Abstract
Hepatic allograft-derived lymph nodes were examined in the post-transplant period on order to determine the origin of lymphocytes and structural elements of the lymph node. Histologic assessment and immunohistochemical studies verified that T-cell infiltration of donor lymph nodes by recipient-derived lymphocytes occurred early in the post-transplant period. These T cells bore T-cell activation markers, e.g. TAC receptor and HLA-DR antigens. In addition, functional analysis demonstrated alloreactive T cells in secondary proliferation assays. The pattern of alloreactivity in these assays was dependent upon the phenotypic make-up (and therefore origin) of the lymphocytes within the lymph node. A gradual shift in predominance of donor-derived lymphocytes to recipient-derived lymphocytes occurred, but even late in the post-transplant course the stromal elements and a residium of lymphocytes within the lymph nodes continued to bear donor HLA antigens. The possible role of these “passenger” lymphocytes in allograft immunity is discussed.
Keywords: HLA, transplantation, lymphocytes
The role of “passenger” lymphocytes in the initiation of allograft rejection is not clear; however, several lines of evidence suggest that these cells are involved in allorecognition. Although these donor lymphocytes are generally rapidly eliminated in the host (review 1, 2), they provide the major stimulus of donor-specific sensitization of the transplant recipient. Experimental depletion of these cells from allografts has been reported to prolong the survival of an allograft in animal models (3, 4). Under certain circumstances, transplanted lymphocytes retain their functional activity to recognize alloantigens of the recipient, as evidenced by graft-versus-host reactions. These observations suggest that mutual interactions can occur between immunocompetent donor and recipient lymphocytes, and that these interactions may potentiate allorecognition (5).
The most significant immunologic sequelae following transplantation of solid organ allografts is rejection of the donor organ by recipient lymphocytes. Yet expression of graft-versus-host immunologic-mediated reactions have been reported following renal (6), hepatic (7), pulmonary (8), splenic (9) and pancreaticoduodenal (10, 11) transplantation. These reactions underscore the immunologic competency of “passenger” lymphocytes. Nevertheless, the generally transient nature of these reactions suggests that interactions between the recipient immune system and transplanted donor lymphocytes occurs. However, little is known regarding the nature of lymphocyte interactions in solid allograft transplantation.
A unique model to study the interactions between recipient lymphocytes and donor “passenger” lymphocytes is found in human hepatic transplantation. Approximately 15% of liver transplant patients undergo allograft hepatectomy with retransplantation, generally for unremitting rejection, but also for primary non-function of the allograft and for technical complications (12, 13). As shown in Fig. 1, the donor liver preparation contains numerous hilar lymph nodes which are transplanted en bloc into the recipient. These lymph nodes therefore retain their normal vascular and lymphatic connections to the liver allograft even following transplantation.
Fig. 1.
Schematic diagram depicting relationship of hilar hepatic lymph nodes to the vascular and biliary anatomoses in liver allografts. Note that the location of these lymph nodes (arrow) is well on the donor side of the anastomoses.
In this report, we describe our findings on the nature of lymphocytes and their interactions in hepatic hilar lymph nodes and the histological changes seen in these lymph nodes following human hepatic transplantation.
Material and methods
Sample/patient profile
Table 1 lists the number of days following liver transplantation and the indications for allograft hepatectomy in the 10 patients studied. In this paper, each lymph node (LN) sample is referred to by a unique number which identifies the patient and the post-transplant day when the hepatectomy was performed. For example, LN1.3, was obtained from patient 1 on the 3rd d post-transplant whereas LN6.17 was obtained from patient 6 on the 17th d following transplantation.
Table 1.
Indications for allograft hepatectomy
| Lymph node | Indication for hepatectomy |
|---|---|
| LN 1.3 | passive venous congestion* |
| LN 2.4 | primary graft non-function |
| LN 3.5 | primary graft non-function |
| LN 4.9 | rejection/hepatic artery thrombosis |
| LN 5.17 | rejection |
| LN 6.19 | rejection/portal vein thrombosis |
| LN 7.59 | rejection |
| LN 8.79 | rejection |
| LN 9.693 | rejection |
| LN 10.755 | rejection |
Passive venous congestion of the hepatic allograft occurred because of size mismatch and subsequent compression of a simultaneously placed heart allograft.
Source material
Liver transplantations were performed by techniques previously developed at our institution (14, 15). All of the grafts used for hepatic recipients were selected without knowledge of their HLA types. Post-transplantation, patients were maintained on a baseline immunosuppression regimen of cyclosporine and steroids. Evidence of allograft dysfunction was monitored by histologic, clinical and laboratory findings and was supplemented with radiographic and ultrasonographic studies (16). Treatment of rejection episodes by steroid boluses and, at times, the monoclonal anti-human T-cell antibody OKT3, has been discussed in detail elsewhere (16). The indication for allograft hepatectomy was failure to control allograft dysfunction along with clinical deterioration.
Lymph node samples were obtained, in a sterile manner, from freshly resected specimens at the time of allograft hepatectomy. Hilar lymph nodes were dissected close to the confluence of the right and left hepatic ducts in order to assure that the lymph nodes were of allograft origin.
