Abstract
Herbal medicines have been used in preventing and treating skin disorders for centuries. It has been demonstrated that systemic administration of chrysanthemum extract exhibits anti-inflammatory properties. However, whether topical applications of apigenin, a constituent of chrysanthemum extract, influence cutaneous inflammation is still unclear. In the present study, we first tested whether topical applications of apigenin alleviate cutaneous inflammation in murine models of acute dermatitis. The murine models of acute allergic contact dermatitis and acute irritant contact dermatitis were established by topical application of oxazolone and phorbol 12-myristate 13-acetate (TPA), respectively. Inflammation was assessed in both dermatitis models by measuring ear thickness. Additionally, the effect of apigenin on stratum corneum function in a murine subacute allergic contact dermatitis model was assessed with an MPA5 physiology monitor. Our results demonstrate that topical applications of apigenin exhibit therapeutic effects in both acute irritant contact dermatitis and allergic contact dermatitis models. Moreover, in comparison with the vehicle treatment, topical apigenin treatment significantly reduced transepidermal water loss, lowered skin surface pH, and increased stratum corneum hydration in a subacute murine allergic contact dermatitis model. Together, these results suggest that topical application of apigenin could provide an alternative regimen for the treatment of dermatitis.
1. Introduction
Increasing evidence demonstrates the beneficial effects of herbal medicines in preventing and treating a variety of disorders, including inflammatory dermatoses. Prior studies have shown that topical applications of herbal extract prevent acute irritant murine contact dermatitis [1] and that oral administration of Cordyceps sinensis extract prevents cutaneous damages from Streptococcus pyogenes infection in an air pouch murine infection model [2]. Likewise, oral administration of Hainosankyuto reduces skin lesion size and increased survival rate in a Streptococcus pyogenes infected murine model [3]. Similarly, oral administration of the water extract of Astragalus membranaceus inhibits the development of atopic dermatitis-like lesions in a murine model [4]. Moreover, topical herbal extracts protect skin from UV radiation [5–7]. Additionally, Scutellaria bardata exhibits a preventive effect on the development of skin cancer [8]. Furthermore, the therapeutic effects of some herbal medicines on cutaneous inflammation have been well studied in both animals and humans. For instance, previous studies demonstrated that either topical or oral applications of herbal extracts inhibited both acute cutaneous inflammation and atopic dermatitis in animal models [9–12]. Study revealed that the majority of atopic dermatitis patients accepted herbal medicines as an alternative treatment approach [13]. Clinically, oral administrations of herbal medicines are effective in treating psoriasis, atopic dermatitis, and glucocorticoid-induced dermatitis [14–17].
Chrysanthemum is a common herbal medicine. The anti-inflammatory effects of chrysanthemum have been documented. For example, systemic administration of chrysanthemum extract inhibits both acute and chronic irritant contact dermatitis in murine models [18]. Topical applications of chrysanthemum extract alleviate diaper dermatitis in infants and newborns with erythema venenatum [19, 20]. Improvement of certain cutaneous drug reactions also has been reported with chrysanthemum [21]. Apigenin is an active constituent that is present in large quantities in chrysanthemum extract [22, 23]. It has been shown that apigenin exhibits preventive activity against UVB-induced cyclooxygenase-2 (COX-2) expression in keratinocyte cultures [24, 25]. In a murine model, an apigenin-enriched diet attenuated the development of atopic dermatitis-like lesions [26]. Although one clinical study showed that an apigenin containing cream inhibited cutaneous inflammation [27], the therapeutic effects of topical apigenin on cutaneous inflammation and barrier function have previously not been elucidated yet. In the present study, we evaluated the effects of topical apigenin on both acute and subacute cutaneous inflammation in murine models.
2. Materials and Methods
2.1. Materials
Both 6–8-week-old female hairless mice (hr/hr) and C57BL/6J mice were purchased from Charles River Laboratories (Wilmington, MA, USA) and fed mouse diet (Ralston-Purina Co., St Louis, MO, USA) and water ad libitum. Apigenin powder was from Sigma Chemical Co. (St Louis, MO, USA). Phorbol 12-myristate 13-acetate (TPA), 4-Ethoxymethylene-2-phenyloxazol-5-one (oxazolone), and ethanol were purchased from Sigma Chemical Co. (St Louis, MO, USA).
