Abstract
Background
Hospital readmissions are a quality of care indicator, yet little is known about their occurrence and predictors after myocardial infarction (MI) in the community.
Objective
Examine 30-day hospital readmissions after incident MI.
Design
Retrospective cohort study
Setting
Population-based registry in Olmsted County, Minnesota
Patients
3010 patients admitted to an Olmsted County hospital with first-ever MI from 1987 to 2010 that survived to hospital discharge.
Measurements
Diagnoses, therapies, and complications during incident and subsequent admissions were identified. Manual chart review was performed to determine the cause of all readmissions. The hazard ratios and cumulative incidence of 30-day readmissions were determined using Cox proportional hazard regression models.
Results
Among 3010 patients (mean age 67 years, 40.5% female) with incident MI (31.2% ST-elevation), 643 readmissions occurred within 30 days in 561 (18.6%) patients. Overall, 30.2% of readmissions were unrelated to the incident MI and 42.6% were related; the relationship was unclear in 27.2% of readmissions. Angiography was performed in 153 (23.8%) readmissions. Revascularization was performed in 103 (16.0%) readmissions, of whom 46 (44.7%) had no revascularization during the index admission. After adjustment for potential confounders, diabetes, COPD, anemia, higher Killip class, longer length of stay during the index hospitalization, and a complication of angiography or reperfusion/revascularization were associated with increased readmission risk. The 30-day incidence of readmission was 35.3% and 31.6% in patients who experienced a complication of angiography or reperfusion/revascularization during the index MI admission, respectively, compared with 16.8% in patients who had reperfusion/revascularization without complications.
Limitations
This represents the experience of a single community.
Conclusions
Comorbidity, longer length of stay, and complications of angiography and revascularization/reperfusion are associated with increased 30-day readmission risk after MI.
INTRODUCTION
Each year, an estimated 785,000 Americans will have a first acute myocardial infarction (MI)(1). With advancement in therapies, in-hospital survival after MI has dramatically improved(2, 3). Thus, a large number of incident MI survivors are being dismissed from the hospital into the community and are at risk for readmissions. As readmissions are costly and frequent in the initial period following MI, 30-day readmission rates have attracted major attention and have become a marker of quality of care(4, 5). However, little is known about the reason for readmission after MI and whether there are patient and/or treatment-specific factors associated with readmission. Furthermore, it is unclear whether the risk of readmission has changed over time.
In order to address these gaps in knowledge, we examined 30-day readmissions following incident MI in our ongoing coronary disease surveillance study in Olmsted County, Minnesota. This study, performed under the auspices of the Rochester Epidemiology Project(6), is uniquely positioned to examine this issue since all incident MIs are identified, and the entire health care experience from diagnosis to death is captured for these patients in a community setting. Among incident MI survivors diagnosed from 1987 to 2010, we sought to examine: (1) 30-day readmission rates after MI, (2) the reasons for readmissions, and (3) factors associated with increased readmission.
METHODS
Study Design and Setting
This study was conducted in Olmsted County, Minnesota (2010 population 144,248). Population-based research is possible because there are few hospitals, namely Olmsted Medical Center and Mayo Clinic. Medical records from all sources of care for residents are linked via the Rochester Epidemiology Project(6). Therefore, patient-level information can be obtained via the medical and administrative records. This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Incident MI Patient Identification and Validation
Olmsted County residents admitted with possible MI to Olmsted County Hospitals from 1987–2010 were identified using methods previously described(3). Briefly, all events with International Classification of Diseases, 9th revision (ICD-9), code 410 (acute MI) were reviewed, along with a 50% random sample of code 411 (other ischemic heart disease) from 1987–1998, a 10% random sample of 411 codes from 1999–2002, and a 100% sample of 411 codes from 2003–2010. Additional codes were not included as they were low yield. Patients were excluded if they declined to provide Minnesota Research Authorization, which is provided by >96% of Olmsted County adults.
MIs were validated using standard epidemiologic criteria(3). Patients diagnosed with MI prior to 1987 were excluded as this study included only patients with incident (first-ever) MI. The diagnosis of MI was verified based on the presence of two out of three of the following: cardiac pain, elevated biomarkers, and ECG changes using standard algorithms(7). The biomarkers creatine kinase (CK) and CKMB were used until 2000, and troponin T thereafter. Case reviews were performed to ensure that alternative causes for biomarker elevation were considered. Troponin T, CK, and CKMB were measured with a sandwich electrochemiluminescence immunoassay on the Elecsys 2010 (Roche Diagnostics Corp, Indianapolis, IN) in the Laboratories of the Department of Medicine and Pathology at Mayo Clinic. Three ECGs per episode were coded using the Minnesota Code Modular ECG analysis system(8).