Histologic and immunohistochemical studies
Lymph node samples, sent for histologic examination using standard hematoxylin and eosin stains, were first fixed in neutral buffered formalin and sectioned at 4 µm. Tissues for immunohistochemical staining were embedded in OCT compound, sectioned at 4–6 µm and stained using an indirect immunoperoxidase technique. A panel of monoclonal antibodies was used that included: OKT11 (pan-T cell), OKT4 (helper/inducer), OKT8 (suppressor/cytotoxic), TAC (IL-2 receptor), B1 (pan-B cell), DR (HLA-DR) (ORTHO Diagnostics) as well as type-specific anti-Class I MHC monoclonal antibodies.
Lymph nodes from donor/recipient combinations, with selected HLA disparities, were studied using monoclonal antibodies with specificities towards either donor and/or recipient HLA phenotypes. Monoclonal antibodies with defined anti-HLA specificities were kind gifts from Drs. John Hansen, Genetic Systems, Seattle WA, and Donna Kostyu, Duke University, Durham NC (17). A double immunoenzymatic method was employed for sequential staining using 2 different monoclonal antibodies and with 2 different enzymes, as previously described (18). The stained sections were analyzed to determine donor or recipient origin of lymph node structures by differentiating between the red and blue color reactions of amino ethyl carbazole (AEC) and naphthol AS-MX phosphate substrates, respectively.
Panel cells
Lymphocytes were obtained by either mechanical disruption of donor spleens, obtained during organ procurement, or from peripheral blood from hepatic recipients prior to transplantation. These cells were isolated by centrifugation over a Ficoll-Hypaque gradient (s.g. 1.077) (Ficoll-Paque, Pharmacia, Piscataway, NJ). Viability was determined with try-pan blue and cell aliquots were frozen in 20% human AB serum and 20% dimethyl sulfoxide, and stored in liquid nitrogen.
Isolation of lymph node lymphocytes
Single cell suspensions of lymphocytes were obtained from hilar lymph nodes by mechanical disruption followed by Ficoll-Hypaque density isolation. A portion was sent for determination of HLA phenotypes. In addition, an aliquot of the lymphocyte suspension was used to determine the secondary proliferation profile (PLT) towards a variety of stimulator cells.
Primed lymphocyte testing (PLT)
The PLT activity of lymphocytes obtained from extracted lymph nodes was measured in 3-d proliferation assays, as previously described for alloreactive T-cell clones (18). The relative proliferation of 5 × 103 isolated hilar lymph node lymphocytes to 5 × 104 irradiated donor stimulator cells, as well as irradiated recipient stimulator cells, was determined following incubation for 72 h. In addition, proliferation to recombinant Interleukin-2 (Sandoz Pharmaceuticals, Basel) and “spontaneous” proliferation in 10% human AB serum was assessed. During the final 20 h of incubation, each culture was pulsed with 1µCi of 3H-thymidine (specific activity, 20 mCi/mmole, New England Nuclear Products, Boston, MA). The cultures were harvested with a multiple sample harvester (Skatron, Inc., Sterling, VA) and uptake was determined by liquid scintillation counting (LKB, Gaitherburg, MD).
HLA phenotyping
Peripheral blood lymphocytes, donor spleen cells and lymph node-derived lymphocytes were typed for HLA-A and -B antigens using standard NIH microcytotoxicity assays with defined HLA-specific antisera. Serologic typing for HLA-DR was done by prolonged incubation microlymphocytotoxicity testing using enriched B-cell preparations obtained following carbonyl-iron treatment and Ficoll-Hypaque sedimentation after rosetting with neuraminidase-treated sheep red cells.
A portion of the cell suspension obtained from allograft lymph nodes was tested in microcytotoxicity assays to determine the HLA phenotypes of these cells. The patterns of reactivity were evaluated in a blinded manner by one of us (M.M.), without prior knowledge of donor or recipient HLA phenotypes.
Results
Histology of lymph nodes
Morphologically, resting hepatic hilar lymph nodes are generally small, measuring 5–10 mm in length. However, shortly after transplantation, marked enlargement of these lymph nodes was seen to occur. The changes in the size of the lymph nodes were correlated with histologic changes. The earliest histologic change observed (LN1.3) was hypertrophy of the sinusoidal lining and high endothelial venules (HEV) in the paracortex. Expansion of the paracortical area was seen in lymph nodes starting 1 wk following transplantation, with an increase in immunoblasts, some with plasmacytoid features. Lymphoid cells could be seen beneath and within the wall of the HEV and sinusoidal lining cells in a pattern similar to that seen during early rejection patterns seen in most solid organ transplants (20–23). Active secondary follicle or germinal center formation in the cortex was not a prominent feature.
