Abstract
In Alzheimer's disease, the key pathological culprit is the amyloid-β protein, which is generated through β- and γ-secretase cleavage of the amyloid-β precursor protein (APP). Both the secretases and amyloid-β precursor protein are transmembrane proteins that are sorted via the trans-Golgi network and the endosome through multiple membranous compartments of the cell. The coat complex clathrin controls the sorting from the cell surface and the trans-Golgi network to the endosome. Instead, the retromer controls the reverse transport from the endosome to the trans-Golgi network. The retromer contains two subprotein complexes: the cargo-selective subcomplex consisting of VPS35, VPS29 and VPS26 and the membrane deformation subcomplex consisting of Vps5p, Vps17p, SNX 1/2 and possibly SNX 5/6 or SNX 32 in mammals. Cargo molecules of the retromer include the VPS10 receptor proteins SORL1, SORT1, SORCS1, SORCS2 and SORCS3. There is increasing evidence through cell biology and animal and genetic studies that components of the retromer and the VPS10d receptor family play a role in the etiology of Alzheimer's disease. This article reviews and summarizes this current evidence.
Keywords: Alzheimer's disease, amyloid, APP processing, intracellular trafficking, retromer, VPS10d receptors
Alzheimer's disease (AD) is highly prevalent, affecting approximately 30% of individuals aged 80 years or older [1]. Genetic factors contribute up to 60–80% of the risk [2] and only a few genes have been robustly confirmed to be involved, leaving several to be identified [3]. The discovery of causative genetic variation in the genes encoding the amyloid-β (Aβ) precursor protein (APP), PSEN1 or PSEN2 in the early onset familial form of AD, which has an onset before 60 years of age, has led to the formulation of the amyloid hypothesis [4]. This hypothesis is considered the main pathological pathway in AD and claims that AD begins with the accumulation of oligomeric forms of the Aβ42 in the cerebral cortex and hippocampus. In line with the hypothesis, it is clear that two cleavage steps liberate Aβ from APP. In the first step, BACE cleaves APP near the N-terminus of the Aβ peptide. In the second step, the membrane-bound C-terminal APP fragment (CTF) generated by BACE is cleaved by the γ-secretase, a multimeric complex that is composed of the essential transmembrane proteins presenilin, nicastrin, APH-1 and PEN-2 [5,6].
It is clear that Aβ accumulation is also involved in the etiology of the late-onset form of Alzheimer's disease (LOAD). However, in LOAD, the mutations in APP, PSEN1 and PSEN2 explain only a minor part of the genetic contribution and are not sufficient to explain the Aβ accumulation observed in this form of the disease. Instead, several other genes have been implicated out of which the ε4 allele of the APOE gene is the strongest risk factor [7,8]. The genes identified to date, however, only explain part of the genetic contribution to the disease [3]. Thus, clarifying how and where in the cell APP is processed needs to be extended beyond the simple molecular defects in APP or the secretases.
APP and the secretases are all integral transmembrane proteins, which are sorted through multiple membranous compartments of the cell. This sorting network, that interconnects the trans-Golgi network (TGN), the cell surface and the endosome, is critically important for APP and BACE sorting. Thus, from a molecular point of view, sorting mechanisms that cause APP and the secretases to colocalize in the same membranous compartment would be expected to affect the regulation of Aβ production. Consistent with this notion is the fact that, among the specific pathways the recent genetic studies of LOAD have identified, the endocytic pathway seems to play a major role. Several novel genes identified by the recent genome-wide association studies (GWAS) are part of this itinerary, including BIN1, PICALM, CD2AP, CD33 and the SORL1 [9–12] BIN1 is an amphiphysin and is expressed most abundantly in the brain and muscle [13]. Besides promoting caspase-independent apoptosis, amphiphysins are involved in neuronal membrane organization and clathrin-mediated synaptic vessel formation [14]. Altered expression of BIN1 has been validated in transgenic mouse models of AD, aging mice and in individuals with schizophrenia [15,16]. PICALM encodes a clathrin assembly protein, and thus plays a direct role in clathrin-mediated endocytosis [17]. In addition, it is involved in synaptic transmission and the removal of apoptotic cells [18]. CD2AP encodes a scaffolding protein regulating the actin cytoskeleton. It directly interacts with filamentous actin and a variety of cell membrane proteins. The cytoplasmic protein localizes to membrane ruffles, lipid rafts and the leading edges of cells. In addition to endocytosis, it is implicated in dynamic actin remodeling. CD33 is a member of the sialic acid-binding immunoglobulin-like lectins (Siglec) family. Lectins promote cell-cell interactions and regulate functions of cells in the innate and adaptive immune systems [19]. Most members of the Siglec family, including CD33, act as endocytic receptors, mediating endocytosis through a mechanism independent of clathrin [19]. SORL1 is one of the five VPS10 domain receptor homologs that are the main focus of this review, and will be discussed in detail below. In brief, SORL1 modulates the processing of the APP holoprotein through the retromer complex, thereby influencing levels of Aβ.
