Abstract
How could the incredible complexity of modern cells evolve from something simple enough to have appeared in a primordial soup? This enduring question has sparked the interest of researchers since Darwin first considered his theory of natural selection. Organic molecules, even potentially functional molecules including peptides and nucleotides, can be produced abiotically. Amphiphiles such as surfactants and lipids display remarkable self-assembly processes including the spontaneous formation of vesicles resembling the membranes of living cells. Nonetheless, numerous questions remain. Given the presumably dilute concentrations of macromolecules in the prebiotic pools where the earliest cells are thought to have appeared, how could the necessary components become concentrated and encapsulated within a semipermeable membrane? What would drive the further structural complexity that is a hallmark of modern living systems? The interior of modern cells is subdivided into microcompartments such as the nucleoid of bacteria or the organelles of eukaryotic cells. Even within what at first appears to be a single compartment, for example, the cytoplasm or nucleus, chemical composition is often nonuniform, containing gradients, macromolecular assemblies, and/or liquid droplets. What might the internal structure of intermediate evolutionary forms have looked like?
The nonideal aqueous solution chemistry of macromolecules offers an attractive possible answer to these questions. Aqueous polymer solutions will form multiple coexisting thermodynamic phases under a variety of readily accessible conditions. In this Account, we describe aqueous phase separation as a model system for biological compartmentalization in both early and modern cells, with an emphasis on systems that have been encapsulated within a lipid bilayer. We begin with an introduction to aqueous phase separation and discuss how this phenomenon can lead to microcompartmentalization and could facilitate biopolymer encapsulation by partitioning of solutes between the phases. We then describe primitive model cells based on phase separation inside lipid vesicles, which mimic several basic properties of biological cells: microcompartmentation, protein relocalization in response to stimulus, loss of symmetry, and asymmetric vesicle division. We observe these seemingly complex phenomena in the absence of genetic molecules, enzymes, or cellular machinery, and as a result these processes could provide clues to possible intermediates in the early evolution of cell-like assemblies.
Introduction
The origin of the first living cells is an intriguing and enduring question at the heart of evolutionary theory. A single cell is the simplest unit of life, but cells as we know them today are already rich in structural and functional complexity. Although we are still far from a complete understanding of the first cell, many important pieces of the puzzle have been reported and/or hypothesized. Relevant organic molecules including peptides and nucleotides have been prepared under conditions thought to be consistent with those present on the early earth.1 The self-assembly properties of amphiphiles suggest routes to formation of semipermeable membranes and encapsulation of macromolecules within vesicles.2 Enzymatic reactions, including transcription and translation, have been performed successfully within lipid vesicles, and routes to membrane permeability and even membrane growth and reproduction have been reported.2−7 Despite these and other exciting advances, questions remain. Among them: how did the many different molecules needed for early life become concentrated and encapsulated together in the same precellular compartment? These molecules were presumably rare on the early earth, present in trace amounts in the aqueous pools where the earliest cells are thought to have appeared. How did these early cells then become further compartmentalized to acquire the intracellular complexity common to modern cells?
The physical and chemical properties of the molecules of life, many of which are polymeric, may provide important clues as to how the earliest cells arose. Macromolecules occupy space, sterically excluding volume that would otherwise be available to other solutes. This excluded volume effect, also termed macromolecular crowding, has considerable impact on a wide range of reactions involving one or more polymers in solution.8 Additionally, intermolecular interactions can lead to complex solution chemistry, including, for example, oligomerization, aggregation, or phase separation. In this Account, we will consider a possible role for phase separation in the formation of early cells and their internal compartments and describe experiments performed in simple artificial cells that harbor coexisting aqueous phases.
Historical Context
The idea that phase separation in polymer solutions may have played a role in the earliest stages of protocell evolution is not new. As early as the 1930s, Oparin hypothesized that “coazervate droplets” formed from organic macromolecules could have developed into the “simplest primary organisms”.9 Complex coacervation is a form of liquid–liquid phase separation that occurs in solutions of oppositely charged polyelectrolytes, leading to formation of a dilute phase and a concentrated coacervate phase enriched in both polyelectrolytes.10 Oparin performed reactions in coacervate systems, for example, enzymatically preparing polyadenylic acid in RNA/histone coacervate droplets.11 Inspired by progress in abiotic synthesis of biologically relevant molecules such as amino acids,1,12 Fox prepared “thermal proteinoids”, microspheres formed when abiotic polypeptides, prepared by heating amino acids, were added to water.13 A recurring theme in this early work was the recognition that polymer solutions form a variety of structures at least superficially reminiscent of cells, which led these authors to hypothesize about a role for such assemblies in the origin of life. Oparin, Fox, and their contemporaries did not address the need for heritable genetic material, and it should be noted that DNA was not known to be the genetic material when Oparin was developing his theory on the origin of life.
Lipid self-assembly to form liposomes was first reported by Bangham and Horne in the mid-1960s,14 and hence it is unsurprising that Oparin, Fox and their contemporaries also did not invoke lipid self-assembly as a major focus in their work of this time. Since then, the importance of lipid or other amphiphile self-assemblies in forming the earliest cells has been widely accepted. Indeed, Lazcano reports a fascinating exchange more than 40 years after Oparin first posited his coacervate theory for precellular life, in which Oparin indicated that, had he the chance to start over, even he would have studied liposomes instead of coacervates.15 Bilayer membranes provide a semipermeable boundary between the inside and outside of a cell, which is important for maintaining the macromolecules necessary for life while allowing exchange of smaller molecules such as nutrients and wastes. Recent work by Luisi and co-workers suggests that encapsulation during vesicle formation could be a viable approach for coencapsulation of complex solute mixtures.16,17 They encapsulated a cell free expression system in 100 nm lipid vesicles under conditions where statistical entrapment of all 80 components was extremely unlikely; protein expression indicated that a subset of vesicles did contain all components.17 Although phospholipids are the primary membrane components in modern cell membranes, early cells likely used other amphiphiles. Mansy and co-workers have demonstrated that fatty-acid-based membranes provide the necessary permeability to small molecule nutrients such as nucleotides, while retaining macromolecules.18
In light of the clear importance of genetic information and surfactant self-assembly, coacervate theory has been largely dismissed as an explanation of the origins of life by modern scientists.15 It is nonetheless worthwhile to recall this early work when we consider possible structural and functional roles for macromolecular assemblies and phases in the development of early cells. Indeed, the importance of solution nonideality due to high concentrations of macromolecular solutes has again come to the forefront, with the realization that macromolecular crowding substantially alters the kinetics and thermodynamics of biochemical reactions as compared to performing these reactions in dilute solutions.8 The time may be right to again consider the behavior of multicomponent solutions of macromolecules, appreciating that these phenomena are not acting alone but in concert with replicating informational molecules and lipid self-assembly.