Killip class (measures the severity of HF in patients with acute MI; higher scores indicate worse prognosis) was ascertained based on documentation. Patients were categorized as ST-segment elevation (STEMI) or non ST-segment elevation MI (NSTEMI) based on whether ST elevation was present on the ECG using the Minnesota code(8). Reperfusion was defined as the receipt of fibrinolytic therapy; revascularization was defined by the receipt of coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI) during hospitalization. Whether a coronary angiogram was performed was documented. Complications were assessed using laboratory data and manual chart review (SMD). For PCI and coronary angiography, we assessed bleeding or vascular complications during the hospitalization, stroke within 72 hours of the procedure, and acute renal failure (new dialysis within 30 days or >0.5 mg/dL increase in serum creatinine within 72 hours post-procedure)(7, 9, 10). Vascular access site complications included groin hematoma resulting in a hemoglobin drop ≥3 grams, new arteriovenous fistula or pseudoaneurysm requiring a procedure to treat, or vascular occlusion or dissection. Additional bleeding complications (retroperitoneal, GI, or other) resulting in a hemoglobin drop ≥3 grams or a procedure to stop the bleeding were assessed(9, 11). As cardiac biomarkers were frequently rising in the setting of acute MI, we could not accurately identify periprocedural MI as a complication(9, 12, 13). Stroke occurring during hospitalization following fibrinolysis or CABG and acute renal failure after CABG were assessed.
Additional Patient-Level Data
Baseline characteristics were abstracted from the medical record. A physician’s diagnosis was used to define hyperlipidemia, chronic obstructive pulmonary disease (COPD), heart failure (HF), cerebrovascular disease, and peripheral vascular disease. Smoking status was classified as ‘current’ if they currently smoked or quit within the last 6 months or ‘prior/never’. Hypertension was defined by physician diagnosis, systolic blood pressure >140mm Hg, or diastolic blood pressure >90mm Hg. Diabetes mellitus was defined by blood glucose or diabetic medication use. Body mass index (BMI) was calculated using weight and height at MI diagnosis. Anemia was defined as hemoglobin <13mg/dL in men or <12mg/dL in women. The Modification of Diet in Renal Disease equation(14) was used to estimate glomerular filtration rate (GFR).
Study Outcomes
Data on all-cause hospitalizations occurring within 30 days after incident MI hospital discharge from 1987–2010 were obtained through the Olmsted County Healthcare Expenditure and Utilization Database(15, 16). Admissions to any Olmsted County hospital within the 30 day period after MI hospital discharge were considered readmissions. In-hospital transfers or between the Olmsted Medical Center and Mayo Clinic hospitals were considered a single hospitalization. Patients who died during the incident MI hospitalization were excluded.
The principal diagnosis for each hospitalization was assessed using the primary ICD-9 code. This code, assigned by trained personnel, reflects the main reason for admission. Chart review was performed for all readmissions by the first author (SMD) to determine the relationship to the index MI; SMD was not blinded to the primary ICD-9 code. Readmissions were categorized as related if they were most likely resulting from ischemic heart disease, the incident MI or its treatment, unrelated if they were very unlikely to be related, or unclear. A 10% sample was reviewed by the senior author (VLR, blinded to SMD’s categorization and the ICD-9 diagnosis) and there was agreement on categorization in 89% of cases; the weighted κ statistic was 0.88 (95% CI 0.79–0.97). In cases of disagreement, the classification of SMD was used.
Death was ascertained from the medical record and through the follow-up infrastructure of the Rochester Epidemiology Project. In addition to deaths noted in clinical care, the Mayo Clinic registration office records obituaries and local death notices, and death data are obtained from the State of Minnesota Department of Vital and Health Statistics quarterly. The National Death Index was used to capture deaths occurring in the 50 U.S. states and District of Columbia.
Statistical Analysis
Differences in baseline characteristics by year of diagnosis were tested using Mantel-Haenszel χ2 for trend (categorical variables) or generalized linear models (continuous variables). Differences in the reason for hospitalization by year, type of MI, and sex were examined using the χ2 test. Cox proportional hazards regression modeling was used to determine the predictors of 30-day readmission. Patients were censored at death or 30 days. The proportional hazards assumption was evaluated using the scaled Schoenfeld residuals and was valid. A subset analysis was performed in patients treated with PCI; the 4 patients suffering a stroke post-PCI were grouped with those with bleeding/vascular complication. As patients may be readmitted multiple times, we performed a sensitivity analysis using Andersen-Gill models(17) which account for multiple events and results were similar. The adjusted cumulative incidence of readmission at 30 days treating death as a competing risk was estimated according to patient characteristics using the direct adjustment method(18). Only 3.4% of patients had any variables missing from the multivariable model. Multiple imputation was used to impute missing values. Five datasets were created and analyzed, with results combined using Rubin's rules(19). Analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC). A p value <0.05 was used as the level of significance.