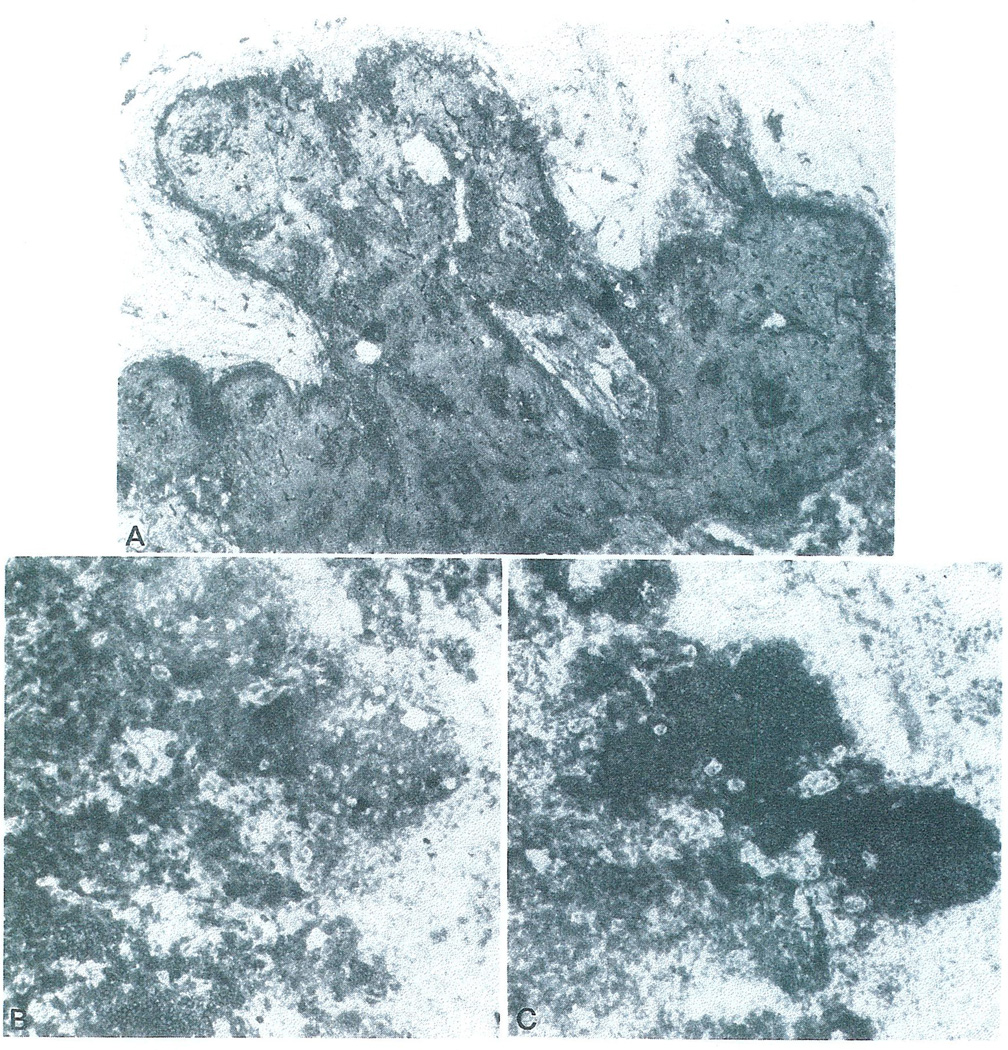
The most striking changes were observed in LN4.9. Grossly, the nodes were enlarged up to 3 cm in size. Microscopically, the nodal architecture was intact. Nodules of small lymphocytes could be seen in the cortex and were identified as B-cell nodules without active secondary follicle formation. There was increased cellularity in the T cell-dependent areas of the lymph nodes, namely the interfollicular and paracortical areas (Fig. 2a). Numerous mitoses could be observed with the presence of immunoblasts and monocytoid cells (Fig. 2b). The nuclei of cells lining the sinuses and HEV were quite prominent and these cells were infiltrated and undermined by lymphoid cells and lifted from the underlying connective tissue (Fig. 2b). Again, the appearance was similar to that seen during rejection reactions in other tissues.
Fig. 2.
A) Histologic section of LN4.9 demonstrating expansion of the interfollicular cortex and paracortex without formation of secondary cortical follicles (40X magnification). B) A higher power magnification of LN4.9 showing hypertrophy of the HEV endothelium and subendothelial lymphocytic infiltrates (arrow head) as well as mitotic activity of lymphoid cells (arrow) found in the paracortical region.
Later in the post-transplant period, hepatic hilar lymph nodes appeared quiescent with relatively normal architecture, as was seen in LN9.693 and LN10.755, and their size was the same or smaller than normal. Microscopically, these nodes had a relative increase in reticuloendothelial cells and a relative decrease in lymphoid elements, but retained the normal segregation of T and B cells (Fig. 3b, 3c).
Fig. 3.
Immunohistochemical staining of LN 9.693. A) Histologic appearance of this lymph node demonstrates normal architecture (not shown). In addition, monoclonal antibodies with specificity to HLA-A3 demonstrate that the stroma, vascular endothelium and scattered foci of lymphoid cells are of donor origin (dark cells) (immunoperoxidase, 40X magnification). B) and C) Monoclonal anti-T cell and anti-B cell antibodies, respectively, were used to demonstrate the normal localization of these lymphocyte subsets within the lymph node.
Immunohistochemical staining of lymph nodes
T cell-specific monoclonal antibodies were used to stain hilar lymph node sections in order to confirm that the early hypertrophy was due to an increase in T cells. The architectural distribution of T lymphocytes was similar to that seen in normal reactive lymph nodes. A preponderance of T4 “hel-per-inducer” phenotypes was found in these lymph nodes. In addition, areas showing T-cell hypercellularity also contained a large number of cells which bore TAC receptors, an indicator of T-cell activation (Fig. 4). Staining of HLA-DR antigens (which is also a marker of T-cell activation) is less specific than TAC staining because of the presence of HLA-DR antigens on B cells and monocytoid cells. Nevertheless, a prominent increase in the relative staining of DR-positive cells could be detected in LN4.9, LN5.17 and LN6.19 (data not shown).
Fig. 4.
Anti-TAC antibodies were used to identify activated T cells within the subcapsular sinus and paracortex in LN4.9.
