Abstract
Owing to the importance of major histocompatibility complex class Ia-restricted CD8+ T cells for host survival following viral, bacterial, fungal, or parasitic infection, it has become largely accepted that these cells should be considered in the design of a new generation of vaccines. For the past 20 years, solid evidence has been provided that the heterologous prime-boost regimen achieves the best results in terms of induction of long-lived protective CD8+ T cells against a variety of experimental infections. Although this regimen has often been used experimentally, as is the case for many vaccines, the mechanism behind the efficacy of this vaccination regimen is still largely unknown. The main purpose of this review is to examine the characteristics of the protective CD8+ T cells generated by this vaccination regimen. Part of its efficacy certainly relies on the generation and maintenance of large numbers of specific lymphocytes. Other specific characteristics may also be important, and studies on this direction have only recently been initiated. So far, the characterization of these protective, long-lived T cell populations suggests that there is a high frequency of polyfunctional T cells; these cells cover a large breadth and display a T effector memory (TEM) phenotype. These TEM cells are capable of proliferating after an infectious challenge and are highly refractory to apoptosis due to a control of the expression of pro-apoptotic receptors such as CD95. Also, they do not undergo significant long-term immunological erosion. Understanding the mechanisms that control the generation and maintenance of the protective activity of these long-lived TEM cells will certainly provide important insights into the physiology of CD8+ T cells and pave the way for the design of new or improved vaccines.
Keywords: memory, vaccines, CD8, adenovirus
GENETIC VACCINATION USING THE HETEROLOGOUS PRIME-BOOST REGIMEN
Genetic vaccination using naked DNA or recombinant viruses is being pursued as an alternative to traditional vaccines. This strategy could be particularly important in the case of intracellular pathogens and neoplastic cells, where the effectiveness relies heavily on the capacity of the vaccine to elicit specific immune responses mediated by cytotoxic CD8+ T cells (reviewed in Rice et al., 2008; Lasaro and Ertl, 2009 Bassett et al., 2011; Gómez et al., 2011; Mudd and Watkins, 2011; Saade and Petrovsky, 2012).
Whereas the use of single-vector delivery for priming and boosting is usually the initial option, one of the most prolific areas of genetic vaccine development is the strategy known as the heterologous prime-boost regimen. This strategy uses two different vectors, each carrying a gene that encodes the same antigenic protein for priming and boosting immunizations. The utility and importance of this strategy was first proposed in the early 1990s using a combination of recombinant viruses (influenza and vaccinia) to induce protective immunity against malaria (Li et al., 1993; Rodrigues et al., 1994). Subsequently, this approach was extended and simplified by incorporating naked DNA for priming followed by a booster injection of a recombinant poxviral vector (i.e., modified vaccinia Ankara, MVA); this was also used to generate sterile protective immunity against rodent malaria (Schneider et al., 1998; Sedegah et al., 1998). Collectively, these studies demonstrated that the heterologous prime-boost regimen generated significantly higher immune responses and proved more effective than the use of any of these genetic vectors individually. The initial use of rodent malaria parasites as a model system may have delayed the development of the field, because malaria is not a conventional model system for developing antiviral or antibacterial vaccines. Nevertheless, this fortuitous fact established the important concept that the difficulty in generating immunity to malaria, and perhaps to other intracellular parasites, in many ways recapitulates the struggles encountered to elicit protective immunity against viruses and bacteria that case chronic infections such as HIV and tuberculosis (TB).