RESULTS
Patient Characteristics
A total of 3010 patients were diagnosed with incident MI from 1987–2010 and survived the index MI hospitalization. In-hospital survival improved over time, from 89.0% from 1987–92 to 95.8% from 2005–2010 (p<0.001). The mean age at MI diagnosis was 67 years, 40.5% of patients were female, and 31.2% had ST-elevation MI (Table 1). The frequency of hypertension, hyperlipidemia, diabetes, obesity, COPD, and anemia increased over time. The proportion of patients with STEMI decreased from 39.1% in 1987–1992 to 24.1% from 2005–2010 (p for trend <0.001). Median length of stay was 5 (25th–75th percentile 3–8) days during the index MI admission and was shorter for those undergoing revascularization (median 5 vs. 6 days, p=<0.001). Length of stay varied by treatment strategy with median length of stay of 4, 7, and 11 days for those treated with PCI, fibrinolysis, and CABG, respectively.
Table 1.
Baseline Patient Characteristics
| Year of Diagnosis | ||||||||
|---|---|---|---|---|---|---|---|---|
| N Missing |
Overall (n=3010) |
1987–92 (n=591) |
1993–98 (n=694) |
1999–2004 (n=874) |
2005–10 (n=851) | P for trend |
||
| Age at MI diagnosis (years) | -- | 67.3 (14.6) | 67.1 (14.3) | 67.1 (13.9) | 68.2 (15.1) | 66.8 (14.8) | 0.20 | |
| Male sex | -- | 1791 (59.5) | 345 (58.4) | 386 (55.6) | 522 (59.7) | 538 (63.2) | 0.01 | |
| Risk Factors and Comorbidities | ||||||||
| Hypertension | 1 | 1839 (61.1) | 291 (49.3) | 400 (57.6) | 563 (64.4) | 585 (68.7) | <0.001 | |
| Current smoker | 5 | 742 (24.7) | 171 (29.1) | 190 (27.4) | 190 (21.7) | 191 (22.4) | 0.002 | |
| Hyperlipidemia | 2 | 1502 (49.9) | 138 (23.4) | 270 (38.9) | 517 (59.2) | 577 (67.8) | <0.001 | |
| Heart failure (pre-existing) | 13 | 321 (10.7) | 52 (8.8) | 69 (9.9) | 118 (13.5) | 82 (9.8) | 0.28 | |
| Diabetes mellitus | 1 | 657 (21.8) | 93 (15.8) | 150 (21.6) | 212 (24.3) | 202 (23.7) | <0.001 | |
| Body mass index ≥ 30 kg/m2 | 4 | 999 (33.2) | 124 (21.1) | 230 (33.2) | 320 (36.6) | 325 (38.2) | <0.001 | |
| COPD | 2 | 391 (13.0) | 58 (9.9) | 83 (12.0) | 142 (16.3) | 108 (12.7) | 0.04 | |
| Cerebrovascular disease | 3 | 425 (14.1) | 71 (12.1) | 93 (13.4) | 147 (16.8) | 114 (13.4) | 0.27 | |
| Incident MI Characteristics | ||||||||
| ST elevation | 38 | 926 (31.2) | 229 (39.1) | 261 (38.5) | 231 (27.0) | 205 (24.1) | <0.001 | |
| Killip class 2–4 | 16 | 831 (27.8) | 184 (31.6) | 204 (29.7) | 252 (28.8) | 191 (22.4) | <0.001 | |
| Q waves | 203 | 1496 (53.3) | 258 (46.3) | 388 (58.4) | 455 (57.0) | 395 (50.2) | 0.51 | |
| Reperfusion/revascularization during hospitalization | 5 | 1928 (64.2) | 347 (58.9) | 437 (63.2) | 553 (63.3) | 591 (69.5) | <0.001 | |
| Fibrinolysis (N, % those with STEMI) | -- | 261 (28.2) | 118 (51.5) | 121 (46.4) | 20 (8.7) | 2 (1.0) | <0.001 | |
| CABG | -- | 282 (9.4) | 71 (12.1) | 72 (10.4) | 73 (8.4) | 66 (7.8) | 0.03 | |
| PCI | -- | 1541 (51.3) | 224 (38.0) | 303 (43.9) | 479 (54.8) | 535 (62.9) | <0.001 | |