The TGN-cell surface-endosome triangle & retromer complex
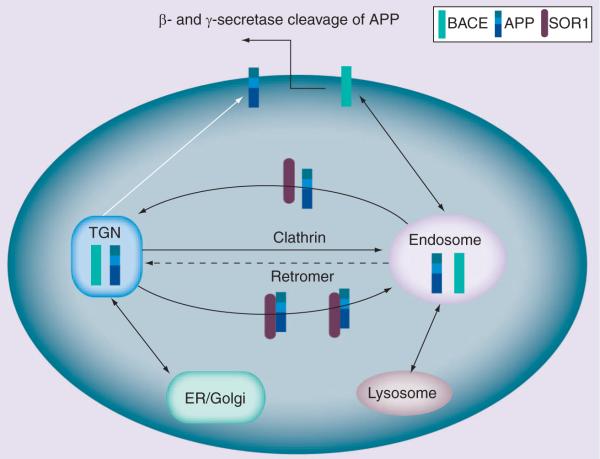
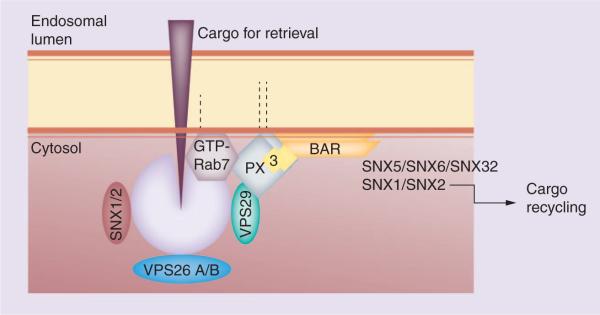
As described above, the sorting triangle that interconnects the TGN, the cell surface and the endosome is critically important for APP and BACE sorting. In addition, it is critically important for the production of the putative culprit in AD, Aβ, as APP and BACE initiate the amyloidogenic pathway by interacting within the membranes of the endosomal system. Clathrin is the coat complex that regulates transport from the cell surface and the TGN to the endosome (Figure 1), while the retromer is the coat complex that selectively regulates the transport of multiple transmembrane proteins from the endosome back to the TGN. The retromer in turn contains two subprotein complexes: the cargo-selective subcomplex and the membrane deformation subcomplex (Figure 2) [20]. The cargo-selective complex is a trimer of vacuolar protein sorting proteins VPS35, VPS29 and VPS26 that sort cargo into tubules for retrieval to the Golgi apparatus. The membrane deformation subcomplex, consisting of sorting nexin dimers (Vps5p and Vps17p in yeast; and sortinnexins 1/2 and likely 5/6 or 32 in mammals), deforms the donor membrane into a tubular profile [20]. The transmembrane proteins transported via the retromer from the endosome to the TGN include mannose 6-phosphate receptors, wntless (a receptor for Wnt morphogens), Ced1 (a phagocytic receptor) and VPS10 family proteins, such as VPS10 in yeast, sortilin and the sortilin-related VPS10d receptor family in vertebrates. The VPS10d receptor family is a group of five type I membrane homologs (SORL1, SORT1, SORCS1, SORCS2 and SORCS3), which are all expressed in the CNS and contain the luminal, extracellular VPS10 domain.
Figure 1. APP sorting in Alzheimer's disease (simplified model to aid clarity).
APP and BACE are sorted through the TGN–cell surface–endosome network. The coat complex, clathrin, controls the transport from both the cell surface and the TGN to the endosome (solid black arrows). The retromer instead controls the reverse transport from the endosome to the TGN (dashed arrow). APP and BACE interact within the endosomal system, representing the first step of the amyloidogenic pathway. SORL1 modulates the sorting of APP. When SORL1 is absent, APP holoprotein is switched away from the recycling pathway through the retromer but is instead directed into the β-secretase cleavage pathway (white arrow), thereby increasing APP's Aβ production. Subsequently, APP enters the γ-secretase cleavage pathway to generate Aβ.
BACE: β-secretase; TGN: Trans-Golgi network.
Figure 2. The distinct cargo-selective and membrane deformation subcomplexes that together form the mammalian SNX-BAR-retromer.
The mammalian genome contains two VPS26 genes.
Recycling of APP through the TGN-cell surface-endosome triangle
After exiting the TGN, some APP molecules enter the nonamyloidogenic pathway, meaning that they are transported through the secretory pathway to the plasma membrane where they can be cleaved within the Aβ domain by one of the α-secretases. α-secretase cleavage of APP results in the formation of a cell-retained α-CTF and in the shedding of the APP ectodomain (soluble APPα). However, APP that does not enter this secretory pathway is transported via clathrin coat vesicles from the TGN to the endosmome, thereby entering the endocytic pathway (i.e., the potentially amyloidogenic [Aβ-forming] pathway). Here, APP can be cleaved by BACE, resulting in the formation of a soluble APPβ fragment and a membrane-bound β-CTF, the amino terminus of which is identical to that of Aβ [21]. Of note, APP that is not cleaved at the plasma membrane by the α-secretases can be reinternalized into the endocytic pathway and can enter the amyloidogenic pathway.
Within the endosomal system, γ-secretase then cleaves the α- and β-CTFs generated by BACE. While cleavage of the α-CTF results in nonamyloidogenic p3 and APP intracellular domain, cleavage of the β-CTF results in APP intracellular domain but also toxic Aβ40 and Aβ42, which in turn are believed to be the key putative culprits in AD. As a consequence, reducing the dwelling time of APP and/or the β-CTF within the endocytic pathway decreases Aβ generation. Retromer-mediated trafficking of APP and/or its CTFs away from the endosome to the TGN plays a key role in modulating this dwelling time in the endocytic system [21].