In subsequent years, the heterologous prime-boost vaccination regimen was adopted worldwide as a powerful means to elicit strong type 1 effector CD8+ T cell-mediated immune responses (Tc1) against viral, parasitic, and neoplastic antigens in rodents and non-human primates (NHP; Irvine et al., 1997; Amara et al., 2001; McShane et al., 2001; Zavala et al., 2001; Moore and Hill, 2004; Ellenberger et al., 2006; Gilbert et al., 2006; Aidoo et al., 2007; Nigam et al., 2007; Martinon et al., 2008; Sadagopal et al., 2008). Based on pre-clinical studies, a number of human clinical trials have also been initiated. However, to our knowledge, heterologous prime-boost regimens using plasmid DNA and recombinant poxviruses have not yet provided meaningful protective immunity in humans (McConkey et al., 2003; Moorthy et al., 2004; Keating et al., 2005; Vuola et al., 2005; Cebere et al., 2006; Dunachie et al., 2006; Goonetilleke et al., 2006). The precise reason for such failures is not yet clear. It may be due to the target antigens chosen or to the possibility that the combination of vectors may elicit a type of effector CD8+ T cells in humans that are not functionally and/or phenotypically related to mice, as discussed below.
A number of possible vector combinations that significantly improved cell-mediated immunity, particularly the generation of specific CD8+ T cells, have been described in parallel. Among them, heterologous prime-boost vaccination using naked plasmid DNA for priming followed by a booster injection of recombinant replication-deficient human adenovirus 5 (AdHu5) has recently received significant attention. This strategy has proved successful in some relevant experimental models such as simian immunodeficiency virus (SIV), malaria, Marburg, and Ebola virus infection, and Chagas disease (American trypanosomiasis), providing a considerable degree of protective immunity (Gilbert et al., 2002; Casimiro et al., 2003, 2005; Santra et al., 2005; Acierno et al., 2006; Letvin et al., 2006; Mattapallil et al., 2006; Sun et al., 2006; Wilson et al., 2006; de Alencar et al., 2009; Geisbert et al., 2010; Hensley et al., 2010; Martins et al., 2010; Dominguez et al., 2011; Rigato et al., 2011). These relative successes obtained in pre-clinical experimental models fueled human phase I trials (Freel et al., 2010; Jaoko et al., 2010; Koup et al., 2010; Schooley et al., 2010; Churchyard et al., 2011; De Rosa et al., 2011).
Very recently, improved vector combinations have yielded results (measured in terms of protective immunity) that are slightly better than the results obtained by using plasmid DNA followed by replication-deficient AdHu5 viruses. These new strategies include (i) prime with plasmid DNA in the presence of cytokine genes such as IL-12 or GM-CSF (Lai et al., 2011; Winstone et al., 2011), (ii) genes encoding multimeric proteins (Lakhashe et al., 2011), (iii) boost of adenovirus-immunized animals with an optimized plasmid DNA (Hutnick et al., 2012), (iv) prime with rhesus cytomegalovirus (Hansen et al., 2011), and (v) prime with a different heterologous strain of adenovirus (Barouch et al., 2012).
The precise reason for the superior performance of the heterologous prime-boost vaccination compared to the sequential use of the same vector is still a matter of controversy. Some evidence indicates the possibility that the intense immunity to epitopes present on the priming vector prevents the boosting effect. For example, a recent study in humans shows that a second dose of a recombinant AdHu5 does not provide significant boosting. In parallel, recombinant AdHu5 boosting of DNA-primed individuals resulted in significantly higher immune responses (De Rosa et al., 2011). The anti-vector immunity can be either antibody mediated or independent (Cockburn et al., 2008; Schirmbeck et al., 2008; Frahm et al., 2012). Haut et al. (2011) found that in B cell-deficient mice transgene-specific CD8+ T cell responses were significantly higher in systemic compartments. In contrast, recent studies in humans showed that neutralizing antibodies titers to AdHu5 did not correlate with the magnitude of specific CD8+ T cell of priming after immunization with a recombinant AdHu5. In these experiments, the frequency of specific CD4+ T cells negatively correlated with the intensity of specific CD8+ T cells priming (Frahm et al., 2012).
In spite of the clear evidences that pre-existing immunity may interfere with the use of viral vectors, still, the heterologous prime-boost regimen of immunization is described as a possible solution to this problem. This can be achieved by strong priming with cytokine genes (for example, see Barouch et al., 2003).