| Angiogram during hospitalization | 1 | 2288 (76.0) | 398 (67.3) | 483 (69.7) | 684 (78.3) | 723 (85.0) | <0.001 | |
| Length of stay (days), median (IQR) | -- | 5 (3–8) | 8 (6–11) | 5 (4–8) | 4 (3–7) | 3 (3–6) | <0.001 | |
| Laboratories | ||||||||
| Peak troponin T | 1448 | 0.8 (0.3–2.7) | NA | NA | 0.9 (0.3–3.0) | 0.8 (0.3–2.5) | -- | |
| CKMB ratio | 389 | 5.8 (2.1–16.9) | 7.9 (3.1–16.0) | 10.3 (3.9–22.6) | 4.5 (1.7–18.1) | 3.6 (1.5–10.6) | <0.001 | |
| Anemia | 32 | 808 (27.1) | 123 (20.9) | 162 (23.5) | 286 (33.1) | 237 (28.3) | <0.001 | |
| Estimated GFR < 60 mL/min | 17 | 1482 (49.5) | 317 (54.2) | 346 (50.1) | 510 (58.7) | 309 (36.4) | <0.001 | |
Age at diagnosis is shown as mean (standard deviation). Peak troponin T and CKMB ratio are shown as median (25th, 75th percentile). All others are shown as N(%). COPD=chronic obstructive pulmonary disease, MI=myocardial infarction, CABG=coronary artery bypass grafting, PCI=percutaneous coronary intervention, GFR=glomerular filtration rate, STEMI= ST segment elevation myocardial infarctio
The proportion receiving a coronary angiogram and/or reperfusion/revascularization in-hospital after MI increased over time (p for trend<0.001). The majority (n=1541, 79.9%) of patients receiving reperfusion and/or revascularization had PCI, while 282 (14.6%) had CABG and 261(13.5%) fibrinolysis. There were 1144 (56.0%) patients with NSTEMI and 636 (68.8%) with STEMI who had PCI or CABG. A total of 158 (10.3%) patients undergoing PCI suffered a complication; 91 (5.9%) had a vascular or bleeding complication, 4 (0.3%) had stroke, and 72 (4.7%) had acute renal failure, though none had to start dialysis. Among patients with vascular or bleeding complications, 57 (62.6%) had access site complications; most were groin hematomas (n=38). A total of 34/91 patients (37.4%) had gastrointestinal bleeding requiring transfusion, while the remainder had bleeding at other sites. Only 5 (1.9%) patients receiving fibrinolysis and 5 (1.8%) undergoing CABG suffered a periprocedural stroke. There were 439 patients who underwent angiogram without reperfusion or revascularization. Among them, 13 (3.0%) had a vascular or bleeding complication, and 22 (5.0%) had acute renal failure, 3 of whom had to start dialysis.
30-day Readmissions After MI
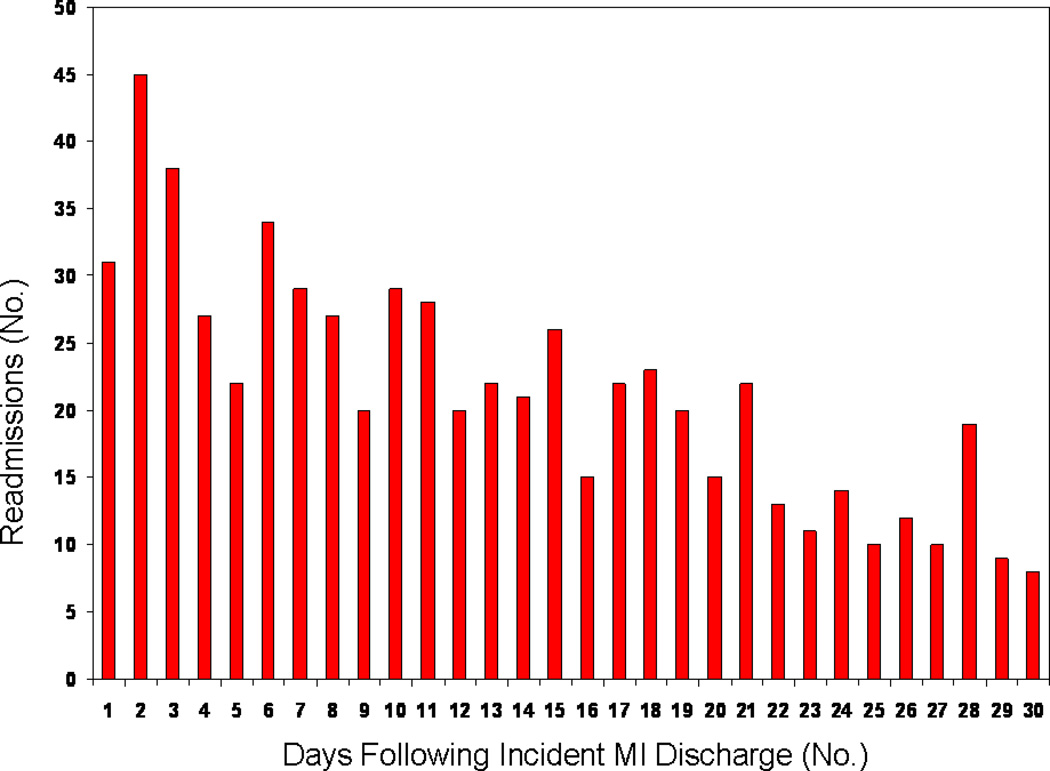
A total of 643 readmissions occurred among 561 (18.6%) patients within 30 days of MI hospital discharge. Of the patients readmitted, 484 were readmitted once, 72 twice, and 5 were readmitted 3 times. Eighty-seven (2.9%) patients died within 30 days, of whom 19 were readmitted at the time of death. The number of readmissions by day within the 30 days following hospital discharge is shown in the Figure. The readmission rates by time period were 137/591 (23.2%) for 1987–1992, 152/694 (21.9%) for 1993–1998, 193/874 (22.1%) for 1999–2004, and 161/851 (18.9%) for 2005–2010. Length of stay for the first readmission ranged from 0 to 64 days (median 3 days, 25th–75th percentile 1–7 days), and was similar for related and unrelated readmissions.