Phase Separation in Aqueous Solutions of Macromolecules
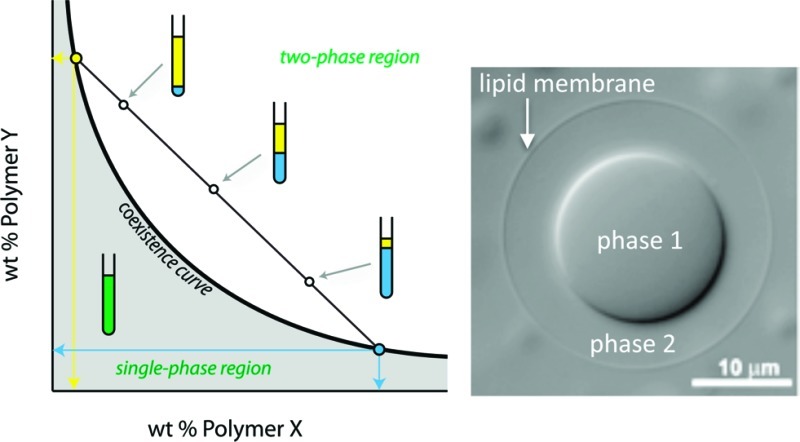
Interactions between macromolecules in aqueous solution can lead to the formation of distinct thermodynamic phases, all aqueous but each harboring a different polymer composition.19 Perhaps the most common and best understood example is the aqueous two-phase system (ATPS) formed when poly(ethylene glycol) and dextran are mixed at concentrations above a few weight percent in each polymer. Other solutions containing two neutral polymers with predominantly repulsive interactions also result in ATPS with one phase enriched in each of the polymers. Figure 1 illustrates the phase diagram for a solution of two uncharged polymers in water. At low concentrations of polymer, the solution exists as a single phase, and at high concentrations phase separation occurs. A coexistence curve, or binodal, separates these two regions of the diagram; compositions for each phase of a phase separated ATPS lie on this curve.19 Tie lines relate the overall composition of a solution to the concentration of each of the polymers in the top (yellow) and bottom (blue) phases, as illustrated by the blue and yellow lines in Figure 1. The length of the tie line therefore gives an indication of the compositional difference between top and bottom phases. For example, points along tie line b will have more similar top and bottom phase compositions than those along tie line a. All compositions located on the same tie line (e.g., 2–4) have the same composition of top (yellow) and bottom (blue) phases, corresponding to the points where the tie line intersects the binodal, but differing in their phase volumes as illustrated in Figure 1. When more than two polymers are present, additional phases can be obtained.19
Figure 1.
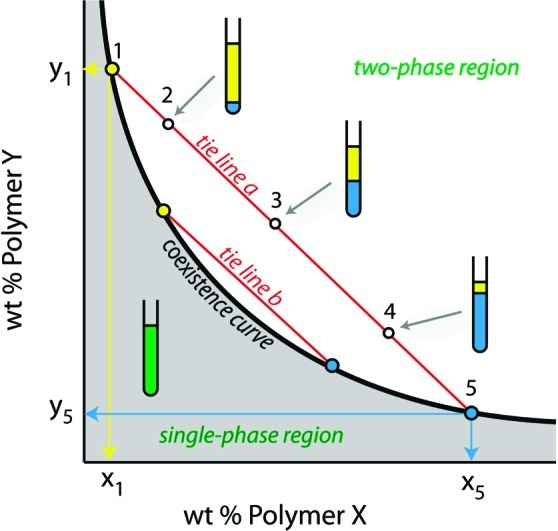
Generic phase diagram for an aqueous solution of two neutral polymers. The concentration of each polymer in the top and bottom phase is given by the intersection of the tie line on which that composition lies with the coexistence curve. Here, points 2, 3, and 4 are above the coexistence curve and therefore exist as two phases. These points lie on the same tie line and consequently their top and bottom phases are each given by points 1 (top phase) and 5 (bottom phase) but differ in volume as indicated in the illustration.
More complex phase behavior is possible for strongly interacting polymers such as oppositely charged polyelectrolytes, which can form complex coacervates as mentioned above.10 Charge screening by dissolved salts controls the interactions between the polyelectrolytes, such that phase behavior is strongly dependent on the solution ionic strength.19,20 Coacervates can also form between strongly hydrogen-bonded polymer pairs. Finally, aqueous phase separation can occur in solutions that contain a single polymer as a function of temperature or at high salt concentration.20
The polymers in any of the aforementioned systems can be biological macromolecules such as polysaccharides, proteins, or nucleic acids, and need not have especially high molecular weights. Mann et al. recently reported reaction compartmentalization in coacervate microdroplets formed by mixing nucleotides with low molecular weight cationic peptides.21 Biomacromolecular phase systems have received less attention from a thermodynamic standpoint than simple polymer systems due to their greater complexity, but data are available on their phase behavior due largely to their importance in applications such as food science.22 Most pairs of polymers will form a phase system if sufficiently concentrated, typically several weight percent of each polymer. Hence, Walter and Brooks in 1995 hypothesized that the cytoplasm of living cells, which contains on the order of 30 wt % total macromolecules, must consist of coexisting aqueous phases.23 If phase separation did occur in cytoplasm, one would expect multiple phases due to the large number of different biomacromolecules, and the various phases could be expected to wet the membrane, organelles, cytoplasm, and each other, depending on their chemical composition. Whether this phenomenon is in fact common in contemporary cells is not known, only a very few examples have been reported.24−26 Regardless, the simplicity of aqueous phase separation as a means of controlling local solution properties is very attractive both as a model system for understanding the effect of intracellular microcompartmentalization and as a possible way to achieve it in early cells.
