that unsaturated species (i.e. 32:0, 34:0 and 36:0) are significantly increased in the KDO2-Lipid A treated cells. Surprisingly the SIMS data are not consistent with this trend. Since the analysis was performed on the surface of a lawn of cells and not on whole cell extracts, we are not observing intercellular lipids. If the discrepancy is in fact the result of intercellular lipids, then further investigation with the 3D cellular imaging capabilities of SIMS will help to elucidate this observation.
Conclusions
In this investigation, a number of glycerophospholipid species in RAW 264.7 cells were characterized with C60-SIMS and tandem MS. This led to the identification of over 50 glycerophospholipid species from the RAW cell lipid mixture, including 22 PC, 9 PG, 12 PI and 9 PA species. At the single cell level, sensitivity is limited by the amount of material available to ionize. In this experiment, numerous intact lipid species were detected with as few as 500 cells. Although a promising starts, efforts to improve the ionization efficiency and molecular ion sensitivity of the technique are needed in order to achieve lipidomic characterization on the single cell level. In addition this investigation found that lipid alterations associated with KDO2-Lipid A stimulated RAW cells measured with C60-SIMS was in generally good agreement with LC-MS measurements, particularly for the PI class of lipids. Based on this study, the RAW 264.7 cell-KDO2 Lipid A stimulation model system should be further investigated, especially with SIMS-based cell imaging experiments.
Acknowledgments
The authors would like to thank Professor Robert Murphy and Dr. Joseph Hankin at the University of Colorado for insight on lipid chemistry. The authors acknowledge the LIPID MAPS Consortium (GM 069338-07) for financial support and LC-MS data. Also NW acknowledges additional financial support from the National Institutes of Health (2R01 EB002016-16) and the National Science Foundation (# CHE-0908226) and AGE acknowledges the European Research Council, the Knut and Alice Wallenberg Foundation, and the Swedish Research Council (VR) for support.
References
- 1.Passarelli MK, Winograd N. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbalip.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winograd N. Analytical Chemistry. 2005;77:142A. doi: 10.1021/ac051263k. [DOI] [PubMed] [Google Scholar]
- 3.Carado A, Passarelli MK, Kozole J, Wingate JE, Winograd N, Loboda AV. Analytical Chemistry. 2008;80:7921. doi: 10.1021/ac801712s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher JS, Rabbani S, Henderson A, Blenkinsopp P, Thompson SP, Lockyer NP, Vickerman JC. Analytical Chemistry. 2008;80:9058. doi: 10.1021/ac8015278. [DOI] [PubMed] [Google Scholar]
- 5.Dennis EA, Deems RA, Harkewicz R, Quehenberger O, Brown HA, Milne SB, Myers DS, Glass CK, Hardiman G, Reichart D, Merrill AH, Sullards MC, Wang E, Murphy RC, Raetz CRH, Garrett TA, Guan Z, Ryan AC, Russell DW, McDonald JG, Thompson BM, Shaw WA, Sud M, Zhao Y, Gupta S, Maurya MR, Fahy E, Subramaniam S. Journal of Biological Chemistry. 2010;285:39976. doi: 10.1074/jbc.M110.182915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raetz CRH, Garrett TA, Reynolds CM, Shaw WA, Moore JD, Smith DC, Ribeiro AA, Murphy RC, Ulevitch RJ, Fearns C, Reichart D, Glass CK, Benner C, Subramaniam S, Harkewicz R, Bowers-Gentry RC, Buczynski MW, Cooper JA, Deems RA, Dennis EA. Journal of Lipid Research. 2006;47:1097. doi: 10.1194/jlr.M600027-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Andreyev AY, Fahy E, Guan ZQ, Kelly S, Li XA, McDonald JG, Milne S, Myers D, Park H, Ryan A, Thompson BM, Wang E, Zhao YH, Brown HA, Merrill AH, Raetz CRH, Russell DW, Subramaniam S, Dennis EA. Journal of Lipid Research. 2010;51:2785. doi: 10.1194/jlr.M008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivanova PT, Milne SB, Byrne MO, Xiang Y, Brown HA. Lipidomics and Bioactive Lipids: Mass-Spectrometry-Based Lipid Analysis. 2007;432:21. [Google Scholar]
- 9.Brewer TM, Szakal C, Gillen G. Rapid Communications in Mass Spectrometry. 2010;24:593. doi: 10.1002/rcm.4423. [DOI] [PubMed] [Google Scholar]