Independent of the reasons why the heterologous prime-boost vaccination regimen is superior to the sequential use of the same vector, the main purpose of this review is to examine the characteristics of the protective CD8+ T cells generated by this vaccination regimen.
CHARACTERISTICS OF PROTECTIVE CD8+ T CELLS ELICITED AFTER HETEROLOGOUS PRIME-BOOST VACCINATION
HIGH FREQUENCIES OF SPECIFIC CD8+ T CELLS
One hallmark of the heterologous prime-boost regimen is the elicitation of a higher frequency of epitope-specific CD8+ T cells across multiple models. This high number of effector T cells was initially estimated by the presence of epitope-specific IFN-γ-producing cells using the ex vivo ELISPOT assay (Murata et al., 1996; Schneider et al., 1998; Sedegah et al., 1998; Bru n, 2001). Subsequently, the hypothesis was further validated by intracellular staining for IFN-γ (Pinto et al., 2003) and tetramer staining of epitope-specific CD8+ T cells (Tao et al., 2005). More recently, intracellular staining for TNF, IL-2, MIP1-β, T cell surface mobilization of CD107a, and in vivo cytotoxicity provided extended evidence (for examples, see Masopust et al., 2006; Mattapallil et al., 2006; Cox et al., 2008; de Alencar et al., 2009; Freel et al., 2010; Reyes-Sandoval et al., 2010; Rigato et al., 2011).
Because most studies are performed with T cells collected from the spleen or peripheral blood lymphocytes (PBL) of mice or NHP, respectively, it is not clear whether these results reflect an overall increase in every tissue. The presence of a large number of epitope-specific T cells in several tissues has been documented in the case of mouse lung, liver, intraepithelial lymphocytes, and PBL (Masopust et al., 2006; Reyes-Sandoval et al., 2011); however, because parallel comparison was not performed with animals that were immunized with a single vector, it is not clear whether these levels were particularly higher than the other vaccination protocols. Conversely, the frequency of epitope-specific CD8+ T cells seems to decrease in mouse lymph nodes (Masopust et al., 2006). This may be due to the lack of CD62L expression on the surface of these activated T cells, as discussed below. In addition, the pattern of circulation and recirculation of these lymphocytes has been poorly explored. A single study, however, demonstrated that, after a recombinant plasmid DNA prime-AdHu5 boost, CD8+ T cells need to recirculate in order to exert protective immunity against an infectious challenge with the protozoan parasite Trypanosoma cruzi. In these vaccinated mice, treatment with the drug FTY720 significantly reduced the efficacy of the protective immunity by interfering specifically with signaling through sphingosine-1-phosphate receptor-1, thereby inhibiting the egress of T cells from the lymph nodes (Dominguez et al., 2012). This observation is important because immunity to certain pathogens is not dependent on the recirculation of T lymphocytes (Pinschewer et al., 2000; Kursar et al., 2008; Jiang et al., 2012).
The importance of recirculation is likely dependent on factors such as (i) the host, (ii) the vectors used for prime and/or boost, and (iii) the route of administration of each vector (for examples, see Mattapallil et al., 2006; Kaufman et al., 2010). These are important characteristics that will certainly influence the protective immunity, as pathogens can cause either tissue-specific or systemic infections.
The T cell protective immunity elicited by heterologous prime-boost regimen is not only significantly higher than the immunity elicited by the traditional vaccine regimen but is also longer-lived. Several experimental models have shown that the immunity lasts for significant periods of time (Amara et al., 2001; Bru n, 2001; de Alencar et al., 2009; Reyes-Sandoval et al., 2010; Rigato et al., 2011).
POLYFUNCTIONALITY OF SPECIFIC CD8+ T CELLS
Based on the assays described above, it became clear that the specific CD8+ T cells elicited by the heterologous prime-boost regimen could exert different immunological functions, as measured by ex vivo or in vivo assays. Confirmation of the polyfunctionality of these cells was made possible through FACS analyses coupling intracellular cytokine staining and cell surface mobilization of the degranulation marker CD107a.