Retromer complex components & APP processing
As described above, the retromer sorts APP away from the endosome and amyloidogenic pathway, and it does this by indirect binding to its cargo, such as the VPS10d receptors. Consistent with this notion, a deficiency in the cargo-selective core components of the retromer VPS35 and VPS26 is associated with hippocampal progression of AD pathology [22]. Furthermore, VPS26 heterozygote mice show increased levels of endogenous Aβ and impaired long-term potentiation [23], and in a Tg2576 mouse model of AD [24] hemizygous deletion of VPS35 also leads to an earlier-onset of phenotypes commonly associated with AD, including reduced memory function, compromised long-term potentiation and reduced postsynaptic glutamatergic neurotransmission. These phenotypes, in turn, correlate with an Aβ increase in the hippocampus. VPS35 is primarily expressed in the hippocampus and interacts with BACE. As a consequence, loss of VPS35 function in the mouse hippocampus leads to an increase of the activity of BACE [24]. In flies in which the VPS35 homolog has been knocked-out, overexpression of human APP and BACE results in an increase of total Aβ and neurodegeneration [23].
Besides playing a role in the anterograde sorting from the TGN network to the endosome, the retromer also modulates APP processing by modifying retrograde trafficking of BACE [25–28]. This sorting itinerary, in turn, is modulated by several components of the retromer complex including ***SNX6 and VPS26, but also various other factors such as sortilin (i.e., one of the VPS10d receptors) and the small GTPase ARF6 [25–28].
Genetic association studies exploring the relationship between variation in the genes encoding VPS35 or VPS26 with AD have been inconclusive, possibly due to a lack of power or genetic or phenotypic heterogeneity [29–32]. Recently, genetic variation in these genes has, however, been related to familial [33] and sporadic late-onset Parkinson's disease [34], which likely overlap etiologically with AD, suggesting that in these diseases, mutations disturb cargo recognition and binding, resulting in deficient receptor recycling. In line with this notion, α-synuclein, which has been implemented in parkinsonism, may modulate levels of SNARE proteins, which in turn play a role in vesicle fusion and exocytosis [35]. In addition, another protein implemented in this disorder, LRRK2, partly modifies neuritic outgrowth, morphology and cellular homeostasis through endosomal trafficking [36]. Overall, the abovementioned functional, animal and genetic data support an association between retromer deficiency and neurodegeneration in AD and suggest that this effect may be exerted through an affect on dwelling time of APP and BACE in the endosomes and BACE activity.
The VPS10 receptor proteins, APP processing & AD risk
SORL1
A study by Scherzer et al. performed in 2004 provided the first suggestions that SORL1 may be involved in the etiology of AD [37]. The study compared the gene expression patterns derived from lymphocytes of individuals with AD with controls and found that the fluency intensity ratio for sporadic AD patients was 1.8-fold higher than in controls. In order to minimize false-positive results for low intensity genes, this study applied a selective intensity filter (absolute fluorescence intensities ≥800) to exclude genes with low hybridization signal intensities. Then, genes with an AD-to-control fluorescence intensity ratio (fold change) of 1.8 or greater were considered significant.
Six genes were differentially expressed, one of which was SORL1. Subsequent immunohistochemistry validated a reduction of SORL1 expression in histologically normal neurons in AD brains compared with control brains, including neurons in the hippocampus. Evidence that the neuronal retromer might be involved in controlling the sorting of the VPS10 receptors was provided by microarray studies on human brain tissue [22]. Small et al. demonstrated that out of several potential retromer cargo molecules, the expression levels of SORL1 and BACE had the strongest correlation with neuronal VPS35 levels [22].
In 2007, Rogaeva et al. first reported an association between genetic variation in SORL1 and AD [29]. The original study included >6000 subjects from four different ethnic groups (Caucasians, Israeli–Arabs, African–Americans, Caribbean–Hispanics) and identified two haplotypes associated with both familial and sporadic forms of AD: SNPs 8–10 (alleles C-G-C) in the 5′ end of the gene and SNPs 22–25 (alleles T-T-C) in the 3′ end of the gene. In various subsequent studies of independent cohorts [101], the associations with SNPs in the same two SORL1 regions with AD were replicated in case–control and family samples of various ethnic groups. Reitz et al. further validated these haplotypes by a collaborative, meta-analysis of the published Caucasian and Asian datasets (12,464 cases; 17,929 controls; 0.7 < odds ratio < 1.2; p ≤ 0.001) [38]. Both the same alleles and soluble SORL1 levels have also been associated with various AD endophenotypes. Variants in SORL1 have been associated with a lower age-of-onset of AD and also a lower age-of-onset of AD in individuals with Down's syndrome [39,40]. Adults with Down's syndrome overexpress APP and have an early onset and increased risk of AD compared with those individuals without Down's syndrome [41]. In a GWAS of cognitive and MRI measures performed on 705 nondemented persons participating in the Framingham study, variants in SORL1 were associated with abstract reasoning and total cerebral brain volume [42]. In white and African–American sib-ships from the MIRAGE study, SORL1 variants were furthermore associated with white matter lesions and hippocampal atrophy [43], and this association with hippocampal volume was also observed in a study of young healthy adults [44]. In several studies that explored the association of SORL1 variants with CSF biomarkers of AD pathology, variants were associated with cerebrospinal fluid-Aβ42 and tau levels (i.e., markers of the disease) [45–48]. In a study that evaluated SORL1 expression and splicing as a function of AD and AD neuropathology, expression of total SORL1 as well as the full-length SORL1 isoform, which contains exon 2, was reduced in individuals with AD but also cognitively healthy individuals with moderate AD neuropathology, suggesting that SORL1 declines early in the disease process [49] A recent major GWAS also identified SORL1 as a late-onset AD risk gene [10]. Finally, a study that performed exome sequencing in 14 autosomal dominant early-onset AD cases without APP, PSEN1 or PSEN2 mutations identified several nonsense and missense mutations, suggesting that this pathway may also play a role in the early onset form of the disease [50].