Partitioning
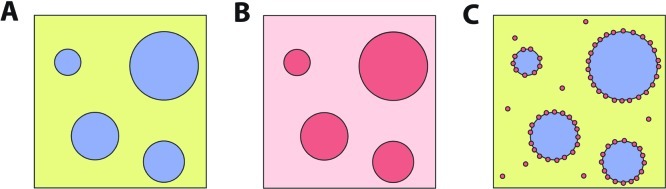
As illustrated in Figure 2, the presence of distinct liquid phases means that molecular solutes and colloidal particulates will partition between the two phases or between one of the phases and the aqueous/aqueous interface.20,27 This provides a simple and powerful mechanism for concentration of biopolymers or their precursors by partitioning into one of the phases. Partitioning is quantified in terms of the partitioning coefficient, K, which equals the concentration of solute in the top phase divided by its concentration in the bottom phase. In the case of PEG/dextran ATPS, where the PEG-rich phase is on top, K > 1 indicates partitioning into the PEG-rich phase while K < 1 indicates partitioning into the dextran-rich phase. Most native proteins will partition into the dextran-rich phase. Denatured proteins, having exposed their more hydrophobic interior amino acid residues, typically accumulate in the PEG-rich phase, due to the greater hydrophobicity of this phase.28,29 Whether a given solute partitions strongly into one of the aqueous phases or is more uniformly distributed depends on the properties of the ATPS (e.g., polymer structure, polymer molecular weight, and concentration) as well as the chemistry of the solute itself.20,27 In general, solute partitioning scales with tie line length (TLL), because TLL is a measure of the difference in composition between the top and bottom phases of the ATPS.19 Solute properties of particular importance include charge, size, and any binding interactions with the polymers that make up the ATPS. Charged solutes can partition quite strongly between the phases, particularly in ATPS containing charged polymers either as one of the major polymeric components or as a low concentration additive.19,20 Partitioning depends on solute size, because larger solutes experience a greater interaction area with the phases.30
Figure 2.
Agitation of a bulk ATPS results in micrometer-scale droplets of one phase suspended in the other phase (A). Two examples of partitioning are shown: partitioning between the two aqueous phases with no significant accumulation at the interface (B) and particulate partitioning between one of the phases and the interface (C).
When partitioning to the interface is negligible and K is concentration-independent, which is the case for most proteins and other solutes of interest in the absence of aggregation, predictions about the solute concentration in each phase may be made for any phase volumes. Relative phase volume is important because for a fixed total volume and amount of solute, maintaining constant K requires that the solute concentrations in the two phases vary as volumes are changed. Decreasing the relative volume of the phase into which the solute preferentially accumulates results in an increase in solute concentration in both phases. If we posit the formation of aqueous phases in a primordial soup with dilute organic macromolecules, two observations can be made: (1) a small volume of the phase into which partitioning is to occur is desirable, and (2) the most-studied ATPS where both polymers are neutral and each phase is enriched in one of the polymers is perhaps the least likely scenario. This is because ATPS formed from oppositely charged polyelectrolytes or one polyelectrolyte and a salt could be achieved at lower overall concentrations of organic macromolecules.10
Aqueous phase droplets could collect and concentrate organic macromolecules from a dilute solution; however, these structures are unstable with respect to coalescence. Additionally, complex coacervates and polymer/salt ATPS are very sensitive to salt concentration and can be readily dissolved by changes in ionic strength.10 Indeed, instability to changes in salt concentration was one of the early criticisms of Oparin’s coacervate theory for protocell development. How might such droplets be stabilized? One possibility is the formation of a surface coating to form a stabilized emulsion.
Assembly of Material at the Aqueous/Aqueous Interface
Partitioning of material to the aqueous/aqueous interface of an ATPS can be significant, especially for large solutes such as protein aggregates, liposomes, whole cells, and other particulates. This has been used in whole cell and organelle separations19 and as a way to control biorecognition-driven particle assembly at the aqueous–aqueous interface.31 Driving forces for interfacial assembly include reducing the interfacial tension at the aqueous–aqueous interface and volume exclusion from the aqueous phases, one or both of which has high macromolecule concentrations. ATPS interfacial tensions are substantially lower than organic/aqueous interfaces and depend on the composition of the ATPS.31
Assembly of material at the ATPS interface could provide a means of encapsulating preconcentrated biopolymers or their precursors within a semipermeable shell. Jin and co-workers recently demonstrated the formation of polymersomes with asymmetric bilayers around dispersed dextran-rich phase droplets of a PEG/dextran ATPS.32 The bilayer was composed of block copolymers designed to interact with each of the phases such that each copolymer would preferentially contact one of the phases: poly(ethylene glycol)-block-poly (ε-caprolactone) was thought to make up the outer leaflet which contacted the PEG-rich continuous phase while dextran-block-poly(ε-caprolactone) formed the inner leaflet in contact with the dextran-rich phase.32
Microcompartmentalized Artificial Cells Formed by Encapsulating ATPS within a Lipid Membrane
Phase-separating aqueous systems have been encapsulated within lipid bilayers to form compartmentalized primitive artificial cells that mimic some aspects of intracellular organization. To date, this work has focused almost exclusively on PEG/dextran systems because they are well understood, and can be encapsulated within cell-sized giant lipid vesicles during formation by gentle hydration or electroformation.33−35 In addition to limiting the encapsulated volume, the lipid membrane provides a semipermeable, flexible boundary around the polymer solution. Water molecules can pass the membrane but the polymers cannot; hence, the interior volume and polymer concentrations can be controlled by changing external osmolarity.
ATPS Encapsulation within Lipid Vesicles
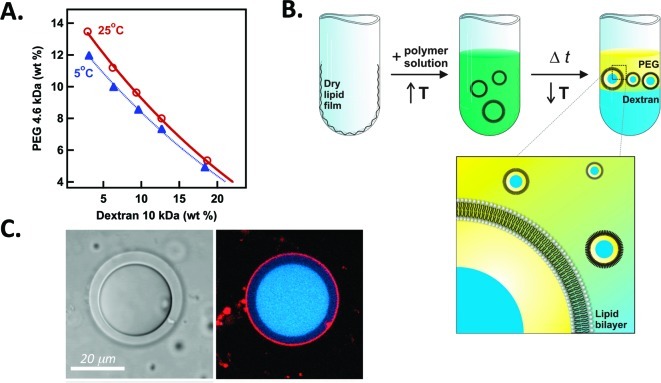
Lipid vesicles larger than 5 μm in diameter are referred to as giant vesicles (GVs).36 Populations of ATPS-containing GVs can be prepared by selecting a polymer solution that exists as a single phase at the preparation temperature and can subsequently be heated or cooled to induce phase separation (Figure 3). Careful attention to the phase diagram is necessary because this approach is viable for only a narrow range of solution compositions, e.g. those between the 25 and 5 °C binodal curves in Figure 3A.33,34 Another route to ATPS encapsulation is to hydrate lipids in a polymer solution corresponding to the single-phase region of the phase diagram and then expose them to a solution of higher osmotic pressure. The resulting concentration of the internal polymer solution can be used to move into a region of the phase diagram where separation occurs.33,35,37
Figure 3.