2.2. Experimental Protocols and Functional Studies
All animal procedures were approved by the Animal Studies Subcommittee (IACUC) of the San Francisco Veterans Administration Medical Center and performed in accordance with their guidelines. For anti-inflammatory studies in irritant contact dermatitis model, ear inflammation on C57BL/6J mice was induced by a topical application of 15 μL of 0.03% TPA to both the inner and outer surfaces of both ears [28, 29]. 20 μL of 0.1% (about 0.67 mg/kg body weight) apigenin in ethanol was applied to both surfaces of the right and 20 μL of ethanol alone was applied to both surfaces of the left ear at 45 min and 2 hours following TPA treatment. Additionally, the oxazolone-induced ear inflammation model (allergic contact dermatitis model) was also used to assess the anti-inflammatory effects of apigenin. C57BL/6J mice were sensitized by topical application of 3% oxazolone to the back once daily for two days. One week later, 15 μL of 0.5% oxazolone was applied to both the inner and outer surfaces of both ears. 20 μL of 0.1% apigenin or ethanol alone was applied to both surfaces of the right or left ear at 45 min and 2 hours following oxazolone treatment. Ear thickness was measured with a digital caliper (Mitutoyo, Tokyo, Japan) before and 20 hours after the challenge with oxazolone or TPA application. Under anesthesia (4% chlorohydrate, IP injection) ear samples were taken with surgical scissors for hematoxylin and eosin staining (H&E) staining of 5 μm paraffin-enabled sections [30].
For the subacute dermatitis model, 6–8-week-old female hairless mice (hr/hr) with body weight of 28–30 g were sensitized by topical application of 3% oxazolone to the back once daily for two days. One week later, 60 μL of 0.01% oxazolone was applied to both flanks of mice once every other day for 4 applications. One group of oxazolone-treated mice was topically treated with 60 μL of 0.1% (about 2 mg/kg body weight) apigenin twice daily for 7 days. The other group of oxazolone-treated mice was topically treated with ethanol alone and served as the control. In the case that oxazolone and apigenin or ethanol were applied on the same day, apigenin and ethanol were applied one hour after oxazolone application. On the 8th day, 18 hours after the last apigenin or ethanol application, basal transepidermal water loss (TEWL), stratum corneum hydration, and skin surface pH were measured using their respective probes connected to MPA5 (C&K, Cologne, Germany) as described earlier [31, 32].
2.3. Statistics
Data are expressed as the mean ± SEM. GraphPad Prism 4 software (San Diego, CA, USA) was used for all statistical analyses. Unpaired two-tailed Student's t-test with Welch's correction was used to determine the statistical significances when two groups were compared. One-Way ANOVA with Tukey correction was used when three or more groups were compared.
3. Results
3.1. Topical Apigenin Inhibits Acute Cutaneous Inflammation in Murine Contact Dermatitis Models
We first assessed whether topical applications of apigenin inhibit acute irritant contact dermatitis (AICD) and acute allergic contact dermatitis (AACD) in murine dermatitis models. As seen in Figure 1, ear thickness increased following either TPA or oxazolone treatment. Topical applications of apigenin almost completely normalized ear thickness in a murine AACD model (0.186 ± 0.003 for normal; 0.189 ± 0.003 for oxazolone + apigenin). Similarly, topical apigenin significantly reduced ear thickness in murine AICD model (0.485 ± 0.013 for TPA + vehicle; 0.409 ± 0.016 for TPA + apigenin, P < 0.001) (Figure 1). The anti-inflammatory effects of apigenin on acute cutaneous inflammation were further confirmed by H&E staining (Figure 2). These results demonstrate that topical apigenin inhibits acute cutaneous inflammation in murine dermatitis models.
Figure 1.
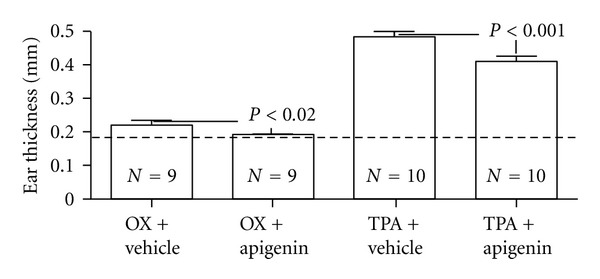
Topical apigenin reduces ear thickness in acute murine dermatitis models. Acute irritant contact dermatitis and allergic contact dermatitis models were established as described in Materials and Methods. 20 μL of 0.1% apigenin in ethanol or ethanol alone was applied to both surfaces of the right or left ear, respectively, 45 min and 2 hours following TPA or oxazolone challenge. Ear thickness was measured with a digital caliper (Mitutoyo, Tokyo, Japan) before and 20 hours after challenge with oxazolone or TPA application. The dotted line represented the normal ear thickness. Numbers and significances are indicated in the figures.