Rogaeva et al. also demonstrated, through functional cell biology studies, that SORL1 modifies the sorting of APP from the cell surface to the Golgi–endoplasmic reticulum complex, and that a lower expression of SORL1 leads to higher Aβ levels as well as increased AD risk [29]. In addition, they demonstrated that SORL1 is colocalized to the same subcellular departments as APP. Together with the findings that SORL1 expression levels cross-correlate with expression levels of VPS35, this suggests that SORL1 may modulate APP metabolism and Aβ generation [29,51–53] via an interaction with Vps [21,35,54]. As described above, human studies have shown that VPS35 and other components of the retromer complex are deficient in the brains of AD patients. In addition, animal model studies have shown that retromer deficiency results in key features of AD [23]. When the SORL1–APP interaction is eliminated by mutation of the FANSHY domain in the cytoplasmic tail of SORL1 that specifies its binding to VPS26, the FANSHY domain loses its capability to regulate APP metabolism [54].
In addition to its role in retromer-mediated retrieval of APP, there is also evidence that SORL1 plays a gating/retention function for APP at the TGN [55] by interaction with the adaptors GGA and PACS-1, controlling the exit of APP into the amyloidogenic and nonamyloidogenic pathways [55]. Finally, there is further evidence that PKC and ROCK2 modulate APP metabolism by phosphorylation of SORL1. ROCK2 phosphorylation of SORL1 at serine 2206 regulates shedding of the SORL1 ectodomain as well as SORL1-mediated effects on Aβ production [56]. PKC and ROCKs are two `third messenger' signaling molecules that control the relative utilization of the secretory and endocytic pathways. All of these different action models for SORL1 are not mutually exclusive and can be complementary, and their relative significance is unknown.
SORCS1
There is also evidence that some of the four SORL1 homologs are involved in AD etiology through similar mechanisms. Reitz et al. analyzed the associations of 16 SORCS1-SNPs in exon 23 and introns 1–3, with AD and memory retention in six independent family and case–control datasets (2809 cases and 3482 controls) that comprised Caucasian, Caribbean–Hispanic and African–American cohorts [57,58]. In addition, they compared SORCS1 expression levels of affected and unaffected brain regions in AD and control brains, explored the effects of SORCS1-SNPs on SORCS1 brain expression levels and explored the effect of suppression and overexpression of two SORCS1 isoforms (splice variants SORCS1a and SORCS1b) on APP processing and Aβ generation [58]. All results were consistent with the findings on the homolog SORL1. Inherited variants in all assessed exons and introns were associated with AD. In addition, they were associated with memory retention, the key cognitive endophenotype of AD [57]. In conditioned media of HEK293 APPsw cells transfected with SorCS1a and SorCS1b, overexpression of both isoforms resulted in ~30% reductions in both Aβ40 and Aβ42. Conversely, RNAi knockdown of SorCS1 on APP processing had the inverse observation of a two- to three-fold increase in Aβ levels. In addition, the APP-γ-secretase assay demonstrated that the reduction of SorCS1 leads to a >threefold increase of γ-secretase activity on APP processing. Reminiscent of AD, level and maturation of APP was unaffected. Although the exact mechanism remains to be clarified, this suggests that either altered trafficking and/or increased activity of γ-secretase leads to the generation of Aβ. These data by Reitz et al. are supported by three major studies reporting associations between SORCS1-SNPs in the same linkage disequilibrium blocks and AD [31,59,60]. In addition, they are supported by a study by Lane et al. in which overexpression of SorCS1cβ-myc in cultured cells caused a significant reduction in Aβ generation, while, conversely, endogenous murine Aβ40 and Aβ42 levels were increased in the brains of female SORCS1 hypomorphic mice [61]. Of note, SORCS1 also resides at a quantitative trait locus for Type 2 diabetes mellitus (T2DM) in mice and rats [62,63] and is associated via GWAS to both T1DM and T2DM in humans [64,65]. T2DM in turn, is associated with AD [66]. These data suggest that the sortilin/retromer pathway may link the pathogenesis of AD and sortilin.
There is evidence for an effect of sortilin on β-secretase processing of APP. In a study by Finan et al. [25], sortilin overexpression led to increased BACE1-mediated cleavage of APP in cultured cells while RNAi suppression led to decreased BACE1-mediated cleavage of APP. Furthermore, sortilin interacted with BACE1 and a truncated sortilin construct lacking it's cytoplasmic domain, which in turn contains putative retromer sorting motifs, remained bound to BACE1. Expression of this truncated sortilin redistributes BACE1 from the TGN to the endosomes and substantially reduces the retrograde trafficking of BACE1.
Discussion
As reviewed above, the sorting of APP and BACE through the membranous compartments of the TGN, the cell surface and the endosome is a dynamic and strictly regulated process. As a consequence, even modest sorting defects that alter the trafficking in these compartments would be expected to modulate Aβ generation. In theory, changes in any factor that is part of this sorting itinerary could modulate Aβ generation.