Figure.
Distribution of 643 Readmissions within 30 Days of Incident Myocardial Infarction.
Causes of readmissions
The reasons for readmission by 1st ICD-9 code are shown in Appendix Table 1. The most common reasons were ischemic heart disease, respiratory/chest symptoms, and heart failure. Upon manual review of readmissions, there were 9 readmissions (1.4%) where the abstracted reason was markedly different than the ICD9 code. Cardiac biomarkers were measured in 74.8% of readmissions. Angiography was performed in 23.8% of readmissions (153/643). PCI was performed in 9.5% (61/643) of readmissions, and 6 of these were planned. A total of 24.6% (15/61) of PCIs were repeat PCI of the same culprit vessel treated at index MI because of occlusion. A total of 44 underwent CABG, and 28 of these were planned admissions for surgery. Among those undergoing revascularization during readmission, 46/103 (44.7%) were treated medically during index hospitalization (Appendix Table 2).
Overall, 42.6% of readmissions after MI were related to the incident MI or its treatment, while 30.2% were unrelated, and 27.2% were unclear (Table 2). The proportion of unrelated readmissions was higher in women and patients with NSTEMI. The proportion of related readmissions decreased over time. The most common reason for “unclear” readmissions was atypical chest pain (frequently coded as ICD9 786). Narrative examples of readmissions are shown in the Appendix.
Table 2.
Association Between Index MI Characteristics and Reason for 30-Day Readmissions
| Reason for Readmission*, N (%) | ||||
|---|---|---|---|---|
| Related | Unrelated | Unclear | P value† | |
| Overall | 274 (42.6) | 194 (30.2) | 175 (27.2) | |
| Year of Index MI Diagnosis | ||||
| 1987–1992 | 65 (47.5) | 38 (27.7) | 34 (24.8) | 0.01 |
| 1993–1998 | 81 (53.3) | 40 (26.3) | 31 (20.4) | |
| 1999–2004 | 69 (35.8) | 67 (34.7) | 57 (29.5) | |
| 2005–2010 | 59 (36.7) | 49 (30.4) | 53 (32.9) | |
| Sex | ||||
| Male | 154 (46.5) | 84 (25.4) | 93 (28.1) | 0.02 |
| Female | 120 (38.5) | 110 (35.3) | 82 (26.3) | |
| Index MI Type | ||||
| Unknown | 7 (63.6) | 2 (18.2) | 2 (18.2) | 0.046 |
| STEMI | 84 (46.4) | 42 (23.2) | 55 (30.4) | |
| NSTEMI | 183 (40.6) | 150 (33.3) | 118 (26.2) | |
| Reperfusion/revascularization/ angiography performed during index MI hospitalization | ||||
| None | 70 (39.1) | 81 (45.3) | 28 (15.6) | --- |
| Angiogram Alone | 44 (47.8) | 25 (27.2) | 23 (25.0) | |
| Thrombolytics | 33 (61.1) | 8 (14.8) | 13 (24.1) | |
| PCI | 104 (37.6) | 72 (26.0) | 101 (36.5) | |
| CABG | 32 (51.6) | 11 (17.7) | 19 (30.7) | |
MI= myocardial infarction, STEMI=ST-segment elevation myocardial infarction, NSTEMI=non ST-segment elevation myocardial infarction, PCI=percutaneous coronary intervention
The proportion of readmissions that are related (related, unrelated, unclear) to the incident MI or its treatment are shown according to sex and characteristics of the index MI.
P value compares the proportions of related vs. unrelated vs. unclear readmissions by comparison group in the left column (e.g. sex). No p value is shown for reperfusion/revascularization as some patients received more than one treatment (e.g. thrombolytics and PCI).
Risk Factors for Readmission After MI
Diabetes mellitus, COPD, anemia, higher Killip class at presentation, longer length of stay during incident MI admission, and a complication of either angiography or reperfusion/revascularization during the index admission were independently associated with increased risk of readmission after MI (Table 3). After adjustment for potential confounders, the risk of readmission did not change over the study period. To investigate the impact that death had on readmission, we performed two sensitivity analyses (Appendix Table 4). First, we assumed that patients who died within 30 days survived and were never hospitalized. This resulted in no appreciable differences in results. Second, we assumed that those who died within 30 days were hospitalized on the day of death. Overall results were similar, though there was an increased risk of readmission for patients who had not undergone reperfusion/revascularization or angiogram (adjusted HR 1.42, 95% CI 1.14–1.78). The cumulative incidence of readmission within 30 days stratified by patient and MI characteristics is shown in Table 3.
Table 3.