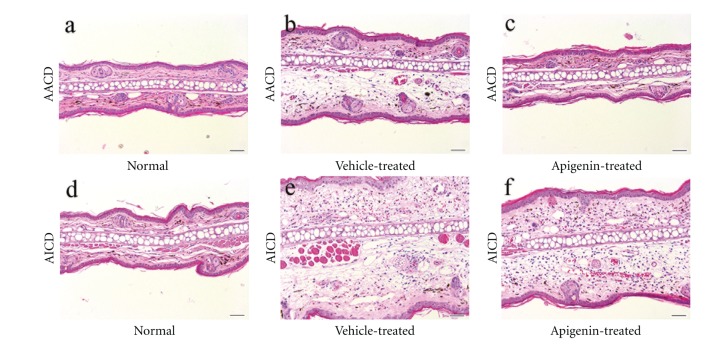
Figure 2.
Topical applications of apigenin reduce edema in acute murine dermatitis models. Acute irritant contact dermatitis and allergic contact dermatitis models were established as described in Materials and Methods Ear samples for H&E staining were taken immediately after measurements of ear thickness. (a) and (d) are normal ears. (b) and (c) are acute allergic contact dermatitis (AACD) treated with vehicle and apigenin, respectively. (e) and (f) are acute irritant contact dermatitis (AICD) treated with vehicle and apigenin, respectively. A remarkable reduction in ear thickness and edema were evident in apigenin-treated ear. The magnifications for all images are the same. Scale bar = 50 μm.
3.2. Topical Apigenin Lowers Transepidermal Water Loss in a Subacute Murine Allergic Contact Dermatitis Model
Previous studies have demonstrated that apigenin attenuates the development of atopic dermatitis-like lesions [26] and that transepidermal water loss positively correlates with the severity of subacute and chronic dermatitis [33, 34]. We next determined whether topical apigenin influences transepidermal water loss in subacute cutaneous inflammation in a murine model. As shown in Figure 3(a), repeated topical oxazolone treatment markedly increased transepidermal water loss as compared with vehicle-treated control (P < 0.001). Topical apigenin treatment dramatically prevented the increase in transepidermal water loss induced by oxazolone treatment (P < 0.001 versus oxazolone + vehicle treatment). This result indicates that topical apigenin improves epidermal permeability barrier function in murine subacute dermatitis model.
Figure 3.
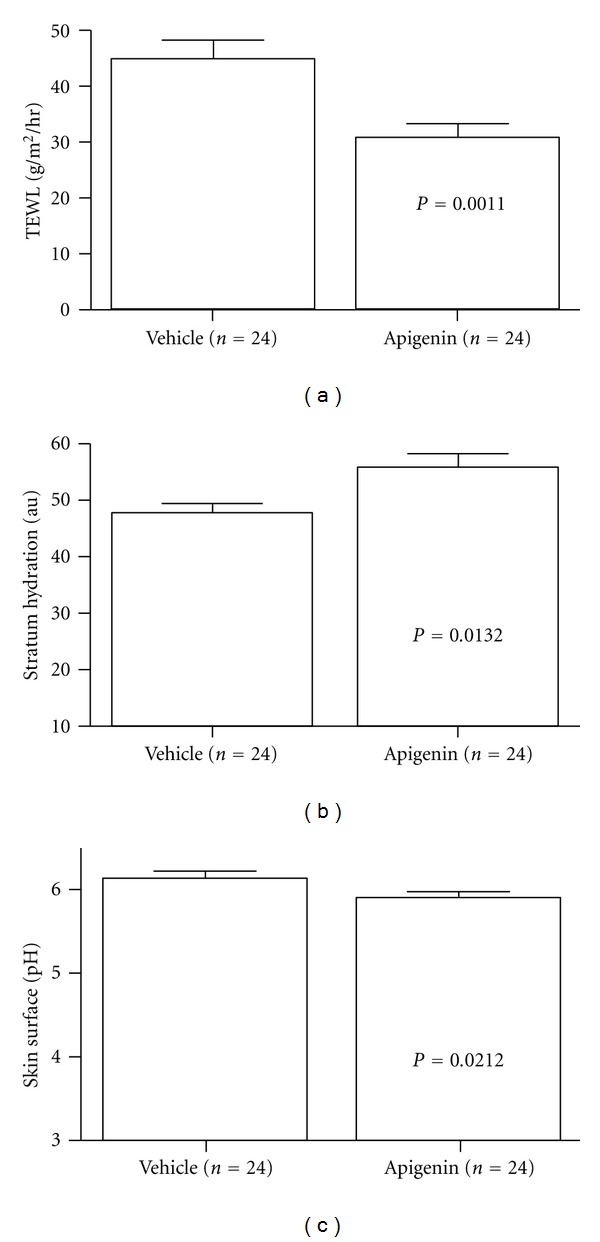
Topical apigenin improves stratum corneum function in murine model of subacute allergic contact dermatitis. Subacute dermatitis model was established as described in Materials and Methods. On the 8th day, basal transepidermal water loss, skin surface pH, and stratum corneum (SC) hydration were assessed with an MPA5 (CK electronic GmbH, Cologne, Germany) connected to TM 300, pH905, and Corneometer 825. Two readings were taken from each mouse for basal TEWL, hydration, as well as pH. (a) indicates a reduction in transepidermal water loss following apigenin treatment; (b) shows apigenin induced an increase in stratum corneum hydration; (c) exhibits a lower skin surface pH after apigenin treatment. Numbers and significances are indicated in the figures.