The architecture of the lymph nodes remained intact throughout the post-transplant period. Staining with B1 revealed that the B cell-dependent areas were primarily located in the cortex and retained their normal configuration and did not show activated germinal centers. This is in contrast to findings previously reported with immunosuppression consisting of azathioprine and steroids where activated germinal centers were a prominent feature (23).
HLA phenotypes of hilar lymph node lymphocytes
Using standard lymphocyte microcytotoxicity assays, the HLA reactivity pattern of the lymphocytes extracted from hilar lymph nodes was compared to the HLA phenotypes of the donor and recipient. As shown in Table 2, as early as 3 d post-transplant, hepatic hilar lymph nodes apparently contained a mixture of donor and recipient lymphocytes, as determined by reactivity towards HLA-defined antisera. This mixture of donor and recipient lymphocytes appeared to phenotypically shift from predominantly donor origin to predominantly recipient origin during the post-transplant period. This was determined by comparison of the strength of reaction patterns by complement-mediated lymphocytotoxicity assays. Early in the post-transplant period, the strongest reactivity was towards donor cells, while later the strength of reactivity shifted towards recipient patterns (data not shown).
Table 2.
HLA phenotypes of transplanted allograft lymph node lymphocytes*
| Patient HLA | Donor HLA | Lymph node HLA | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymph node | A | A | B | B | Bw | Bw | A | A | B | B | Bw | Bw | A | A | A | B | B | B | Bw | Bw |
| LN 1.3 | 3 | 28 | 44 | 35 | 4 | 6 | 1 | 24 | 13 | 44 | 4 | – | 1 | 3 | 28 | 44 | 35 | – | 4 | 6 |
| LN 2.4 | 1 | 2 | 14 | w60 | 6 | – | 2 | 3 | 27 | 35 | 4 | 6 | 2 | 3 | – | 27 | 35 | w60 | 4 | 6 |
| LN 3.5 | 1 | 24 | 35 | w55 | 6 | – | 29 | 31 | 7 | w60 | 6 | – | 1 | 24 | 31 | 35 | w60 | w55 | 6 | – |
| LN 4.9 | 2 | – | w62 | 35 | 6 | – | 26 | 11 | 18 | w41 | 6 | – | 2 | 11 | – | w62 | 35 | 18 | 6 | – |
| LN 5.17 | 2 | 28 | 27 | w60 | 4 | 6 | 2 | 28 | 44 | w57 | 4 | – | 2 | 28 | – | 27 | w60 | 44 | 4 | 6 |
| LN 6.19 | 2 | – | 35 | – | 6 | – | 25 | 32 | 18 | w60 | 4 | 6 | 2 | 25 | – | 35 | – | – | 4 | 6 |
| LN 7.59 | 2 | w33 | 44 | 38 | 4 | – | 2 | 29 | 7 | – | 6 | – | ND | ND | ND | ND | ND | ND | ND | ND |
| LN 8.79 | 2 | 24 | 8 | 35 | 6 | – | 2 | 28 | 44 | 35 | 4 | 6 | 2 | 24 | 28 | 8 | 35 | – | 4 | 6 |
| LN 9.693 | 1 | – | w57 | – | 4 | – | 3 | 29 | 7 | 44 | 4 | 6 | 1 | – | – | w57 | – | – | 4 | – |
| LN 10.755 | 2 | – | 51 | 35 | 6 | – | 3 | 11 | 7 | w48 | 6 | – | ND | ND | ND | ND | ND | ND | ND | ND |
Denotes HLA antigen reactions by microlymphocytotoxicity assays.
While microlymphocytotoxicity assays were used to detect qualitative changes in the reactivity pattern of lymph node lymphocytes, these findings were verified in several instances by immunohistochemical staining using donor-specific and/or recipient-specific monoclonal anti-HLA antibodies (Figs. 3a, 5a, 5b). In this manner a quantitative change in the phenotypic makeup of these cells could be analyzed. This was particularly evident in those lymph nodes which were stained using a double immunoenzymatic method with 2 monoclonal antibodies, one of donor specificity and the other of recipient specificity.
Fig. 5.