Preparation of artificial cells in which ATPS are encapsulated by a lipid membrane. (A) phase diagram for a PEG/dextran ATPS showing temperature dependence. (B) Vesicles are formed by gentle hydration in a warm polymer solution, after which cooling leads to phase separation. Reprinted with permission from ref (33). Copyright 2005 National Academy of Sciences. (C) Partitioning in an ATPS-containing giant vesicle. Fluorescently labeled dextran is shown in blue, and lipid in red. Image acquired by M. Andes-Koback.
Microcompartmentalization
ATPS-containing lipid vesicles provide a versatile and biophysically reasonable experimental system for cytoplasmic organization. Each aqueous phase serves as a distinct compartment, different in chemical composition. Solute partitioning leads to differences in local concentration on subcellular length scales. This can be seen in optical microscope images for a representative ATPS-containing vesicle (Figure 3C). PEG-rich and dextran-rich phases are distinguishable in transmitted light with differential interference contrast (DIC); the dextran-rich phase, which contains a greater concentration of the optically active dextran, appears “thicker” in DIC. Locations of dye-labeled lipid and polymer are imaged via fluorescence (Figure 3C).
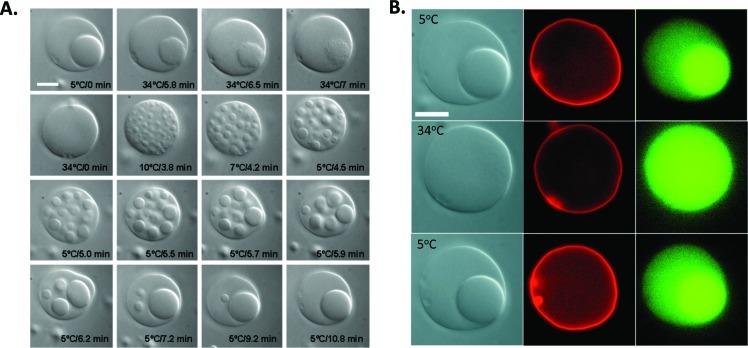
Local solute concentrations within this model cytoplasm can be changed by undergoing a phase transition (Figure 4). The concentration of a protein, concanavalin A (green), is initially much higher locally in the dextran-rich phase compartment, but after heating to convert the internal ATPS to a single phase the protein concentration is uniform throughout the vesicle interior. Cooling reforms the dextran-rich phase, resulting in recompartmentalization of concanavalin A (Figure 4B). Osmotic pressure induced phase transitions can also be used to reversibly compartmentalize the vesicle interior.33,35 Although dynamic compartmentalization is common in biological cells, it does not generally occur in response to temperature or osmotic pressure differences. A more biologically relevant model would be one in which proteins undergo changes in local concentration in response to alterations in their structure such as may occur through denaturation or post-translational modifications. An experimental model for structure-dependent compartmentalization has also been prepared using ATPS-containing vesicles.28
Figure 4.
Reversible microcompartmentalization in an ATPS-containing vesicle. (A) Optical microscopy images during heating and subsequent cooling show phase transitions in the interior ATPS. (B) Fluorescence microscopy indicates the location of the lipid membrane (red) and a protein concanavalin A (green) before and after temperature changes. Scale bars are 10 μm. Reprinted with permission from ref (33). Copyright 2005 National Academy of Sciences.
Changes in molecular structure can alter protein partitioning between the phase compartments. We demonstrated this using the pH-sensitivity of protein folding.28 Many proteins undergo unfolding transitions when the pH is very high or low. This effect has been used to separate different serum proteins in bulk ATPS, based on slight differences in their pH-dependent partitioning.29 When human serum albumin (HSA) was encapsulated in an ATPS-containing vesicle at pH 4, it partitioned largely to the PEG-rich phase and at the interface, presumably as aggregates.28 Changing the external pH to 6.5 renatured the protein, resulting in its migration to the dextran-rich phase (Figure 5). Protein relocalization to the dextran-rich phase was complete in just over 2 min.28
Figure 5.
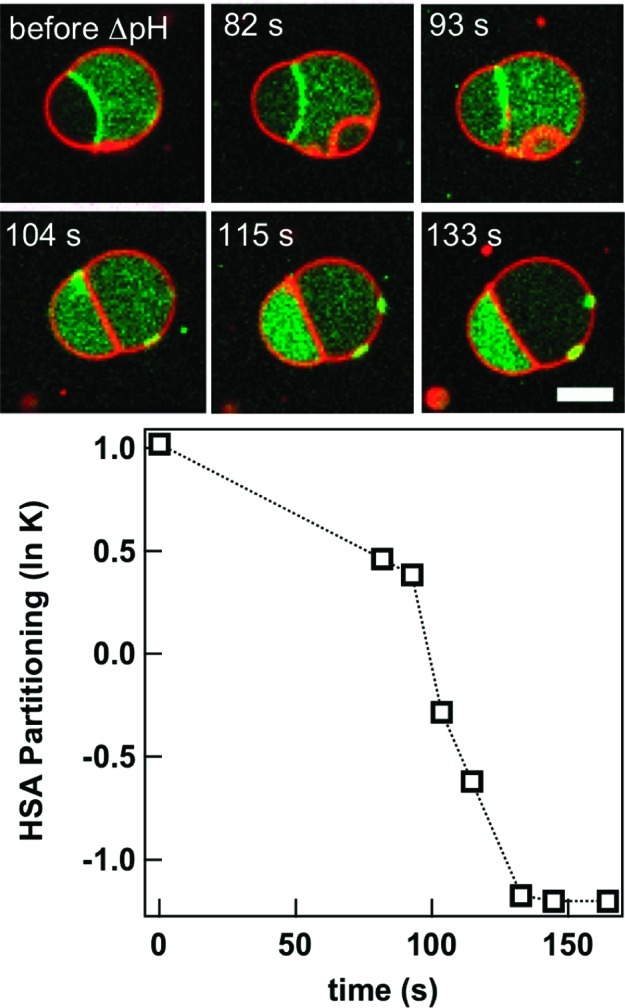
Protein migration between aqueous phase microcompartments within a giant lipid vesicle. Human serum albumin (HSA, green) moves from the PEG-rich compartment and ATPS interface at pH 4.1 to the dextran-rich compartment at pH 6.5. Lipid membrane is shown in red; scale bar is 5 μm. Reprinted with permission from ref (28). Copyright 2010 American Chemical Society.