Cumulative Incidence of Readmission at 30 Days After Incident MI Discharge According to Baseline/Incident MI Characteristics
| Characteristic | Hazard Ratio (95% CI) |
Cumulative Incidence (95% CI) |
|
|---|---|---|---|
| Age (years) | |||
| <60 | 1 (referent) | 19.1 (16.0–22.3) | |
| 60–79 | 0.90 (0.71–1.14) | 17.4 (15.5–19.4) | |
| ≥80 | 1.07 (0.80–1.42) | 19.9 (16.9–22.8) | |
| Sex | |||
| Female | 1 (referent) | 20.2 (18.0–22.4) | |
| Male | 0.83 (0.69–1.00) | 17.2 (15.4–19.1) | |
| Hypertension | |||
| No | 1 (referent) | 17.1 (14.7–19.5) | |
| Yes | 1.15 (0.94–1.40) | 19.3 (17.5–21.1) | |
| Diabetes mellitus | |||
| No | 1 (referent) | 17.3 (15.8–18.8) | |
| Yes | 1.34 (1.10–1.63) | 22.6 (19.5–25.6) | |
| Chronic obstructive pulmonary disease | |||
| No | 1 (referent) | 17.6 (16.2–19.0) | |
| Yes | 1.43 (1.15–1.79) | 23.9 (19.9–27.9) | |
| Current smoker | |||
| No | 1 (referent) | 18.2 (16.7–19.7) | |
| Yes | 1.11 (0.89–1.39) | 19.7 (16.4–22.9) | |
| Cerebrovascular Disease | |||
| No | 1 (referent) | 18.4 (16.9–19.9) | |
| Yes | 1.04 (0.83–1.30) | 19.2 (15.8–22.5) | |
| Heart failure | |||
| No | 1 (referent) | 18.3 (16.8–19.8) | |
| Yes | 1.12 (0.87–1.43) | 20.0 (16.2–23.7) | |
| Body mass index (kg/m2) | |||
| <20 | 1.03 (0.70–1.52) | 18.4 (12.9–23.9) | |
| 20 to 24.9 | 1.19 (0.94–1.50) | 20.1 (17.4–22.9) | |
| 25 to 29.9 | 1.07 (0.87–1.32) | 18.5 (16.2–20.8) | |
| ≥30 | 1 (referent) | 17.3 (14.9–19.7) | |
| Type of myocardial infarction | |||
| ST-segment elevation | 0.87 (0.71–1.06) | 17.1 (14.6–19.5) | |
| Non ST-segment elevation | 1 (referent) | 19.2 (17.5–20.9) | |
| Killip class | |||
| 1 | 1 (referent) | 17.6 (16.0–19.3) | |
| 2–4 | 1.22 (1.01–1.46) | 20.5 (17.9–23.0) | |
| Angiography/ Reperfusion/revascularization | |||
| Reperfusion/revascularization no complications | 1 (referent) | 16.8 (14.8–18.7) | |
| Angiogram no complications | 1.13 (0.91–1.40) | 16.3 (12.8–19.8) | |
| No angiogram/reperfusion/revascularization | 1.17 (0.92–1.48) | 18.7 (15.7–21.8) | |
| Angiogram with complications | 2.40 (1.43–4.01) | 35.3 (21.6–49.0) | |
| Reperfusion/ revascularization with complications | 2.12 (1.61–2.80) | 31.6 (25.4–37.9) | |
| Anemia* | |||
| No | 1 (referent) | 20.9 (18.3–23.6) | |
| Yes | 1.26 (1.03–1.50) | 17.4 (15.8–19.1) | |
| Estimated glomerular filtration rate (mL/min)* | |||
| ≥60 | 1 (referent) | 17.5 (15.4–19.6) | |
| <60 | 1.13 (0.93–1.37) | 19.4 (17.4–21.3) | |
| Length of stay during incident MI admission (days) | |||
| 0–3 | 1 (referent) | 14.4 (11.8–17.0) | |
| 4–7 | 1.34 (1.05–1.70) | 18.5 (16.4–21.7) | |
| >7 | 1.65 (1.27–2.14) | 22.0 (19.3–24.7) | |
| Year of diagnosis | |||
| 1987–92 | 1 (referent) | 18.6 (15.6–21.7) | |
| 1993–98 | 1.03 (0.80–1.33) | 18.7 (15.8–21.5) | |
| 1999–2004 | 1.02 (0.79–1.32) | 18.8 (16.2–21.3) | |
| 2005–10 | 0.98 (0.74–1.29) | 18.1 (15.3–20.9) | |
The estimated cumulative incidence and 95% CI of readmission at 30 days for each characteristic are adjusted for all other variables shown as well as site of care. CI= confidence interval
Anemia and estimated glomerular filtration rate are based on hemoglobin and creatinine from laboratory measurements on the index MI date or the closest date when measured during the index admission.