It has been shown that subacute and chronic dermatitis are characterized by lower stratum corneum hydration and higher skin surface pH [30, 35]. Therefore, stratum corneum hydration and skin surface pH were also evaluated following vehicle and apigenin treatment in oxazolone-treated mice. As reported previously [30], repeated oxazolone applications significantly decreased stratum corneum hydration (Figure 3(b)). Although the apigenin treatment did not normalize stratum corneum hydration, a notably higher stratum corneum hydration was observed following apigenin treatment (Figure 3(b), P < 0.05 for oxazolone + vehicle versus oxazolone + apigenin). In agreement with prior findings [30], repeated oxazolone treatment caused a significant increase in skin surface pH (Figure 3(c), P < 0.001 for normal versus oxazolone + vehicle). In comparison with the vehicle treatment, a substantially lower skin surface pH was apparent in apigenin-treated mice (Figure 3(c), P < 0.05 for oxazolone + vehicle versus oxazolone + apigenin; unpaired Student's t-test). These results suggest that topical apigenin partially inhibits the changes of stratum corneum hydration and skin surface pH induced by repeated oxazolone applications.
Together, these results demonstrate that topical apigenin attenuates the changes of stratum corneum function induced by repeated oxazolone applications.
4. Discussion
It has been shown that topical applications of herbal extracts inhibit cutaneous inflammation and improve both the epidermal permeability barrier and the antimicrobial barrier function [1, 9, 32]. However, the active constituents of herbal medicines have not yet been well defined. Recently, an active ingredient, hesperidin, in orange peel has been shown to improve the epidermal permeability barrier function [31]. In the present study, we first demonstrated that topical applications of apigenin, an extract from chrysanthemum, inhibit both acute irritant and acute allergic dermatitis in murine models. Although the exact mechanisms by which apigenin inhibits acute cutaneous inflammation are not clear, several potential mechanisms could be involved. It is well known that matrix metalloproteinase-1 is involved in cutaneous inflammation [36, 37]. It has been reported that apigenin inhibits matrix metalloproteinase-1 expression induced by 12-O-tetradecanoylphorbol 13-acetate in dermal fibroblasts [38]. Secondly, in addition to inhibiting TNF-alpha gene expression induced by lipopolysaccharide [39], apigenin also inhibits TNF-alpha secretion in vitro [40]. Similarly, oral administrations of apigenin reduce the high serum TNF-alpha levels induced by romurtide in mice [41]. Thirdly, apigenin inhibits the expression of inflammation-related molecules, such as intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin, induced by TNF-alpha and IL-1alpha [42, 43]. Studies suggest that inhibition of inflammation by apigenin is via nuclear factor (NF)-κB and MAPKs pathways [44, 45]. All these anti-inflammatory effects induced by apigenin are likely attributed to its antioxidant properties. Apigenin is a well-known antioxidant [46, 47]. Studies have demonstrated that antioxidants such as quercetin (Que) and cromolyn inhibit release of inflammatory mediators including histamine, leukotrienes, IL-6, IL-8, and TNF release from mast cells in vitro [48]. In vivo studies reveal that vitamin E, an antioxidant, improves atopic dermatitis-like inflammation [49]. Therefore, the anti-inflammatory effect of apigenin could be due to its antioxidant effects.
Previous studies revealed that stratum corneum function, especially transepidermal water loss, positively correlates with the severity of atopic dermatitis [50, 51]. And inhibition of cutaneous inflammation could decrease transepidermal water loss [52, 53]. In the present study, the effect of apigenin on subacute dermatitis was evaluated by assessing transepidermal water loss, a parameter of epidermal permeability barrier function. A significantly lower level of transepidermal water loss was observed in apigenin-treated mice. This improvement of transepidermal water loss could be attributable to both the anti-inflammatory and the antioxidant properties of apigenin. Thus, apigenin-induced improvement of transepidermal water loss in the murine subacute dermatitis model is at least partially due to the inhibition of cutaneous inflammation. In addition, apigenin exhibits antioxidant properties [54, 55]. It has been shown that both systemic and topical administration of antioxidants lower transepidermal water loss [56–59]. Hence, the apigenin-induced improvement in epidermal permeability barrier homeostasis could result from its antioxidant properties.