As seen in Figure 5 and described above for bulk ATPS, the aqueous/aqueous interface of encapsulated ATPS serves as an assembly site for particulates such as aggregated protein. Lipid material also accumulates at this interface (see later panels of Figure 5). This interfacial lipid does not necessarily represent a bilayer dividing the two aqueous phases. Dimova and co-workers have shown that lipid material accumulated at the interface between encapsulated PEG-rich and dextran-rich phases is in the form of lipid nanotubes, which are pulled from the vesicle membrane when it is exposed to hypertonic solution and the interior undergoes phase separation.35
Budding and Fission
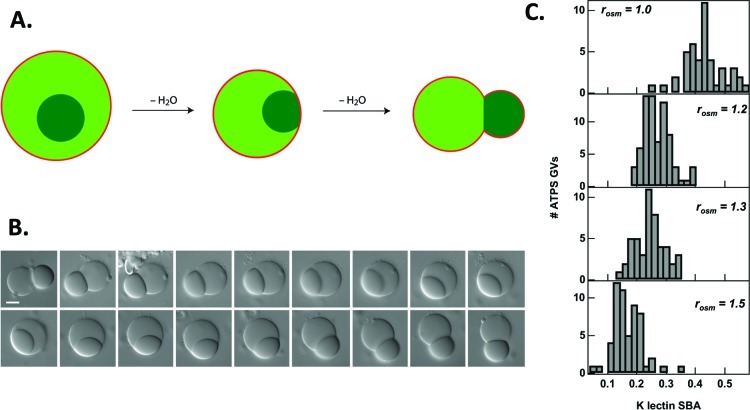
The vesicle shown in Figure 5 is nonspherical. This budded morphology is the result of osmotically induced deflation of an initially spherical ATPS-containing vesicle. Exposure to hypertonic external media draws water out of the vesicles, concentrating the interior polymers and often leading to changes in vesicle shape. As vesicle volume decreases, the amount of membrane surface area available becomes larger than needed to coat the interior volume, allowing the interior aqueous phases to minimize their interfacial area by budding. Interfacial tension at the aqueous/aqueous interface between the two phases increases as the polymers become more concentrated. Initially concentric aqueous phases reduce their ATPS interfacial area by converting to budded geometries as shown in Figure 6. This process requires that both interior phases contact the membrane, and proceeds through a wetting transition in which the contact angle between the dextran-rich aqueous phase droplet and the membrane increases.37,38 A recent theoretical study describes the conditions under which phase droplets adjacent to the membrane can cause changes in membrane curvature that result in budding transitions, depending on droplet size, membrane tension, and intrinsic contact angle of the phases with the membrane.39
Figure 6.
Response of ATPS-containing GVs to osmotic stress. (A) Schematic illustration of budding process. (B) The budding transition is reversible. Here, water is added to the external medium of a budded vesicle, causing a volume increase and retraction of the dextran-rich bud. Subsequent exposure to hypertonic sucrose solution regenerates the budded structure. Scale bar is 10 μm. (C) Histograms for partitioning of a protein, soybean agglutinin as a function of external osmolality; final external/initial internal osmolality ratios, rosm, are given for each panel. Reprinted with permission from ref (38). Copyright 2008 American Chemical Society.
One practical consequence of budding is that proteins or other molecules that are partitioned between the aqueous phases are now asymmetrically distributed in the budded structures, forming a polarized artificial cell that harbors different protein concentrations at its poles.38 The ability to osmotically control interior polymer concentrations also provides a route to improving partitioning in an already phase-separated ATPS. Figure 6C shows the effect of external solution osmolality on partitioning for an encapsulated protein; as the external osmolality increases, the partitioning of soybean agglutinin (SBA) into the dextran-rich phase increases.38 Partitioning varies between individual vesicles within a batch due to a distribution in encapsulated concentrations of each of the polymers.33,40
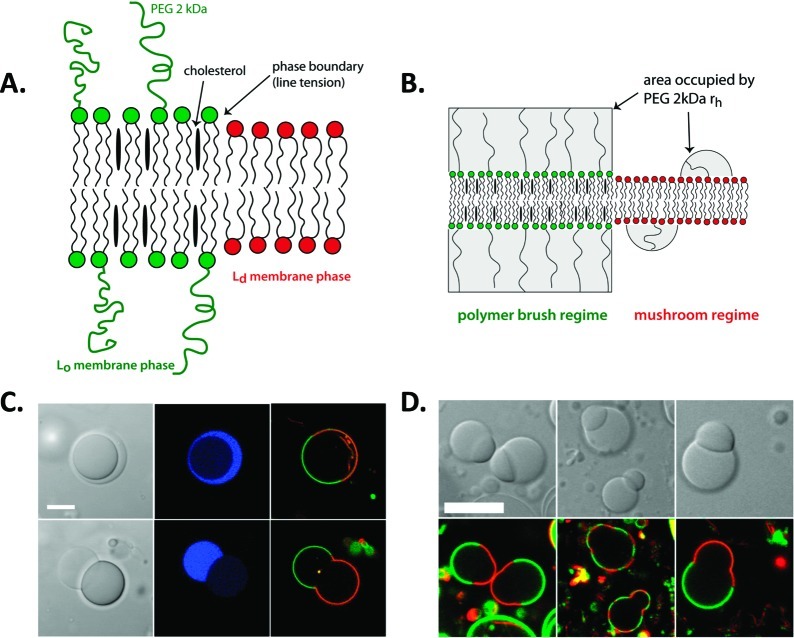
GVs can be prepared with coexisting phase domains in both the aqueous interior and the lipid membrane.41 Ternary mixtures of a high-melting lipid, a low-melting lipid, and cholesterol form liquid ordered (Lo) and liquid disordered (Ld) membrane domains, the latter of which have greater fluidity. We used partitioning between Lo and Ld to control the surface density of pendant PEG groups extending from the membrane surface of GVs (Figure 7A,B).42 PEG 2 kDa-DPPC was added to a DPPC/DOPC/cholesterol lipid system, which resulted in a higher lateral density of PEGylated headgroups in the Lo domain (3.9 vs 1.1 mol % PEG 2 kDa-DPPC in Lo vs Ld). The PEG density in Lo corresponded to the polymer brush regime, while the Ld domain was in the mushroom regime. This difference was sufficient to cause Lo to preferentially wet the PEG-rich phase of the interior ATPS (Figure 7C).42 Similar morphologies can be observed for Lo/Ld membranes in the absence of an internal ATPS: budding occurs to minimize line tension at the boundary between the two membrane domains.43 When an interior ATPS is present, however, the interfacial tension at the aqueous/aqueous phase boundary dominates. This can be seen in Figure 7C and D, where the buds correspond to the internal aqueous phases rather than the Lo and Ld membrane domains. Indeed, budded morphologies persist when the vesicles are heated to disrupt membrane phase domains (Figure 7D).42 These simple model cells, based on phase domains in the membrane and aqueous interior of giant lipid vesicles, display several important attributes of biological cells: (1) asymmetry in shape, aqueous interior composition, local protein concentration, and membrane composition, and (2) communication between membrane and internal microcompartments via noncovalent interactions that result in “pinning” of a membrane domains to internal microcompartments.