Among those treated with PCI initially (n=1541), complications were associated with increased readmission risk. A stroke, vascular complication, or bleeding event after PCI (adjusted HR 1.66, 95% CI 1.09–2.52, p=0.018) and acute kidney injury (HR 1.92, 95% CI 1.25–2.95, p<0.001) were strongly associated with increased readmission compared with those who had PCI without complications.
DISCUSSION
In this community incidence cohort, nearly one in five patients was readmitted within the 30 days following incident MI. Overall, 42.6% of readmissions were related to the index MI or its treatment, while 30.2% were unrelated, and the relationship with the index MI was unclear in 27.2% of readmissions. Diabetes mellitus, COPD, anemia, higher Killip class at MI presentation, a longer index MI admission, and an angiogram or reperfusion/revascularization complication were independent predictors of readmission. The adjusted risk of readmission did not change over the 22-year study period.
Readmission after Incident MI
Readmission rates following illness have been examined in prior studies. Jencks reported that 19.6% of Medicare fee-for-service beneficiaries were readmitted within 30 days, though no specific rates were reported following acute MI(20). Joynt reported 30-day readmission rates after MI of 24.8% among black patients and 22.6% among white Medicare patients(5). For Medicare patients treated with PCI following MI in 2005, the 30-day readmission rate was slightly lower at 17.5%(21). We found that 18.6% of patients were readmitted within 30 days, and 2.6% were readmitted multiple times. While length of stay declined markedly over the study period, there was no change in 30-day readmission risk over time. Despite advances in therapies and improved in-hospital outcomes after MI, readmissions remain a serious problem and have shown no signs of abating.
Reason for Hospitalization and Predictors
In contemporary practice, most patients receive angiography, revascularization and/or reperfusion therapy after MI, though little is known regarding their impact on readmission. Curtis et al reported that among Medicare patients treated with PCI, one-quarter of 30-day readmissions had an associated revascularization procedure, though the analysis was not specific to those admitted with MI and did not assess whether readmissions differed for patients who did not have PCI(21). Herein, we found that a serious complication following angiography or revascularization/reperfusion therapy was strongly associated with readmission. After PCI, acute kidney injury, which occurred in 5% of patients, was associated with increased readmission risk (adjusted HR 1.9), even after adjustment for baseline renal dysfunction. Acute kidney injury has been linked to an increased risk of death following PCI(10) and MI(22), but is also an important risk factor for readmission. However, acute kidney injury was infrequent as a primary reason for readmission, suggesting that although acute kidney injury following PCI is a marker of increased readmission risk, the exact causality is less clear.
Comorbidity appears to play a major role in care utilization following hospitalization for treatment of MI. There has been debate regarding whether readmission should be used as a quality marker of care following admission for acute MI or HF(4, 23), as patients may be readmitted for conditions which may be unrelated to MI or its treatment. Herein, 42.6% of 30-day readmissions were related to the incident MI, though this proportion decreased over time, and coincided with an increase in unrelated and unclear readmissions. The shift in the biomarker definition of acute MI, now relying on troponin, may contribute to the identification of smaller infarcts (NSTEMI)(3), in patients with increased comorbidity. In addition, as the prevalence of some comorbidities such as diabetes and COPD increase over time(24, 25), readmissions after acute MI may continue to shift toward non-cardiovascular causes. Compared with our previous report on readmissions after incident HF diagnosis in Olmsted County(16), COPD, diabetes, and anemia were common risk factors for readmission among patients with incident MI and HF, and may be of particular importance as future targets in preventing readmissions in patients hospitalized with cardiovascular disease.
Limitations, Strengths and Clinical Implications
Some limitations should be acknowledged in interpreting these data. First, patients experiencing MIs not requiring hospitalization were not captured. Second, if a readmission occurred outside of Olmsted County, it would not be captured. However, the county is relatively isolated, thus hospitalizations elsewhere are likely rare within the 30-days post-MI. While classification of the reason for readmission into related, unrelated, and unclear categories could lead to misclassification, there was a high level of agreement in the subset reviewed by the senior author. These data reflect the practice of providers in this community. Care systems where revascularization is performed less commonly following MI may have different rates of complications and readmissions. These patients may be younger than some hospitalized acute MI cohorts as we included only first-ever MI and excluded those who died during index hospitalization, as they are not at risk for readmission. Finally, these results may not be applicable to non-Caucasian populations. While these potential limitations should be acknowledged, the outcome of an entire community-based MI incidence cohort is presented, representing the comprehensive experience of incident MI patients over more than two decades.
There are direct clinical implications of these data. First, patients affected by a vascular or bleeding complication, stroke, or acute kidney injury following angiography or revascularization/reperfusion represent a high-risk population for readmission. Prevention of complications and close follow-up for patients who have suffered a complication may be of particular importance for preventing readmissions. Second, MI patients have a large number of comorbidities which may impact readmission. The management of patients with multiple comorbidities and competing risks are of increasing importance as the population ages(26), and these data underscore the importance of implementing a comprehensive management strategy in patients following incident MI to help prevent rehospitalizations.