Although the factors reviewed above serve multiple functions, they all play a role in the TGN-cell surface-endosome trafficking pathway. VPS35, VPS29 and VPS26 are core molecules of the cargo-selective part of the retromer complex, while VPS5p, VPS17p and the sortinnexins 1/2 (and likely five out of six and 32) compose the membrane deformation complex. SORL1 is a putative direct cargo of the neuronal retromer. SORCS1, SORCS2 and SORCS3 are likely not engaged in Golgi-endosomal transport but may tie APP to the cellular surface, promote its internalization and target APP to specific endosomal compartments.
Taken together, the functional, animal and genetic studies reviewed above strongly suggest that the TGN-cell surface-endosome trafficking pathway, in particular the retromer, plays a primary role in Aβ generation and the etiology of LOAD. They suggest that defects in this trafficking pathway elevate the residence time of APP and/or BACE in the endosome, thereby increasing the β-cleavage step of APP processing. Consistent with this notion, a number of studies have suggested that β-cleavage is enhanced in LOAD [67–69]. The fact that several of the genes discovered in the recent large GWAS on AD are involved in endocytosis provides further support for this notion [9,10,42].
Conclusion
Transmembrane protein sorting is a fundamental property of normal cellular function, thus it is not surprising that a growing number of diseases are found to be associated with, or directly caused by, defects in sorting molecules [62,70,71]. Since APP and its cleaving enzymes are transmembrane proteins, and because Aβ liberation is a membrane-dependent event, protein sorting is a biological process uniquely vulnerable to AD pathogenesis. Specifically, the trafficking of APP and BACE among the TGN, cell surface and endosome are predicted to be the sorting pathways most relevant to AD. Indeed, recent studies have begun confirming this prediction, isolating disease-related defects in protein sorting, and future studies are expected to identify additional defects that regulate the transport of APP and its cleaving enzymes through its sorting itinerary.
Elucidating the mechanisms that sort APP and the secretases through the TGN, cell surface and endosome has significantly expanded the understanding of AD cell biology. Identifying the specific causative defects in protein sorting in addition will lead to novel targets for therapeutic interventions that may accelerate the development of effective treatments for this devastating disease.
Future perspective
Despite the evidence summarized above, several questions remain about the intracellular trafficking pathway in AD. First, further confirmation is required to establish whether the retromer affects the sorting of APP and/or BACE and whether it does so by directly binding either of these type-I transmembrane proteins. Second, although studies agree that SORL1 modulates the production of Aβ and binds APP, there are discrepancies in the currently existing data as to whether SORL1 downregulation missorts APP to the TGN or to the endosome [52,72]. Third, it has to be clarified how SORCS1, SORCS2 and SORCS3, which do not seem to be engaged in Golgi-endosomal transport, affect APP processing and whether they tie APP to the cellular surface, promote its internalization and target APP to specific endosomal compartments.
Finally, it has to be clarified whether retromer elements and its cargo undergo accelerated degradation in the disease state as the underexpression of VPS35, SORL1 and SORCS1 in AD and genetic studies relating these components to AD status suggest.
Beyond implicating a specific pathway, the recent convergence of studies on AD provide convincing evidence that protein sorting is an important cell biological mechanism that contributes to LOAD. AD is a complex disorder to which many molecular defects are expected to contribute. Consequently, additional sorting molecules are expected to contribute to disease pathogenesis.
Executive summary.
Alzheimer's disease & the amyloid cascade hypothesis
-
■
The putative culprit in Alzheimer's disease (AD), amyloid-β, is generated through β- and γ-secretase cleavage (BACE) of the amyloid precursor protein (APP).
The trans-Golgi network-cell surface-endosome triangle & retromer complex
-
■
Transmembrane protein sorting is a fundamental property of normal cellular functioning.
-
■
The triangle interconnecting the trans-Golgi network (TGN), cell surface and the endosome is critically important for APP and BACE sorting.
-
■
The clathrin coat regulates transport from the cell surface and the TGN to the endosome; the retromer regulates transport from the endosome back to the TGN.
-
■
The retromer contains the cargo-selective subcomplex (a trimer of VPS35, VPS29 and VPS26) and the membrane deformation subcomplex (VPS5p, VPS17p, sortinnexins 1/2, 5/6, 32).
-
■
Cargo molecules of the retromer include members of the VPS10 receptor protein family (SORL1, SORT1, SORCS1, SORCS2 and SORCS3).
Recycling of APP through the TGN-cell surface-endosome triangle
-
■
After exiting the TGN, APP either enters the nonamyloidogenic pathway to the plasma membrane where it can be cleaved by α-secretase, or is transported by clathrin to the endosomes where it is cleaved by BACE and γ-secretase resulting in Aβ (amyloidogenic endocytic pathway).
-
■
Thus, retromer-mediated trafficking of APP and/or its C-terminal APP fragments away from the endosome to the TGN plays a key role in modulating dwelling time for APP and/or its C-terminal APP fragments in the endocytic system.
-
■
The retromer sorts APP or BACE either by direct binding to the retromer or by indirect binding to its cargo molecules, such as the VPS10d receptors.
Retromer complex components & APP processing
-
■
Deficiency in the cargo-selective core components of the retromer VPS35 and VPS26 hippocampal subregion is linked to progression of AD pathology.
-
■
VPS26 or VPS35 deficient mice and flies show increased levels of BACE activity, endogenous Aβ and an earlier onset of AD-related phenotypes, including memory deficits.