Figure 7.
Correspondence between interior aqueous microcompartments and membrane phase domains. Illustrations of (A) phase separation in ternary lipid mixture, and (B) higher surface density of PEGylated headgroups leading to polymer brush regime for Lo domain while surface densities for Ld domain are in the mushroom regime. (C) Optical microscope images for an ATPS-containing GV before and after undergoing a budding transition. (D) Effect of temperature on lipid phase domain distribution on budded GVs. Blue indicates the PEG-rich aqueous phase, green fluorescence indicates PEGylated Lo lipid domain, and red indicates Ld lipid domain. Reprinted with permission from ref (42). Copyright 2008 American Chemical Society.
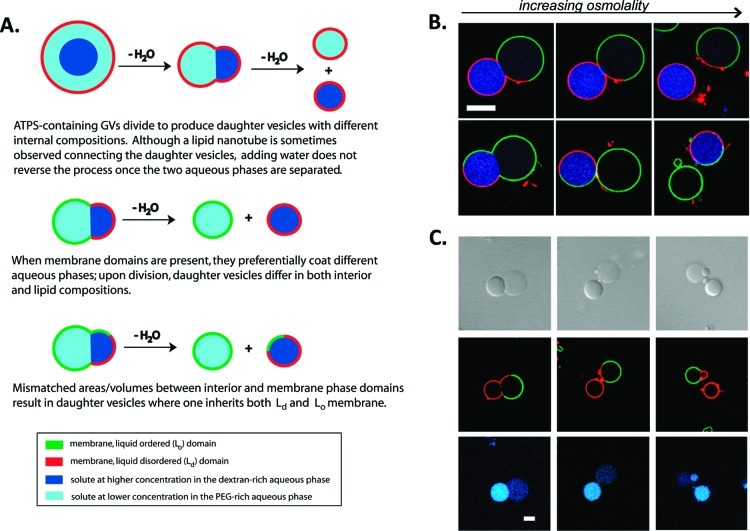
Further osmotically induced volume loss ultimately leads to fission of the model cells to produce nonidentical daughter vesicles, each containing one of the aqueous phases.44 Fission occurs at the aqueous/aqueous phase boundary. Thus, one daughter vesicle contains the PEG-rich phase and any molecules partitioned into this phase, while the other contains the dextran-rich phase and its contents. If membrane domains are also present, these are also inherited asymmetrically. By controlling the relative volumes of the two interior aqueous phases and the relative areas of the Lo and Ld membrane domains, it is possible to dictate which of the daughter cells inherits an asymmetric membrane (Figure 8A,B).44 In these examples, the dextran-rich daughter vesicle contains most of the SBA protein (blue) that was in the initial vesicle, and is coated by either Ld or a mixture of Ld and Lo, depending on the Lo/Ld area coverage in the initial vesicle. Membrane-bound protein (streptavidin, green, bound to biotinylated lipid in the Lo domain) is inherited by the PEG-rich daughter vesicle. When one of the daughter vesicles inherits an asymmetric membrane, it can serve as a polarity cue for the next round of budding (Figure 8C). The fact that very simple artificial cells composed of only a lipid membrane surrounding a polymer solution can display seemingly complex behaviors such as reversible microcompartmentalization and asymmetric division is remarkable. These observations underscore the important roles that can be filled by lipids and macromolecular solutions as a function of their inherent physical and chemical properties.
Figure 8.
Asymmetric fission of ATPS-containing GV. (A) Illustration of outcomes based on initial conditions. (B) Fluorescence microscopy showing two division events yielding nonidentical daughter vesicles, one of which is already polarized due to the presence of both membrane domains. (C) Budding of a daughter vesicle after initial fission event. Panels top to bottom are transmitted light (DIC), membrane fluorescence, and interior protein fluorescence. Red indicates Ld domain lipid (DOPE-rhodamine), and green indicates protein bound to Lo domain lipid (streptavidin-Alexa 488, bound to DSPE-PEG 2000-biotin), and blue indicates a protein, SBA-Alexa 647, which is partitioned into the dextran-rich interior aqueous phase. T = 5 °C. Scale bar is 10 μm. Reprinted with permission from ref (44). Copyright 2011 American Chemical Society.
Relevance to Modern Cells
Today’s cells are known to require many specific proteins, working seamlessly together, to accomplish major tasks such as metabolism and cell division. These processes are sufficiently complex that intermediate evolutionary forms can be difficult to envision. If tiny changes in DNA sequence that encode for as little as a single amino acid residue can be enough to interfere with these biochemical processes, how could significantly more primitive forms have functioned at all? An attractive hypothesis is that the fundamental properties of the molecules involved (self-assembly phase separation, partitioning, and macromolecular crowding) provided initial complexity that was then built upon as more sophisticated molecules such as proteins became available. Hence, investigations into the solution chemistry and self-assembly properties of the molecular classes that make up living systems may point toward possible evolutionary routes.
The physical properties of macromolecules, including volume exclusion and phase separation, remain important for modern cells.45,46 Phase separation has not been observed in most cell types, perhaps because the large number of different macromolecules, interaction types, and intracellular structures precludes its occurrence or its observation. A few examples of aqueous phase separation have been observed in biological cells. Phase separation is the cause of “cold cataracts”, a disease state in which the normally clear eye lens cytoplasm becomes cloudy, interfering with vision.47 The eye lens is a special type of cell, in which phase separation is presumably more detrimental as compared to other cell types. It is also easier to detect in these cells, which have a transparent cytoplasm. What of other cell types? Do they incorporate phase separation as type of microcompartment or have they evolved to avoid phase separation, as do healthy eye lens cells? A recent paper provides a possible answer. Brangwynne et al. demonstrated that RNA- and protein-rich bodies termed P-granules found in C. elegans embryos were liquid droplets.25 P-granules had been imaged previously but it was only through sophisticated fluorescence microscopic methods that their liquid nature was apparent. This finding suggests that aqueous phase separation can and does occur in modern cells and furthermore that it may not be obvious on simple inspection. Whether other intracellular structures observed via fluorescence microscopy and presumed to be complexes turn out to be aqueous phase droplets remains to be seen.