It is worth noting that apigenin is a relatively safe agent. Singh et al. reported that 50 mg/kg body weight of apigenin caused no changes in serum biomarkers (alanine amino transferase, aspartate amino transferase, and alkaline phosphatase) of hepatotoxicity at 48 hours after intraperitoneal injection in Swiss mice [60]. Likewise, no sign of illness was observed in mice after 10 days of single intraperitoneal injection of 40 mg/kg body weight of apigenin [61]. However, apigenin at dosage of 10 mg/kg body weight significantly inhibits cytokines such as TNF, IL-1 and IL-6 expression in vivo [61]. In the present, our results indicated that a lower dose (1.23 mg/kg body weight) apigenin could improve acute dermatoses and topical applications of apigenin at dosage of 4 mg/kg body weight per day for 7 days could relieve subacute dermatitis. Taken together, these results strongly suggest that apigenin is a safe and effective anti-inflammatory agent, especially for topical use.
5. Conclusions
The present study demonstrates that topical apigenin inhibits acute inflammation and subacute dermatitis as indicated by improved epidermal permeability barrier function. Therefore, apigenin could be useful in treating acute and subacute dermatitis.
Conflict of Interests
All authors have no conflict of interests.
Acknowledgment
This work was partially supported by Grants AR19089, PEM; AR051930, TM from the National Institutes of Health. The authors are grateful to Dr. Shunpeng Song for his useful advice and suggestions.
References
- 1.Man W, Man M, Hupe M, et al. Topical herbal extract (Huangdang mixture) exhibits both preventive and therapeutic effects in murine acute irritant contact dermatitis. International Journal of Dermatology. 2011;50(11):1421–1427. doi: 10.1111/j.1365-4632.2011.04970.x. [DOI] [PubMed] [Google Scholar]
- 2.Kuo CF, Chen CC, Luo YH, et al. Cordyceps sinensis mycelium protects mice from group A streptococcal infection. Journal of Medical Microbiology. 2005;54, Part 8:795–802. doi: 10.1099/jmm.0.45704-0. [DOI] [PubMed] [Google Scholar]
- 3.Minami M, Ichikawa M, Hata N, Hasegawa T. Protective effect of hainosankyuto, a traditional japanese medicine, on streptococcus pyogenes infection in murine model. PLoS ONE. 2011;6(7, article e22188) doi: 10.1371/journal.pone.0022188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SJ, Oh SG, Seo SW, et al. Oral administration of Astragalus membranaceus inhibits the development of DNFB-induced dermatitis in NC/Nga mice. Biological and Pharmaceutical Bulletin. 2007;30(8):1468–1471. doi: 10.1248/bpb.30.1468. [DOI] [PubMed] [Google Scholar]
- 5.Korać RR, Khambholja KM. Potential of herbs in skin protection from ultraviolet radiation. Pharmacognosy Reviews. 2011;5(10):164–173. doi: 10.4103/0973-7847.91114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaid M, Sharma SD, Katiyar SK. Honokiol, a phytochemical from the Magnolia plant, inhibits photocarcinogenesis by targeting UVB-induced inflammatory mediators and cell cycle regulators: development of topical formulation. Carcinogenesis. 2010;31(11):2004–2011. doi: 10.1093/carcin/bgq186. [DOI] [PubMed] [Google Scholar]
- 7.Hwang YP, Choi JH, Kim HG. et al. Cultivated ginseng suppresses ultraviolet B-induced collagenase activation via mitogen-activated protein kinases and nuclear factor κB/activator protein-1-dependent signaling in human dermal fibroblasts. Nutrition Research. 2012;32(6):428–438. doi: 10.1016/j.nutres.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Suh SJ, Yoon JW, Lee TK, et al. Chemoprevention of Scutellaria bardata on human cancer cells and tumorigenesis in skin cancer. Phytotherapy Research. 2007;21(2):135–141. doi: 10.1002/ptr.2010. [DOI] [PubMed] [Google Scholar]
- 9.Man MQ, Shi Y, Man M, et al. Chinese herbal medicine (Tuhuai extract) exhibits topical anti-proliferative and anti-inflammatory activity in murine disease models. Experimental Dermatology. 2008;17(8):681–687. doi: 10.1111/j.1600-0625.2007.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung YY, Yoon T, Jang JY, Park SJ, Kim HK. Topical application of Rehmannia glutinosa extract inhibits mite allergen-induced atopic dermatitis in NC/Nga mice. Journal of Ethnopharmacology. 2011;134(1):37–44. doi: 10.1016/j.jep.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 11.Yoon T, Lee DY, Lee AY, Choi G, Choo BK, Kim HK. Anti-inflammatory effects of Glehnia littoralis extract in acute and chronic cutaneous inflammation. Immunopharmacology and Immunotoxicology. 2010;32(4):663–670. doi: 10.3109/08923971003671108. [DOI] [PubMed] [Google Scholar]