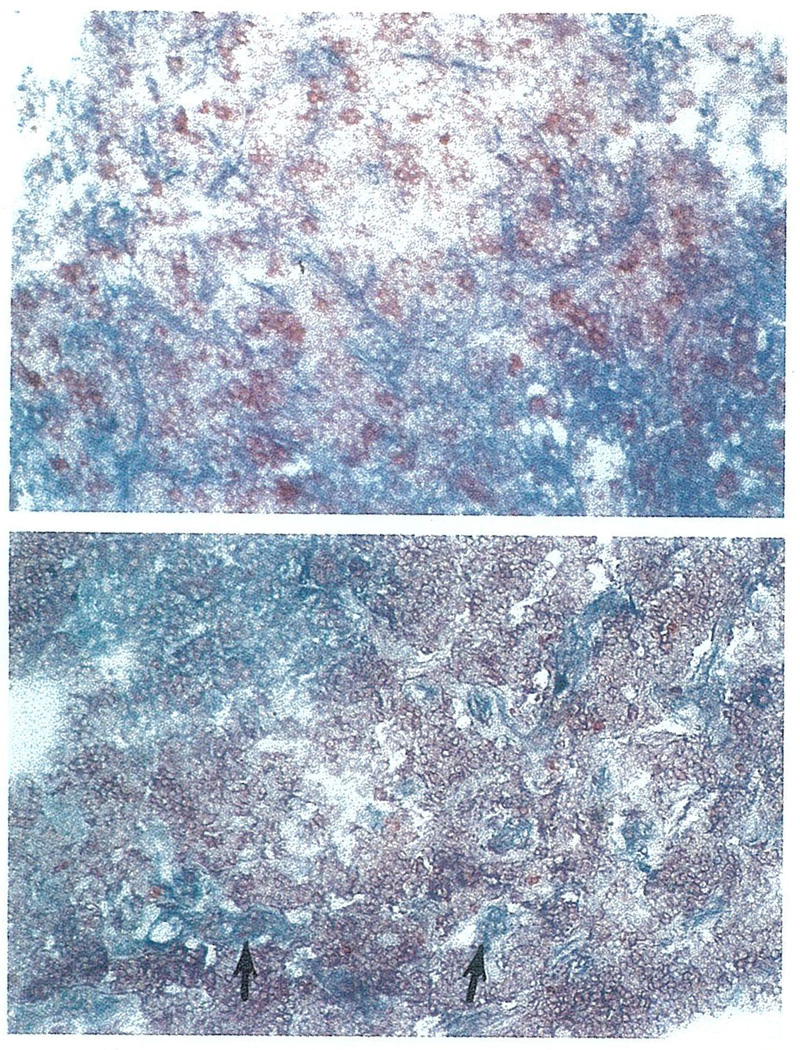
A) Two-color immunohistochemical staining of LN6.19, using monoclonal anti-donor and recipient HLA antibodies, show that the lymphoid cells contain a mixture of donor (anti-HLA A25, blue) and recipient (anti-HLA A2, red) lymphocytes, while the stromal elements are of donor origin. B) Similarly, in LN 10.755, while the majority of lymphoid cells are of recipient origin (anti-HLA A2, red), the stromal elements and isolated nests of lymphoid cells are of donor origin (anti-HLA A3, blue).
Two such examples are shown in Fig. 5a and 5b. In LN 6.19, the donor was HLA typed as A25,32,B18,w60(Bw4,6), while the recipient was typed as A2,–;B35,–(Bw6). An HLA-A2-specific monoclonal antibody, P5.1, was used to first stain recipient-derived cells with a red reaction product, which was followed by an HLA-A25-specific monoclonal antibody, 1125, to determine structures which were of donor origin (blue reaction product). In this early post-transplant specimen, in agreement with cytotoxicity data, both donor and recipient lymphocytes can be detected with the stromal elements being of donor origin. Similar staining of lymph node samples obtained in the 1st month post-transplantation verified a mixture of lymph node lymphocytes of both donor and recipient origin. In the early lymph node specimens (e.g. LN2.4 and LN4.9), the incoming recipient lymphocytes could be seen in the peripheral and trabecular sinuses. By the 2nd wk, the larger population of recipient lymphocytes was seen in the paracortex with lesser numbers in the sinuses. The dual staining of these early post-transplant lymph nodes confirmed that resident and infiltrating lymphocytes were of exclusive origins.
In LN 10.755, the donor was HLA typed as A2,–;B51,35;(Bw6);DR2,5 and the recipient was typed as A3,11;B7,w48;(Bw6);DR4,w6. An HLA-A3-specific monoclonal antibody, GAP-A3, was used to first stain recipient-derived cells with a red reaction product, which was followed by an HLA-A2-specific monoclonal antibody, P5.1, to determine structures which were of donor origin (blue reaction product). It is evident that the majority of lymphocytes were of recipient origin, while the stromal elements, e.g. blood vessels, capsule and trabeculae, and residual nests of lymphocytes were persistently of donor origin.
Correlation with proliferate reactivity
Lymphocyte suspensions were tested in PLT assays to examine the pattern of proliferation towards donor or recipient stimulators. As shown in Table 3, all lymph node-derived lymphocytes demonstrated IL-2 responsiveness in PLT assays. Lymphocytes, from lymph nodes taken from transplants greater than 1 month old, showed primarily reactivity towards donor cells in PLT assays. On the other hand, lymphocytes extracted from lymph nodes removed in the early post-transplant period showed mixed proliferative responses, varying from primarily reactivity towards recipient cells to an equal reactivity towards either cell. The functional studies verified the complimentary HLA phenotype and PLT alloreactivity of the lymphocytes within the lymph node.
Table 3.
Proliferative reactivities of transplanted allograft lymph node lymphocytes
| 3H-thymidine uptake (CPM)* | ||||
|---|---|---|---|---|
| Lymph node | 10% AB (spontaneous) |
Recombinant interleukin-2 |
Donor stimulated† |
Recipient stimulated† |
| LN 1.3 | 1,444 | 6,232 | 4,293 | 9,547 |
| LN 2.4 | 4,242 | 7,083 | 1,563 | 12,506 |
| LN 3.5 | 11,008 | 53,769 | 13,521 | 12,258 |
| LN 4.9 | 2,302 | 25,130 | 13,927 | 4,711 |
| LN 5.17 | 6,723 | 16,801 | 18,070 | 19,375 |
| LN 6.19 | 10,066 | 17,180 | 26,772 | 8,581 |
| LN 7.59 | 877 | 17,347 | 26,336 | 3,202 |
| LN 8.79 | 1,612 | 35,6911 | 51,620 | 3,787 |
| LN 9.693 | 1,002 | 12,556 | 14,434 | 2,090 |
| LN 10.755 | 1,773 | 21,944 | 8,805 | 1,266 |
Uptake of 3H-thymidine was determined in 3 d proliferation assays.