The VPS10 receptor proteins & APP processing
-
■
SORL1 and SORCS1 gene expression is reduced in AD brains compared with control brains.
-
■
Two haplotypes in the SORL1 gene and several variants in SORCS1 are associated with AD and AD endophenotypes in various ethnic groups.
-
■
Underexpression of SORL1 or SORCS1 leads to increased production of Aβ and an increased risk of AD in humans.
-
■
SORL1 expression levels cross-correlate with expression levels of VPS35 suggesting that SORL1 may modulate Aβ generation via an interaction with this core component of the retromer complex.
-
■
PKC and ROCK2, which control the relative utilization of the secretory and endocytic pathways, may modulate APP metabolism by phosphorylation of SORL1.
Future perspective
-
■
Further confirmation is required to establish whether the retromer affects the sorting of APP and/or BACE and whether it does so by directly binding either of these type-I transmembrane proteins.
-
■
It must be clarified whether SORL1 downregulation missorts APP to the TGN or to the endosome.
-
■
It must be elucidated how SORCS1, SORCS2 and SORCS3, which do not seem to be engaged in Golgi-endosomal transport, affect APP processing and whether they tie APP to the cellular surface, promote its internalization and target APP to specific endosomal compartments.
-
■
It has to be clarified whether retromer elements and its cargo undergo accelerated degradation in the disease state.
-
■
Additional sorting molecules contributing to disease pathogenesis have to be identified.
Footnotes
Financial & competing interests disclosure C Reitz was supported by a Paul B Beeson Career Development Award (K23AG034550). The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry. 2006;63(2):168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 3.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68(2):270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of γ-secretase activity. Nat. Cell. Biol. 2003;5(5):486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 6.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. γ-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc. Natl Acad. Sci. USA. 2003;100(11):6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pericak-Vance MA, Bebout JL, Gaskell PC, Jr, et al. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am. J. Hum. Genet. 1991;48(6):1034–1050. [PMC free article] [PubMed] [Google Scholar]
- 8.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer disease meta analysis consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 9.Hollingworth P, Harold D, Sims R, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat. Genet. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat. Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seshadri S, Fitzpatrick AL, Ikram MA, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303(18):1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert JC, Heath S, Even G, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat. Genet. 2009;41(10):1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 13.Wechsler-Reya R, Sakamuro D, Zhang J, Duhadaway J, Prendergast GC. Structural analysis of the human BIN1 gene. Evidence for tissue-specific transcriptional regulation and alternate RNA splicing. J. Biol. Chem. 1997;272(50):31453–31458. doi: 10.1074/jbc.272.50.31453. [DOI] [PubMed] [Google Scholar]
- 14.Wigge P, Kohler K, Vallis Y, et al. Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Mol. Biol. Cell. 1997;8(10):2003–2015. doi: 10.1091/mbc.8.10.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S, Liu T, Li S, et al. Comparative proteomic analysis of brains of naturally aging mice. Neuroscience. 2008;154(3):1107–1120. doi: 10.1016/j.neuroscience.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 16.English JA, Dicker P, Focking M, Dunn MJ, Cotter DR. 2-D DIGE analysis implicates cytoskeletal abnormalities in psychiatric disease. Proteomics. 2009;9(12):3368–3382. doi: 10.1002/pmic.200900015. [DOI] [PubMed] [Google Scholar]
- 17.Tebar F, Bohlander SK, Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Mol. Biol. Cell. 1999;10(8):2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harel A, Wu F, Mattson MP, Morris CM, Yao PJ. Evidence for CALM in directing VAMP2 trafficking. Traffic. 2008;9(3):417–429. doi: 10.1111/j.1600-0854.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- 19.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007;7(4):255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 20.Bonifacino JS, Hurley JH. Retromer. Curr. Opin. Cell. Biol. 2008;20(4):427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Small SA, Gandy S. Sorting through the cell biology of Alzheimer's disease: intracellular pathways to pathogenesis. Neuron. 2006;52(1):15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Small SA, Kent K, Pierce A, et al. Model-guided microarray implicates the retromer complex in Alzheimer's disease. Ann. Neurol. 2005;58(6):909–919. doi: 10.1002/ana.20667. [DOI] [PubMed] [Google Scholar]
- 23.Muhammad A, Flores I, Zhang H, et al. Retromer deficiency observed in Alzheimer's disease causes hippocampal dysfunction, neurodegeneration, and Aβ accumulation. Proc. Natl Acad. Sci. USA. 2008;105(20):7327–7332. doi: 10.1073/pnas.0802545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen L, Tang FL, Hong Y, et al. VPS35 haploinsufficiency increases Alzheimer's disease neuropathology. J. Cell. Biol. 2011;195(5):765–779. doi: 10.1083/jcb.201105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finan GM, Okada H, Kim TW. BACE1 retrograde trafficking is uniquely regulated by the cytoplasmic domain of sortilin. J. Biol. Chem. 2011;286(14):12602–12616. doi: 10.1074/jbc.M110.170217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X, Li F, Chang WP, Tang J. GGA proteins mediate the recycling pathway of memapsin 2 (BACE) J. Biol. Chem. 2005;280(12):11696–11703. doi: 10.1074/jbc.M411296200. [DOI] [PubMed] [Google Scholar]