- 12.Chung TH, Kang TJ, Cho WK, et al. Effectiveness of the novel Herbal medicine, KIOM-MA, and its bioconversion product, KIOM-MA128, on the treatment of atopic dermatitis. Evidence-Based Complementary and Alternative Medicine. 2012;2012:9 pages. doi: 10.1155/2012/762918.762918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston GA, Bilbao RM, Graham-Brown RAC. The use of complementary medicine in children with atopic dermatitis in secondary care in Leicester. British Journal of Dermatology. 2003;149(3):566–571. doi: 10.1046/j.1365-2133.2003.05471.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheng HM, Chiang LC, Jan YM, Chen GW, Li TC. The efficacy and safety of a Chinese herbal product (Xiao-Feng-San) for the treatment of refractory atopic dermatitis: a randomized, double-blind, placebo-controlled trial. International Archives of Allergy and Immunology. 2011;155(2):141–148. doi: 10.1159/000318861. [DOI] [PubMed] [Google Scholar]
- 15.May BH, Zhang AL, Zhou W, Lu CJ, Deng S, Xue CC. Oral herbal medicines for psoriasis: a review of clinical studies. Chinese Journal of Integrative Medicine. 2012;18:172–178. doi: 10.1007/s11655-012-1008-z. [DOI] [PubMed] [Google Scholar]
- 16.Bai YS, Zhou CY, Wang JQ. Clinical observation on auxiliary treatment of hormone dependence dermatitis by shufeng liangxue decoction. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28(12):1121–1123. [PubMed] [Google Scholar]
- 17.Chen SY, Song WM, Du XH. Effect of modified wuhua decoction on skin barrier function in facial corticosteroid addictive dermatitis patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28(5):410–413. [PubMed] [Google Scholar]
- 18.Lee DY, Choi G, Yoon T, Cheon MS, Choo BK, Kim HK. Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. Journal of Ethnopharmacology. 2009;123(1):149–154. doi: 10.1016/j.jep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Yuan F. Nursing care for babies with diaper dermatitis treated with chrysanthemum flower water. China Foreign Medical Treatment . 2010;13, article 117 [Google Scholar]
- 20.Sun T, Meng L, Wang S. Bathing with different concentrations of wild chrysanthemum soup for newborn erythema venenatum: comparison of the effect. Journal of Nursing Sciences. 2011;32:39–40. [Google Scholar]
- 21.Zhou M. Treatment of drug dermatitis with chrysanthemum bathing. Jiangxi Journal of Traditional Chinese Medicine. 2001;32:60–61. [Google Scholar]
- 22.Zhang J, Qiang DW, Li YB, Duan ZQ. Chemical constituents from chrysanthemum morifolium ramat. Natural Product Research and Development. 2006;18:71–73. [Google Scholar]
- 23.Shen H, Guo Q, Fang H, Wang Y, Jin M. Determination of quercetin, luteolin, apigenin and acacetin in Flos Chrysanthemi Indici by RP-HPLC. Zhongguo Zhongyao Zazhi. 2010;35(2):191–193. doi: 10.4268/cjcmm20100216. [DOI] [PubMed] [Google Scholar]
- 24.Tong X, Van Dross RT, Abu-Yousif A, Morrison AR, Pelling JC. Apigenin prevents UVB-induced cyclooxygenase 2 expression: coupled mRNA stabilization and translational inhibition. Molecular and Cellular Biology. 2007;27(1):283–296. doi: 10.1128/MCB.01282-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Dross RT, Hong X, Essengue S, Fischer SM, Pelling JC. Modulation of UVB-induced and basal cyclooxygenase-2 (COX-2) expression by apigenin in mouse keratinocytes: role of USF transcription factors. Molecular Carcinogenesis. 2007;46(4):303–314. doi: 10.1002/mc.20281. [DOI] [PubMed] [Google Scholar]
- 26.Yano S, Umeda D, Yamashita S, Yamada K, Tachibana H. Dietary apigenin attenuates the development of atopic dermatitis-like skin lesions in NC/Nga mice. Journal of Nutritional Biochemistry. 2009;20(11):876–881. doi: 10.1016/j.jnutbio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Arsić I, Tadić V, Vlaović D, et al. Preparation of novel apigenin-enriched, liposomal and non-liposomal, antiinflammatory topical formulations as substitutes for corticosteroid therapy. Phytotherapy Research. 2011;25(2):228–233. doi: 10.1002/ptr.3245. [DOI] [PubMed] [Google Scholar]