Likelihood of Phase Separation in Early Cells or Precellular Assemblies
Given that aqueous phase separation is common in relatively concentrated polymer solutions, we can speculate about possible roles for this phenomenon in the formation of the earliest cell-like assemblies. Even if early polymers formed at initially low concentrations, it is not difficult to imagine that evaporation could reduce the volume of polymer-containing pools sufficiently to enable phase separation, perhaps by the formation of coacervate droplets.11,21 Partitioning of biopolymer precursors into these droplets could, in turn, have increased reaction rates, facilitating biopolymer production. Accumulation of material such as amphiphiles or their assemblies at the aqueous–aqueous interface could have played a role in high-yield encapsulation of the newly formed biopolymers or their precursors. Subsequent dehydration could have triggered further phase separation of the encapsulated material, leading to interior microcompartments. We cannot verify that aqueous phase separation was present in precellular assemblies. If present, however, this phenomenon could provide answers to longstanding questions about how the necessary molecules for life became sufficiently concentrated and how they became encapsulated in the earliest cells.
Several possible mechanisms have been proposed for the encapsulation of macromolecules in the earliest cells. Here, we focused on aqueous phase separation. We refer the interested reader to also consider alternatives such as amphiphile self-assembly based mechanisms, aqueous/organic phase separation, and macromolecule gelation.5,6 Because all of these rely on the physical and chemical properties of molecules themselves, one can reasonably anticipate that multiple mechanisms were operative and may have operated synergistically in protocell assembly.
Acknowledgments
This work was funded by the National Science Foundation (CHE-0750196, cofunded by MCB) and National Institutes of Health (R01GM078352). I am grateful to current and former collaborators and members of my research group for their scientific contributions, enthusiasm, and creativity. Additional thanks to M. Andes-Koback for the image in Figure 3C.
Biography
Christine D. Keating received her B.S. in Biology and Chemistry from Saint Francis College (1991) and her Ph.D. in Chemistry from Penn State University (1997). She is currently an Associate Professor of Chemistry at Penn State University. Her research interests include cytomimetic materials chemistry and biointerfaces.
Funding Statement
National Institutes of Health, United States
References
- Follmann H.; Brownson C. Darwin’s warm little pond revisited: from molecules to the origin of life. Naturwissenschaften 2009, 96, 1265–1292. [DOI] [PubMed] [Google Scholar]
- Pohorille A.; Deamer D. Self-assembly and function of primitive cell membranes. Res. Microbiol. 2009, 160, 449–456. [DOI] [PubMed] [Google Scholar]
- Noireaux V.; Libchaber A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 17669–17674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara K.; Tamura M.; Shohda K.-i.; Toyota T.; Suzuki K.; Sugawara T. Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat. Chem 2011, 3, 775–781. [DOI] [PubMed] [Google Scholar]
- The Origins of Life. Cold Spring Harbor Perspectives in Biology; Deamer D., Szostak J. W., Eds.; Cold Spring Harbor Press: Cold Spring Harbor, NY, 2010; 318 pp. [Google Scholar]
- The Minimal Cell: The Biophysics of Cell Compartment and the Origin of Cell Functionality; Luisi P. L., Stano P., Eds.; Springer: Dordrecht, 2011; 298 pp. [Google Scholar]
- Chen I. A.; Salehi-Ashtiani K.; Szostak J. W. RNA catalysis in model protocell vesicles. J. Am. Chem. Soc. 2005, 127, 13213–13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.-X.; Rivas G.; Minton A. P. Macromolecular crowding and confinement: Biochemical, biophysical and potential physiological consequences. Annu. Rev. Biophys. 2008, 37, 375–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparin A. I.The Origin of Life (English Translation); Macmillan: New York; 1938. [Google Scholar]
- a van der Gucht J.; Spruijt E.; Lemmers M.; Stuart M. A. C. Polyelectrolyte complexes: Bulk phases and colloidal systems. J. Colloid Interface Sci. 2011, 361, 407–422. [DOI] [PubMed] [Google Scholar]; b Chollakup R.; Smitthipong W.; Eisenbach C. D.; Tirrell M. Phase behavior and coacervation of aqueous poly(acrylic acid)–poly(allylamine) solutions. Macromolecules 2010, 43, 2518–2528. [Google Scholar]
- Oparin A. I.; Serebrovskaya K. B.; Pantskhava S. N.; Vasileva N. V. Enzymatic synthesis of polyadenylic acid in coacervate drops. Biochemistry 1963, 28, 550–554. [PubMed] [Google Scholar]
- Miller S. L. A production of amino acids under possible primitive earth conditions. Science 1953, 117, 528–529. [DOI] [PubMed] [Google Scholar]
- a Fox S. W. A theory of macromolecular and cellular origins. Nature 1965, 205, 328–340. [DOI] [PubMed] [Google Scholar]; b Fox S. W. How did life begin?. Science 1960, 132, 200–208. [DOI] [PubMed] [Google Scholar]
- Bangham A. D.; Horne R. W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668. [DOI] [PubMed] [Google Scholar]
- Lazcano A. Historical development of origins research. Cold Spring Harb. Perspect. Biol. 2010, 2, 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Pereira de Souza T.; Stano P.; Luisi P. L. The minimal size of liposome-based model cells brings about a remarkably enhanced entrapment and protein synthesis. ChemBioChem 2009, 10, 1056–1063. [DOI] [PubMed] [Google Scholar]; b Luisi P. L.; Allegretti M.; Pereira de Souza T.; Steiniger F.; Fahr A.; Stano P. Spontaneous protein crowding in liposomes: A new vista for the origin of cellular metabolism. ChemBioChem 2010, 11, 1989–1992. [DOI] [PubMed] [Google Scholar]
- Stano P.; Pereira de Souza T.; Allegretti M.; Kuruma Y.; Luisi P. L.. New and unexpected insights on the formation of protocells from a synthetic biology approach: the case of entrapment of biomacromolecules and protein synthesis inside vesicles. In The Minimal Cell: The Biophysics of Cell Compartment and the Origin of Cell Functionality; Luisi P. L., Stano P., Eds.; Springer: Dordrecht, 2011; Chapter 11, pp 195–216. [Google Scholar]