Irradiated lymphocytes were used as the source for stimulating cells in PLT assays.
Cells from lymph nodes which showed a mixture of lymphocytes bearing HLA phenotypes of both the donor and recipient (e.g. LN3.5, LN5.17) appeared to have equal proliferative reactivity towards both donor and recipient cells. In these cases, one would expect that the “spontaneous” proliferation, i.e. lymph node lymphocytes incubated with 10% human serum only, would be high. This would reflect a phenomenon of mutual stimulation of donor – and recipient-derived lymphocytes, since both populations would be present in culture. Indeed, a high “spontaneous” uptake of thymidine was noted in these early post-transplant samples.
Discussion
Peripheral lymph nodes (including mesenteric lymph nodes) play an important role in the immune system and in the development of humoral and cell-mediated immunity. Particulate and soluble antigens are brought into the lymph node by the afferent lymphatics and are optimally processed by antigen-processing cells in this environment, with subsequent antigenic stimulation of T and B cells. A continuously recirculating pool of mature lymphocytes influx and exit lymph nodes, presumably in order to optimize chances that an antigen-specific lymphocyte population, found in the wide spectrum of antigen specificities, will encounter a specific antigen processed in a lymph node. Lymph nodes draining sites of alloantigen immunization or allograft transplantation have demonstrated the presence of donor-specific alloreactive T cells (24, 25).
The model presented here is unique, in that one can study the interactions of two immunocompetent lymphocyte populations within an environment of optimal alloantigen presentation found within a lymphoid organ. These interactions appear to trigger the mutual alloactivation of both donor and recipient lymphocytes. Functionally, both recipient-specific alloreactive T cells of donor origin can be demonstrated, similar to that seen in two-way mixed lymphocyte reactions (26). The relative intensity of the anti-donor or anti-recipient response appears to be directly correlated to the number of recipient or donor lymphocytes in the lymph node at the time of testing, as determined by immunohistochemical and serologic assays. Therefore lymphocytes obtained fairly early in the post-transplant course, which phenotypically have greater numbers of donor-derived cells, have a correspondingly higher anti-recipient response. On the other hand, later in the post-transplant periods, as continuing replacement of donor lymphocytes by recipient-derived lymphocytes occurs, there is a relatively greater anti-donor response.
The fate of donor-derived lymphocytes in hepatic hilar lymph nodes during the post-transplant period is not known. A wealth of data has been presented by other investigators showing that un-primed allogeneic hosts rapidly eliminate injected donor lymphocytes, termed “natural cytotoxicity” (1). We have been able to demonstrate a low degree of donor-specific lymphocytotoxicity in such lymph nodes (data not shown here), but it is not clear whether this is the primary mode of allogeneic lymphocyte elimination. It is likely that the physiologic process of exodus of lymphocytes from a lymph node into the peripheral circulation continues within these nodes. Transplanted lymph nodes in a rodent model have shown rapid reestablishment of efferent lymphatic channels with functional lymphatic drainage (27–29).
Influx of recipient-derived lymphocytes into allogeneic donor lymph nodes may take place by two mechanisms, one by transit through the high endothelial cells lining the post-capillary venules of the lymph node (30), or via afferent lymphatic channels draining the liver allograft. It is likely that both mechanisms are involved. Histologically, there is massive hypertrophy of T cell-dependent areas and these T cells bear activation markers. The B-cell areas within the lymph nodes appear to be less affected following transplantation. These samples were taken from patients who had been on cyclosporine, and the primary mode of action of cyclosporine is modulation of T-cell activation (31). It is possible that while T-cell activation continues to occur in allograft lymph nodes, the release of lymphokines (such as B-cell growth factor) is modulated and subsequent B-cell responses are diminished. In earlier studies of hepatic-derived lymph nodes taken from patients on azathioprine/steroid therapy, it was noted that substantial hypertrophy occurred in B cell-dependent areas (23).
Several investigators have suggested that specific homing mechanisms or recirculating lymphocytes into peripheral lymph nodes is MHC-restricted (32–34). Rolstad and Ford (2) have forwarded the contrary view that such migration is not MHC-restricted. Certainly the data presented here supports the latter contention, although the infiltration of allospecific recipient lymphocytes may also be modulated by other factors, e.g. soluble chemotactic factors, secreted by alloactivated lymphocytes.
The presence of persistently acquired immuno-globulin of donor allotype in some liver transplant recipients has suggested that donor lymphocytes are present in long-term allografts (35). Indeed, we have been able to detect residual nests of donor lymphocytes within lymph nodes from long-term functioning allografts. However, it cannot be determined at this time whether it is this residuum of lymphocytes or the plasma cells normally found within the liver parenchyma that is responsible for continued antibody secretion following transplantation. Finally, in agreement with studies by Porter (23), we found that structural and stromal elements continue to bear donor phenotypes.