- 28.Schmuth M, Haqq CM, Cairns WJ, et al. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. Journal of Investigative Dermatology. 2004;122:971–983. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- 29.Komuves LG, Hanley K, Lefebvre AM, et al. Stimulation of PPARalpha promotes epidermal keratinocyte differentiation in vivo. Journal of Investigative Dermatology. 2000;115:353–360. doi: 10.1046/j.1523-1747.2000.00073.x. [DOI] [PubMed] [Google Scholar]
- 30.Man MQ, Hatano Y, Lee SH, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. Journal of Investigative Dermatology. 2008;128(1):79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou M, Man M, Man W, et al. Topical hesperidin improves epidermal permeability barrier function and epidermal differentiation in normal murine skin. Experimental Dermatology. 2012;21:337–340. doi: 10.1111/j.1600-0625.2012.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man M, Hupe M, Mackenzie D, et al. A topical Chinese herbal mixture improves epidermal permeability barrier function in normal murine skin. Experimental Dermatology. 2011;20(3):285–288. doi: 10.1111/j.1600-0625.2010.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemoto-Hasebe I, Akiyama M, Nomura T, Sandilands A, McLean WHI, Shimizu H. Clinical severity correlates with impaired barrier in filaggrin-related eczema. Journal of Investigative Dermatology. 2009;129(3):682–689. doi: 10.1038/jid.2008.280. [DOI] [PubMed] [Google Scholar]
- 34.Hatano Y, Man MQ, Uchida Y, et al. Murine atopic dermatitis responds to peroxisome proliferator-activated receptor alpha, ß/delta (but not gamma), and liver-X-receptor activators. Journal of Allergy and Clinical Immunology. 2010;125:160–169. doi: 10.1016/j.jaci.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knor T, Meholjić-Fetahović A, Mehmedagić A. Stratum corneum hydration and skin surface pH in patients with atopic dermatitis. Acta Dermatovenerologica Croatica. 2011;19:242–247. [PubMed] [Google Scholar]
- 36.Herouy Y, Mellios P, Bandemir E, et al. Inflammation in stasis dermatitis upregulates MMP-1, MMP-2 and MMP-13 expression. Journal of Dermatological Science. 2001;25(3):198–205. doi: 10.1016/s0923-1811(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 37.Unemori EN, Ehsani N, Wang M, Lee S, McGuire J, Amento EP. Interleukin-1 and transforming growth factor-α: synergistic stimulation of metalloproteinases, PGE2, and proliferation in human fibroblasts. Experimental Cell Research. 1994;210(2):166–171. doi: 10.1006/excr.1994.1025. [DOI] [PubMed] [Google Scholar]
- 38.Lim H, Kim HP. Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids. Planta Medica. 2007;73(12):1267–1274. doi: 10.1055/s-2007-990220. [DOI] [PubMed] [Google Scholar]
- 39.Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. Journal of Pharmacology and Experimental Therapeutics. 2001;296(1):181–187. [PubMed] [Google Scholar]
- 40.Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1β and tumor necrosis factor-α genes in J774.2 macrophages. Pharmacological Reports. 2005;57(3):390–394. [PubMed] [Google Scholar]
- 41.Ueda H, Yamazaki C, Yamazaki M. A hydroxyl group of flavonoids affects oral anti-inflammatory activity and inhibition of systemic tumor necrosis factor-α production. Bioscience, Biotechnology and Biochemistry. 2004;68(1):119–125. doi: 10.1271/bbb.68.119. [DOI] [PubMed] [Google Scholar]
- 42.Gerritsen ME, Carley WW, Ranges GE, et al. Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. American Journal of Pathology. 1995;147(2):278–292. [PMC free article] [PubMed] [Google Scholar]
- 43.Takano-Ishikawa Y, Goto M, Yamaki K. Inhibitory Effects of Several Flavonoids on E-selectin Expression on Human Umbilical Vein Endothelial Cells Stimulated by Tumor Necrosis Factor-α . Phytotherapy Research. 2003;17(10):1224–1227. doi: 10.1002/ptr.1371. [DOI] [PubMed] [Google Scholar]
- 44.Cheon MS, Yoon T, Lee DY, et al. Chrysanthemum indicum Linné extract inhibits the inflammatory response by suppressing NF-κB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophages. Journal of Ethnopharmacology. 2009;122(3):473–477. doi: 10.1016/j.jep.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 45.Xie C, Kang J, Li Z, et al. The açaí flavonoid velutin is a potent anti-inflammatory agent: blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. The Journal of Nutritional Biochemistry. 2012;23:1184–1191. doi: 10.1016/j.jnutbio.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Khalil MI, Alam N, Moniruzzaman M, Sulaiman SA, Gan SH. Phenolic acid composition and antioxidant properties of malaysian honeys. Journal of Food Science. 2011;76(6):C921–C928. doi: 10.1111/j.1750-3841.2011.02282.x. [DOI] [PubMed] [Google Scholar]