Conclusions
As the costs of healthcare continue to rise, 30-day readmission after MI has been targeted for public reporting. Despite advances in medical care following MI, readmission risk has not declined. Angiography, reperfusion and revascularization are mainstays of therapy in acute MI, and complications are associated with a large risk of readmission. Comorbidities appear to play a central and increasing role in readmissions. Comprehensive strategies of care in patients with MI need to be deployed that incorporate the treatment of both cardiovascular and non-cardiovascular disease in order to prevent future hospitalizations.
Supplementary Material
Acknowledgments
Study Support. This study was supported by grants from the National Institute of Health (RO1-HL59205), and was made possible by the Rochester Epidemiology Project (R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases).
Footnotes
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of the record.
Disclosures. Dr. Jaffe has or does consult for most of the major diagnostic companies. Present consulting is with Beckman, Inverness, Amgen, Radiometer, Critical Diagnostics. Dr Jaffe has received stipends for participation in symposia from Roche and Abbott. Dr. Dunlay, Ms. Weston, Ms. Killian, Dr. Bell, and Dr. Roger have no disclosures.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, et al. Heart Disease and Stroke Statistics--2011 Update: A Report From the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kostis WJ, Deng Y, Pantazopoulos JS, Moreyra AE, Kostis JB. Trends in mortality of acute myocardial infarction after discharge from the hospital. Circ Cardiovasc Qual Outcomes. 2010;3:581–589. doi: 10.1161/CIRCOUTCOMES.110.957803. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121(7):863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305:504–505. doi: 10.1001/jama.2011.72. [DOI] [PubMed] [Google Scholar]
- 5.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305:675–681. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 7.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years' experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 8.Kors JA, Crow RS, Hannan PJ, Rautaharju PM, Folsom AR. Comparison of computer-assigned Minnesota Codes with the visual standard method for new coronary heart disease events. Am J Epidemiol. 2000;151:790–797. doi: 10.1093/oxfordjournals.aje.a010279. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed Feb 26, 2012];NCDR Cath PCI Registry Coder's Data Dictionary. 2008 http://www.ncdr.com/WebNCDR/NCDRDocuments/CathPCI_v4_CodersDictionary_4.4.pdf.
- 10.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 11.Smith SC, Jr, Dove JT, Jacobs AK, Kennedy JW, Kereiakes D, Kern MJ, et al. ACC/AHA guidelines for percutaneous coronary intervention (revision of the 1993 PTCA guidelines)-executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) endorsed by the Society for Cardiac Angiography and Interventions. Circulation. 2001;103:3019–3041. doi: 10.1161/01.cir.103.24.3019. [DOI] [PubMed] [Google Scholar]
- 12.Miller WL, Garratt KN, Burritt MF, Lennon RJ, Reeder GS, Jaffe AS. Baseline troponin level: key to understanding the importance of post-PCI troponin elevations. Eur Heart J. 2006;27:1061–1069. doi: 10.1093/eurheartj/ehi760. [DOI] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 15.Leibson CL, Katusic SK, Barbaresi WJ, Ransom J, O'Brien PC. Use and costs of medical care for children and adolescents with and without attention-deficit/hyperactivity disorder. JAMA. 2001;285:60–66. doi: 10.1001/jama.285.1.60. [DOI] [PubMed] [Google Scholar]
- 16.Dunlay SM, Redfield MM, Weston SA, Therneau TM, Hall Long K, Shah ND, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–1702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen PK, Gill RD. Cox's regression model for counting processes: a large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 18.Zhang X, Zhang MJ. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Computer Methods and Programs in Biomedicine. 2011;101:87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin D. Multiple imputation for nonresponse in surveys. New York: J Wiley and Sons; 1987. [Google Scholar]
- 20.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. New Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 21.Curtis JP, Schreiner G, Wang Y, Chen J, Spertus JA, Rumsfeld JS, et al. All-cause readmission and repeat revascularization after percutaneous coronary intervention in a cohort of Medicare patients. J Am Coll Cardiol. 2009;54:903–907. doi: 10.1016/j.jacc.2009.04.076. [DOI] [PubMed] [Google Scholar]
- 22.Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med. 2008;168:987–995. doi: 10.1001/archinte.168.9.987. [DOI] [PubMed] [Google Scholar]
- 23.Gorodeski EZ, Starling RC, Blackstone EH. Are all readmissions bad readmissions? New Engl J Med. 2010;363:297–298. doi: 10.1056/NEJMc1001882. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 25.Gershon AS, Wang C, Wilton AS, Raut R, To T. Trends in chronic obstructive pulmonary disease prevalence, incidence, and mortality in Ontario, Canada, 1996 to 2007: a population-based study. Arch Intern Med. 2010;170:560–565. doi: 10.1001/archinternmed.2010.17. [DOI] [PubMed] [Google Scholar]
- 26.Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. 2008;56:1839–1844. doi: 10.1111/j.1532-5415.2008.01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.