Outside of the obvious interest of using this model to study lymph node physiology and immunology, one can also begin to speculate as to the role of “passenger” lymphocytes in the development of allograft immunity. Lacy et al. (4) first demonstrated that pretreatment of islet allografts, in order to eliminate “passenger” lymphoid cells, could prolong the survival of these allografts in rodents. Prop et al. (3) demonstrated that removal of bronchus-associated lymphoid tissues (BALT) resulted in diminished lung rejection in rodents. The reason(s) for this phenomenon is not clear, but certainly lymphocytes bear HLA antigens and are potent stimulators of alloactivation. One might expect that their removal would diminish the degree of antigenicity of the allograft. The mechanism(s) involved in the infiltration and alloactivation of T cells into lymph nodes may be the same as that seen in allografts. Preliminary data from immunogenetic analysis of cloned T cells from these rejected human liver allografts and hilar lymph nodes has shown similar patterns of reactivity to HLA-defined antigens.
This phenomenon does not appear to be unique to hepatic allografts only; in two instances where hilar lymph nodes were examined from human lung allografts, there appeared to be variable phenotypic expressions of donor- and recipient-derived lymphocytes. In addition, a high degree of “spontaneous” proliferation was seen in the lymph node-derived lymphocyte suspension from a lung allograft removed 10 d post-transplant. We have previously shown that these lung allografts undergo a transition period whereby bronchoalveolar macrophages and lymphocytes (obtained by bronchoalveolar lavage) appear to shift from donor to recipient origin (36, 37). Interestingly, a similar phenomenon of high “spontaneous” proliferation was noted in bronchoalveolar cells phenotypically showing mixtures of donor and recipient cells. As with hepatic hilar lymph nodes, lymphocytes isolated from bronchoalvolar lavages have demonstrated donor-specific secondary proliferation (5, 36).
Since the majority of acute cell-mediated rejection episodes occur within the first few months following transplantation, and because it is during this period that interactions between donor and recipient lymphocytes occur, it is possible that these events may be related. Further study of role of “passenger” lymphocytes in the development of allograft immunity may elucidate the mechanism(s) and possible therapeutic interventions of allograft rejection.
Acknowledgments
Grant Support: Supported by Grants AI-21410 and AI-18923 from NIAID, and Grant AM-29961 from NIH.
References
- 1.Heslop B, McNeilage L. Natural cytotoxicity: Early killing of allogeneic lymphocytes in rats. Immunol Rev. 1983;73:35. doi: 10.1111/j.1600-065x.1983.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 2.Rolstad B, Ford W. The rapid elimination of allogeneic lymphocytes: Relationship to established mechanisms of immunity and to lymphocyte traffic. Immunol Rev. 1983;73:87. doi: 10.1111/j.1600-065x.1983.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 3.Prop J, Kuijpers K, Nieuwenhuis R, Wildevurr C. Why are lung grafts rejected more vigorously than heart grafts? Heart Transplant. 1985;4:433. [PubMed] [Google Scholar]
- 4.Lacy P, Davie J, Finke E. Prolongation of islet allograft survival following in vitro culture (24°C) and a single injection of ALS. Science. 1979;204:312. doi: 10.1126/science.107588. [DOI] [PubMed] [Google Scholar]
- 5.Zeevi A, Fung J, Paradis I, et al. Lymphocytes of bron-choalveolar lavages from heart-lung transplant recipients. Heart Transplant. 1985;4:417. [PubMed] [Google Scholar]
- 6.Stevens J, Callender C, Jilly P. Emergence of red blood cell agglutinins following renal transplantation in a patient with systemic lupus erythematosus. Transplantation. 1981;32:398. doi: 10.1097/00007890-198111000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Ramsey G, Nusbacher J, Starzl T, Lindsay G. Isohemag-glutinins of graft origin after ABO-unmatched liver transplantation. New Engl J Med. 1984;311:1167. doi: 10.1056/NEJM198411013111807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck H, Haines E, Oberman H. Unexpected serologic findings following lung homotransplantation. Abstract presented at Amer Assoc Blood Banks, 24th Annual Meeting; Chicago. 1971. [Google Scholar]
- 9.Marchioro T, Rowlands D, Rifkind D, Waddell W, Starzl T. Splenic homotransplantation. Ann NY Acad Sci. 1964;120:626. doi: 10.1111/j.1749-6632.1964.tb34757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl T, Iwatsuki S, Shaw B, et al. Pancreaticoduodenal transplantation in humans. Surg Gyne Obstet. 1984;159:265. [PMC free article] [PubMed] [Google Scholar]
- 11.Deierhoi M, Sollinger H, Bozdech M, Belzer F. Lethal graft-versus-host disease in a recipient of a pancreas-spleen transplant. Transplantation. 1986;41:544. [PubMed] [Google Scholar]
- 12.Shaw B, Gordon R, Iwatsuki S, Starzl T. Hepatic re-transplantation. Transplant Proc. 1985;17:264. [PMC free article] [PubMed] [Google Scholar]
- 13.Esquivel C, Jaffe R, Gordon R, Iwatsuki S, Shaw B, Starzl T. Liver rejection and its differentiation from other causes of graft dysfunction. Semin Liver Dis. 1985;5:369. doi: 10.1055/s-2008-1040634. [DOI] [PubMed] [Google Scholar]
- 14.Starzl T, Iwatsuki S, Esquivel C, et al. Refinements in the surgical technique of liver transplantation. Semin Liver Dis. 1985;5:349. doi: 10.1055/s-2008-1040632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl T, Iwatsuki S, Shaw B. Technique of liver transplantation. In: Blumgart C, editor. Surgery of the Liver and Biliary Tract. Edinburgh: Churchill Livingston; 1986. [Google Scholar]
- 16.Fung J, Demetris A, Porter K, et al. Use of 0KT3 with cyclosporine and steroid for reversal of acute kidney and liver allograft rejection. Nephron. 1986;46(Suppl):19. doi: 10.1159/000184431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fauchet R, Bodmer JG, Kennedy LJ, et al. Albert ED, Baur MP, Mayr WR, editors. HLA-A, B, C monoclonal antibodies. Report on the Ninth International Histocompatibility Workshop, Histocompatibility Testing. 1984:211–216.