- 47.Romanová D, Vachálková A, Cipák L, Ovesná Z, Rauko P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma. 2001;48(2):104–107. [PubMed] [Google Scholar]
- 48.Weng Z, Zhang B, Asadi S, et al. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PLoS One. 2012;7, article e33805 doi: 10.1371/journal.pone.0033805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayashi D, Sugaya H, Ohkoshi T, et al. Vitamin E improves biochemical indices associated with symptoms of atopic dermatitis-like inflammation in NC/Nga mice. Journal of Nutritional Science and Vitaminology. 2012;58:161–168. doi: 10.3177/jnsv.58.161. [DOI] [PubMed] [Google Scholar]
- 50.Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. Journal of Allergy and Clinical Immunology. 2008;121(3):725–730. doi: 10.1016/j.jaci.2007.12.1161. [DOI] [PubMed] [Google Scholar]
- 51.Kim DW, Park JY, Na GY, Lee SJ, Lee WJ. Correlation of clinical features and skin barrier function in adolescent and adult patients with atopic dermatitis. International Journal of Dermatology. 2006;45(6):698–701. doi: 10.1111/j.1365-4632.2005.02644.x. [DOI] [PubMed] [Google Scholar]
- 52.Le TKM, De Mon P, Schalkwijk J, Van Der Valk PGM. Effect of a topical corticosteroid, a retinoid and a vitamin D3 derivative on sodium dodecyl sulphate induced skin irritation. Contact Dermatitis. 1997;37(1):19–26. doi: 10.1111/j.1600-0536.1997.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 53.Parneix-Spake A, Goustas P, Green R. Eumovate (clobetasone butyrate) 0.05% cream with its moisturizing emollient base has better healing properties than hydrocortisone 1% cream: a study in nickel-induced contact dermatitis. Journal of Dermatological Treatment. 2001;12(4):191–197. doi: 10.1080/09546630152696107. [DOI] [PubMed] [Google Scholar]
- 54.Suh KS, Oh S, Woo JT, et al. Apigenin attenuates 2-deoxy-D-ribose-induced oxidative cell damage in HIT-T15 pancreatic β-cells. Biological and Pharmaceutical Bulletin. 2012;35:121–126. doi: 10.1248/bpb.35.121. [DOI] [PubMed] [Google Scholar]
- 55.Jin BH, Qian LB, Chen S, et al. Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. European Journal of Pharmacology. 2009;616(1–3):200–205. doi: 10.1016/j.ejphar.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 56.Al-Shaal L, Shegokar R, Muller RH. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. International Journal of Pharmaceutics. 2011;420:133–140. doi: 10.1016/j.ijpharm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 57.Pasonen-Seppänen S, Suhonen MT, Kirjavainen M, et al. Vitamin C enhances differentiation of a continuous keratinocyte cell line (REK) into epidermis with normal stratum corneum ultrastructure and functional permeability barrier. Histochemistry and Cell Biology. 2001;116(4):287–297. doi: 10.1007/s004180100312. [DOI] [PubMed] [Google Scholar]
- 58.Jeon HY, Kim JK, Kim WG, Lee SJ. Effects of oral epigallocatechin gallate supplementation on the minimal erythema dose and uv-induced skin damage. Skin Pharmacology and Physiology. 2009;22(3):137–141. doi: 10.1159/000201562. [DOI] [PubMed] [Google Scholar]
- 59.Jerajani H, Mizoguchi H, Li J, Whittenbarger D, Marmor M. The effects of a daily facial lotion containing vitamins B3 and E and provitamin B5 on the facial skin of Indian women: a randomized, double-blind trial. Indian Journal of Dermatology, Venereology and Leprology. 2010;76(1):20–26. doi: 10.4103/0378-6323.58674. [DOI] [PubMed] [Google Scholar]
- 60.Singh P, Mishra SK, Noel S, Sharma S, Rath SK. Acute exposure of apigenin induces hepatotoxicity in Swiss mice. PLoS One. 2012;7, article e31964 doi: 10.1371/journal.pone.0031964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rithidech KN, Tungjai M, Reungpatthanaphong P, Honikel L, Simon SR. Attenuation of oxidative damage and inflammatory responses by apigenin given to mice after irradiation. Mutation Research. 2012;749(1-2):29–38. doi: 10.1016/j.mrgentox.2012.08.001. [DOI] [PubMed] [Google Scholar]