- 27.Okada H, Zhang W, Peterhoff C, et al. Proteomic identification of sorting nexin 6 as a negative regulator of BACE1-mediated APP processing. FASEB J. 2010;24(8):2783–2794. doi: 10.1096/fj.09-146357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sannerud R, Declerck I, Peric A, et al. ADP ribosylation factor 6 (ARF6) controls amyloid precursor protein (APP) processing by mediating the endosomal sorting of BACE1. Proc. Natl Acad. Sci. USA. 2011;108(34):E559–E568. doi: 10.1073/pnas.1100745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat. Genet. 2007;39(2):168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riemenschneider M, Schoepfer-Wendels A, Friedrich P, et al. No association of vacuolar protein sorting 26 polymorphisms with Alzheimer's disease. Neurobiol. Aging. 2007;28(6):883–884. doi: 10.1016/j.neurobiolaging.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Wetten S, Li L, et al. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch. Neurol. 2008;65(1):45–53. doi: 10.1001/archneurol.2007.3. [DOI] [PubMed] [Google Scholar]
- 32.Reiman EM, Webster JA, Myers AJ, et al. GAB2 alleles modify Alzheimer's risk in APOE ε4 carriers. Neuron. 2007;54(5):713–720. doi: 10.1016/j.neuron.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vilarino-Guell C, Wider C, Ross OA, et al. VPS35 mutations in Parkinson disease. Am. J. Hum. Genet. 2011;89(1):162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimprich A, Benet-Pages A, Struhal W, et al. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am. J. Hum. Genet. 2011;89(1):168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. α-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dachsel JC, Behrouz B, Yue M, Beevers JE, Melrose HL, Farrer MJ. A comparative study of Lrrk2 function in primary neuronal cultures. Parkinsonism Relat. Disord. 2010;16(10):650–655. doi: 10.1016/j.parkreldis.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scherzer CR, Offe K, Gearing M, et al. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch. Neurol. 2004;61(8):1200–1205. doi: 10.1001/archneur.61.8.1200. [DOI] [PubMed] [Google Scholar]
- 38.Reitz C, Cheng R, Rogaeva E, et al. Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch. Neurol. 2011;68(1):99–106. doi: 10.1001/archneurol.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JH, Chulikavit M, Pang D, Zigman WB, Silverman W, Schupf N. Association between genetic variants in sortilin-related receptor 1 (SORL1) and Alzheimer's disease in adults with Down syndrome. Neurosci. Lett. 2007;425(2):105–109. doi: 10.1016/j.neulet.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolsch H, Jessen F, Wiltfang J, et al. Association of SORL1 gene variants with Alzheimer's disease. Brain Res. 2009;1264:1–6. doi: 10.1016/j.brainres.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 41.Heinrich G. Amyloid A4 protein and its precursor in Down's syndrome and Alzheimer's disease. N. Engl. J. Med. 1989;321(17):1197. doi: 10.1056/NEJM198910263211715. [DOI] [PubMed] [Google Scholar]
- 42.Seshadri S, DeStefano AL, Au R, et al. Genetic correlates of brain aging on MRI and cognitive test measures: a genome-wide association and linkage analysis in the Framingham Study. BMC Med. Genet. 2007;8(Suppl. 1):S15. doi: 10.1186/1471-2350-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.T Cuenco K, Lunetta KL, Baldwin CT, et al. Association of distinct variants in SORL1 with cerebrovascular and neurodegenerative changes related to Alzheimer disease. Arch. Neurol. 2008;65(12):1640–1648. doi: 10.1001/archneur.65.12.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bralten J, Arias-Vasquez A, Makkinje R, et al. Association of the Alzheimer's gene SORL1 with hippocampal volume in young, healthy adults. Am. J. Psychiatry. 2011;168(10):1083–1089. doi: 10.1176/appi.ajp.2011.10101509. [DOI] [PubMed] [Google Scholar]
- 45.Alexopoulos P, Guo LH, Tsolakidou A, et al. Interrelations between CSF soluble AβPPβ, amyloid-β 1-42, SORL1, and tau levels in Alzheimer's disease. J. Alzheimers Dis. 2012;28(3):543–552. doi: 10.3233/JAD-2011-110983. [DOI] [PubMed] [Google Scholar]
- 46.Guo LH, Westerteicher C, Wang XH, et al. SORL1 genetic variants and cerebrospinal fluid biomarkers of Alzheimer's disease. Eur. Arch. Psychiatry Clin. Neurosci. 2012 doi: 10.1007/s00406-012-0295-x. doi:10.1007/s00406-012-0295-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Alexopoulos P, Guo LH, Kratzer M, Westerteicher C, Kurz A, Perneczky R. Impact of SORL1 single nucleotide polymorphisms on Alzheimer's disease cerebrospinal fluid markers. Dement. Geriatr. Cogn. Disord. 2011;32(3):164–170. doi: 10.1159/000332017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kauwe JS, Cruchaga C, Bertelsen S, et al. Validating predicted biological effects of Alzheimer's disease associated SNPs using CSF biomarker levels. J. Alzheimers Dis. 2010;21(3):833–842. doi: 10.3233/JAD-2010-091711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grear KE, Ling IF, Simpson JF, et al. Expression of SORL1 and a novel SORL1 splice variant in normal and Alzheimers disease brain. Mol. Neurodegener. 