- Mansy S. S.; Schrum J. P.; Krishnamurthy M.; Tobe S.; Treco D. A.; Szostak J. W. Template-directed synthesis of a genetic polymer in a model protocell. Nature 2008, 454, 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertsson P.-Å.Partition of Cell Particles and Macromolecules, 3rd ed.; Wiley-Interscience: New York, 1986. [Google Scholar]
- Partitioning in Aqueous Two-Phase Systems: Theory, Methods, Uses, and Applications to Biotechnology; Walter H., Brooks D. E., Fisher D., Eds.; Academic Press: Orlando, 1985. [Google Scholar]
- Koga S.; Williams D. S.; Perriman A. W.; Mann S. Peptide-nucleotide microdroplets as a step towards a membrane-free protocell model. Nat. Chem 2011, 3, 720–724. [DOI] [PubMed] [Google Scholar]
- de Kruif C. G.; Weinbreck F.; de Vries R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 2004, 9, 340–349. [Google Scholar]
- Walter H.; Brooks D. E. Phase separation, due to macromolecular crowding, is the basis for microcompartmentation. FEBS Lett. 1995, 361, 135–139. [DOI] [PubMed] [Google Scholar]
- Microcompartmentation and Phase Separation in Cytoplasm. In International Review of Cytology; Walter H., Brooks D. E., Srere P. A., Eds.; Academic Press: San Diego, 2000; Vol. 192. [Google Scholar]
- Brangwynne C. P.; Eckmann C. R.; Courson D. S.; Rybarska A.; Hoege C.; Gharakhani J.; Julicher F.; Hyman A. A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324, 1729–1732. [DOI] [PubMed] [Google Scholar]
- Ge X.; Conley A. J.; Brandle J. E.; Truant R.; Filipe C. D. In vivo formation of protein based aqueous microcompartments. J. Am. Chem. Soc. 2009, 131, 9094–9909. [DOI] [PubMed] [Google Scholar]
- Zaslavski B. Y.Aqueous Two-Phase Partitioning: Physical Chemistry and Bioanalytical Applications; Marcel Dekker: New York, 1995. [Google Scholar]
- Dominak L. M.; Gundermann E. L.; Keating C. D. Microcompartmentation in artificial cells: pH-induced conformational changes alter protein localization. Langmuir 2010, 26, 5697–5705. [DOI] [PubMed] [Google Scholar]
- Di Nucci H.; Nerli B.; Pico G. Comparison between the thermodynamic features of α-1-antitrypsin and human albumin partitioning in aqueous two-phase systems of polyethyleneglycol-dextran. Biophys. Chem. 2001, 89, 219–229. [DOI] [PubMed] [Google Scholar]
- Long M. S.; Keating C. D. Nanoparticle conjugation increases protein partitioning in aqueous two-phase systems. Anal. Chem. 2006, 78, 379–386. [DOI] [PubMed] [Google Scholar]
- Helfrich M. R.; El-Kouedi M.; Etherton M. R.; Keating C. D. Partitioning and assembly of metal particles in aqueous two-phase systems. Langmuir 2005, 21, 8478–8486. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Wu F.; Yuan W.; Jin T. Polymersomes of asymmetric bilayer membrane formed by phase-guided assembly. J. Controlled Release 2010, 147, 413–419. [DOI] [PubMed] [Google Scholar]
- Long M. S.; Jones C.; Helfrich M. R.; Mangeney-Slavin L. K.; Keating C. D. Dynamic microcompartmentation within synthetic cells. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 5920–5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich M. R.; Mangeney-Slavin L. K.; Long M. S.; Djoko K. Y.; Keating C. D. Aqueous phase separation in giant vesicles. J. Am. Chem. Soc. 2002, 124, 13374–13375. [DOI] [PubMed] [Google Scholar]
- Li Y.; Lipowsky R.; Dimova R. Membrane nanotubes induced by aqueous phase separation and stabilized by spontaneous curvature. Proc. Natl. Acad. Sci. U.S.A. 2011, 12, 4731–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giant Vesicles, Perspectives in Supramolecular Chemistry 6; Luisi P. L., Walde, Eds.; John Wiley and Sons: West Sussex, 2000. [Google Scholar]
- Li Y.; Lipowsky R.; Dimova R. Transition from complete to partial wetting within membrane compartments. J. Am. Chem. Soc. 2008, 130, 12252–12253. [DOI] [PubMed] [Google Scholar]
- Long M. S.; Cans A.-S.; Keating C. D. Budding and asymmetric protein microcompartmentation in giant vesicles containing two aqueous phases. J. Am. Chem. Soc. 2008, 130, 756–762. [DOI] [PubMed] [Google Scholar]
- Kusumaatmaja H.; Lipowsky R. Droplet-induced budding transitions of membranes. Soft Matter 2011, 7, 6914–6919. [Google Scholar]
- a Dominak L. M.; Keating C. D. Polymer encapsulation within giant lipid vesicles. Langmuir 2007, 23, 7148–7154. [DOI] [PubMed] [Google Scholar]; b Dominak L. M.; Keating C. D. Macromolecular crowding improves polymer encapsulation within giant lipid vesicles. Langmuir 2008, 24, 13565–13571. [DOI] [PubMed] [Google Scholar]
- Veatch S. L.; Keller S. L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 2003, 85, 3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cans A.-S.; Andes-Koback M.; Keating C. D. Positioning lipid membrane domains in giant vesicles by micro-organization of aqueous cytoplasm mimic. J. Am. Chem. Soc. 2008, 130, 7400–7406. [DOI] [PubMed] [Google Scholar]
- Baumgart T.; Hess S. T.; Webb W. W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature 2003, 425, 821–824. [DOI] [PubMed] [Google Scholar]
- Andes-Koback M.; Keating C. D. Complete budding and asymmetric division of primitive model cells to produce daughter vesicles with different interior and membrane compositions. J. Am. Chem. Soc. 2011, 133, 9545–9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keighron J. D.; Keating C. D.. Towards a minimal cytoplasm. In The Minimal Cell: The Biophysics of Cell Compartment and the Origin of Cell Functionality; Luisi P. L., Stano P., Eds.; Springer: Dordrecht, 2011; Chapter 1, pp 3–30. [Google Scholar]
- Harold F. M. Molecules into cells: Specifying spatial architecture. Microbiol. Mol. Biol. Rev. 2005, 69, 544–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. I.; Clark J. M.. Lens cytoplasmic phase separation. In Microcompartmentation and Phase Separation in Cytoplasm; Walter H., Brooks D. E., Srere P. A., Eds.; International Review of Cytology; Academic Press: San Diego, 2000; Vol. 192. [DOI] [PubMed] [Google Scholar]