- 18.Chen K, Demetris A, VanThiel D, Whiteside T. A double immunoenzyme staining method for analysis of tissue and blood lymphocyte subsets with monoclonal antibodies. (Submitted) [PubMed] [Google Scholar]
- 19.Zeevi A, Scheffel C, Annen K, Bass G, Marrari M, Duquesnoy R. Association of PLT specificity of alloreactive lymphocyte clones with HLA-DR MB and MT determinants. Immunogenetics. 1982;16:209. doi: 10.1007/BF00343310. [DOI] [PubMed] [Google Scholar]
- 20.Demetris A, Lasky S, VanThiel D, Starzl R, Dekker A. Pathology of hepatic transplantation: A review of 62 adult allograft recipients immunosuppressed with cyclosporine/steroid regimen. Am J Pathol. 1985;118:151. [PMC free article] [PubMed] [Google Scholar]
- 21.Snover D, Sibley R, Freese D, Bloomer J, Sharp H, Ascher N. Orthotopic liver transplant rejection: A pathological study of 63 serial liver biopsies from 17 patients with special reference to the diagnostic features and natural history of rejection. Hepatology. 1984;4:1212. doi: 10.1002/hep.1840040620. [DOI] [PubMed] [Google Scholar]
- 22.Porter K. Pathological changes in transplanted kidneys. In: Starzl T, editor. Experiences in Renal Transplantation. Philadelphia: Saunders; 1964. p. 299. [Google Scholar]
- 23.Porter K. Pathology of the orthotopic homograft and heterograft. In: Starzl T, editor. Experiences in Hepatic Transplantation. Philadelphia: Saunders; 1969. p. 422. [Google Scholar]
- 24.Cahill R, Frost H, Hay J, Lafleur L, Trnka Z. Changes in mixed lymphocyte culture-reactive lymphocytes following alloimmunization of single lymph nodes in sheep. Transplantation. 1979;27:102. doi: 10.1097/00007890-197902000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Hanto D, Harty J, Simmons R. Lymphocyte recruitment properties of regional lymph node or spleen cells during skin allograft rejection. Transplantation. 1983;36:578. [PubMed] [Google Scholar]
- 26.Bach F, Van Rood J. The major histocompatibility complex: Genetics and biology, Part 1. New Engl J Med. 1976;295:806. doi: 10.1056/NEJM197610072951504. [DOI] [PubMed] [Google Scholar]
- 27.Rabson J, Geyer S, Levine G, Swartz W, Futrell J. Tumor immunity in rat lymph nodes following transplantation. Ann Surg. 1982;196:92. doi: 10.1097/00000658-198207000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kister S, Conklin E, Habif D. Autotransplantation of lymph nodes in the dog. Surg Forum. 1965;16:266. [PubMed] [Google Scholar]
- 29.Tilak S, Howard B. Regeneration and autotransplantation of lymph nodes. Ann Surg. 1965;161:441. doi: 10.1097/00000658-196503000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gowans J, Knight E. The route of recirculation of lymphocytes in the rat. Proc Royal Soc Lond. 1964;159B:257. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 31.Palacios R. Mechanism of T-cell activation: Role and functional relationship of HLA-DR antigens and interleukins. Immunol Rev. 1982;63:73. doi: 10.1111/j.1600-065x.1982.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 32.Neeuwenhuis P, de Vries-Bos L, Opstelten D, Deenen G, Stet R, Rozing I. Lymphocyte migration across major histocompatibility barriers in splenectomized rats. Immunol Rev. 1983;73:53. doi: 10.1111/j.1600-065x.1983.tb01078.x. [DOI] [PubMed] [Google Scholar]
- 33.Zatz M, Gingrich R, Lance E. The effect of histocompatibility antigens on lymphocyte migration in the mouse. Immunology. 1972;23:665. [PMC free article] [PubMed] [Google Scholar]
- 34.Degos L, Pla M, Colombani J. H-2 restriction for lymphocyte homing into lymph nodes. Eur J Immunol. 1979;9:808. doi: 10.1002/eji.1830091012. [DOI] [PubMed] [Google Scholar]
- 35.Kashiwagi N, Porter K, Penn I, Brettschneider L, Starzl T. Studies of homograft sex and of gamma globulin phenotypes after orthotopic homotransplantation of the human liver. Surg Forum. 1969;xx:374. [PMC free article] [PubMed] [Google Scholar]
- 36.Fung J, Zeevi A, Kaufman C, et al. Interactions between bronchoalveolar lymphocytes and macrophages in heart-lung transplant recipients. Human Immunol. 1985;14:287. doi: 10.1016/0198-8859(85)90236-8. [DOI] [PubMed] [Google Scholar]
- 37.Paradis I, Marrari M, Zeevi A, et al. The transition of lung lavage cells from donor to recipient HLA phenotype following heart-lung transplantation. Heart Transplant. 1985;4:422. [PubMed] [Google Scholar]