2009;4:46. doi: 10.1186/1750-1326-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pottier C, Hannequin D, Coutant S, et al. High frequency of potentially pathogenic SORL1 mutations in autosomal dominant early-onset Alzheimer disease. Mol. Psychiatry. 2012 doi: 10.1038/mp.2012.15. doi:10.1038/mp.2012.15. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.Andersen OM, Reiche J, Schmidt V, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc. Natl Acad. Sci. USA. 2005;102(38):13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Offe K, Dodson SE, Shoemaker JT, et al. The lipoprotein receptor LR11 regulates amyloid β production and amyloid precursor protein traffic in endosomal compartments. J. Neurosci. 2006;26(5):1596–1603. doi: 10.1523/JNEUROSCI.4946-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nielsen MS, Gustafsen C, Madsen P, et al. Sorting by the cytoplasmic domain of the amyloid precursor protein binding receptor SORLA. Mol. Cell. Biol. 2007;27(19):6842–6851. doi: 10.1128/MCB.00815-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fjorback AW, Seaman M, Gustafsen C, et al. Retromer binds the FANSHY sorting motif in sorla to regulate amyloid precursor protein sorting and processing. J. Neurosci. 2012;32(4):1467–1480. doi: 10.1523/JNEUROSCI.2272-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt V, Sporbert A, Rohe M, et al. SorLA/LR11 regulates processing of amyloid precursor protein via interaction with adaptors GGA and PACS-1. J. Biol. Chem. 2007;282(45):32956–32964. doi: 10.1074/jbc.M705073200. [DOI] [PubMed] [Google Scholar]
- 56.Herskowitz JH, Seyfried NT, Gearing M, et al. Rho kinase II phosphorylation of the lipoprotein receptor LR11/SORLA alters amyloid-β production. J. Biol. Chem. 2011;286(8):6117–6127. doi: 10.1074/jbc.M110.167239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reitz C, Lee JH, Rogers RS, Mayeux R. Impact of genetic variation in SORCS1 on memory retention. PLoS ONE. 2011;6(10):E24588. doi: 10.1371/journal.pone.0024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reitz C, Tokuhiro S, Clark LN, et al. SORCS1 alters amyloid precursor protein processing and variants may increase Alzheimer's disease risk. Ann. Neurol. 2011;69(1):47–64. doi: 10.1002/ana.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grupe A, Li Y, Rowland C, et al. A scan of chromosome 10 identifies a novel locus showing strong association with late-onset Alzheimer disease. Am. J. Hum. Genet. 2006;78(1):78–88. doi: 10.1086/498851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang X, Slifer M, Martin ER, et al. Genomic convergence to identify candidate genes for Alzheimer disease on chromosome 10. Hum. Mutat. 2009;30(3):463–471. doi: 10.1002/humu.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lane RF, Raines SM, Steele JW, et al. Diabetes-associated SorCS1 regulates Alzheimer's amyloid-β metabolism: evidence for involvement of SorL1 and the retromer complex. J. Neurosci. 2010;30(39):13110–13115. doi: 10.1523/JNEUROSCI.3872-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clee SM, Yandell BS, Schueler KM, et al. Positional cloning of SORCS1, a Type 2 diabetes quantitative trait locus. Nat. Genet. 2006;38(6):688–693. doi: 10.1038/ng1796. [DOI] [PubMed] [Google Scholar]
- 63.Granhall C, Park HB, Fakhrai-Rad H, Luthman H. High-resolution quantitative trait locus analysis reveals multiple diabetes susceptibility loci mapped to intervals <800 kb in the species-conserved Niddm1i of the GK rat. Genetics. 2006;174(3):1565–1572. doi: 10.1534/genetics.106.062208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goodarzi MO, Lehman DM, Taylor KD, et al. SORCS1: a novel human Type 2 diabetes susceptibility gene suggested by the mouse. Diabetes. 2007;56(7):1922–1929. doi: 10.2337/db06-1677. [DOI] [PubMed] [Google Scholar]
- 65.Paterson AD, Waggott D, Boright AP, et al. A genome-wide association study identifies a novel major locus for glycemic control in Type 1 diabetes, as measured by both A1C and glucose. Diabetes. 2010;59(2):539–549. doi: 10.2337/db09-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luchsinger JA. Diabetes, related conditions, and dementia. J. Neurol. Sci. 2010;299(1–2):35–38. doi: 10.1016/j.jns.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. β-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 2002;59(9):1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 68.Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor β-secretase in Alzheimer's disease. Ann. Neurol. 2002;51(6):783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 69.Johnston JA, Liu WW, Todd SA, et al. Expression and activity of β-site amyloid precursor protein cleaving enzyme in Alzheimer's disease. Biochem. Soc. Trans. 2005;33(Pt 5):1096–1100. doi: 10.1042/BST20051096. [DOI] [PubMed] [Google Scholar]
- 70.Olkkonen VM, Ikonen E. Genetic defects of intracellular-membrane transport. N. Engl. J. Med. 2000;343(15):1095–1104. doi: 10.1056/NEJM200010123431507. [DOI] [PubMed] [Google Scholar]
- 71.Cobbold C, Monaco AP, Sivaprasadarao A, Ponnambalam S. Aberrant trafficking of transmembrane proteins in human disease. Trends Cell. Biol. 2003;13(12):639–647. doi: 10.1016/j.tcb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Spoelgen R, von Arnim CA, Thomas AV, et al. Interaction of the cytosolic domains of sorLA/LR11 with the amyloid precursor protein (APP) and β-secretase β-site APP-cleaving enzyme. J. Neurosci. 2006;26(2):418–428. doi: 10.1523/JNEUROSCI.3882-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 101.AlzGene – field synopsis of genetic association studies in AD. www.alzgene.org.