Accordingly, a number of studies confirmed that distinct heterologous prime-boost regimens elicited polyfunctional CD8+ T cells as defined by the cells’ capability to exert two or more functions at the same time. The most frequent example of this across different models is specific CD8+ T cells producing IFN-γ and TNF simultaneously. High frequencies of polyfunctional specific CD8+ T cells were described in (i) mice (Masopust et al., 2006; Duke et al., 2007; de Alencar et al., 2009; Elvang et al., 2009; Reyes-Sandoval et al., 2010; Rigato et al., 2011; Rodríguez et al., 2012; Vijayan et al., 2012), (ii) NHP (Mattapallil et al., 2006; Sun et al., 2006; Cox et al., 2008; Liu et al., 2008; Magalhaes et al., 2008; Cayabyab et al., 2009; Wilks et al., 2010; Hutnick et al., 2012), and (iii) humans (Beveridge et al., 2007; Harari et al., 2008; Winstone et al., 2009; Freel et al., 2010; Jaoko et al., 2010; Koup et al., 2010; Schooley et al., 2010; Churchyard et al., 2011; De Rosa et al., 2011).
It is noteworthy that although these T cells have a high frequency of polyfunctionality, their ability to mediate multiple immunological functions has never been clearly linked to their protective capacity. It is possible that this characteristic aids in but is not critical for their effector functions, depending on the mechanism necessary for pathogen elimination. In a single study performed using genetically deficient mice, in the absence of either IFN-γ or perforin, heterologous prime-boost vaccination failed to mediate protective immunity against infection with the intracellular parasite T. cruzi. In the case of perforin-deficient mice, the lack of protective immunity was associated mainly with a significant decrease in the induction of polyfunctional T cells (de Alencar et al., 2009). A second study correlated the presence of higher frequencies of polyfunctional T cells to protective immunity against liver stages of malaria parasite (Reyes-Sandoval et al., 2010). Although these studies may suggest a role for polyfunctional T cells during protective immunity, it is still too early to conclude that they are critical for the protective immunity exerted by CD8+ T cells elicited following the heterologous prime-boost vaccination regimen.
BREADTH OF SPECIFIC CD8+ T CELLS
T cell immune responses are often restricted to a few immunodominant epitopes, a phenomenon termed immunodominance (Akram and Inman, 2012). The precise reason for such a restriction is not clear; however, it may have evolved to maximize the immune response, while at the same time reducing the risk of autoimmunity. For the purpose of vaccine development, having only a narrow number of recognized epitopes may be dangerous, as the pathogens will rapidly select for escape mutants to avoid effector immune responses (reviewed in Streeck and Nixon, 2010; Chopera et al., 2011).
Although it has been possible to increase the frequency of T cells specific for immunodominant epitopes, it is still a challenge to broaden the vaccine-induced CD8+ T cell response to a number of subdominant T cell epitopes. There is evidence that heterologous rAdHu5 boosting improved not only the magnitude but also the breadth of specific CD8+ cells (Liu et al., 2008). However, the impact of this response on protective immunity is not clear. Two recent studies indicated that immunity to subdominant epitopes might participate in vaccine-induced protective immunity following a DNA prime-AdHu5 boost vaccination regimen. The first study provided a correlation between the breadth of the immune response and the protective immunity observed in individual rhesus monkeys vaccinated with SIV genes (Martins et al., 2010). A second study formally demonstrated that heterologous prime-boost vaccination with plasmid DNA followed by recombinant AdHu5 elicited strong immune response to two subdominant epitopes that were not recognized during infection (Dominguez et al., 2011). Based on these observations, mutant genes were generated in which the dominant epitope was removed. Heterologous prime-boost vaccination with these mutant genes-induced CD8+ T cell immune responses only to the subdominant epitopes. Most importantly, strong CD8+ T cell-mediated immunity was still observed (Dominguez et al., 2011). These results unequivocally demonstrate the importance of immunity to the subdominant epitopes and the ability of the heterologous prime-boost to elicit them. Nevertheless, other groups still have difficulty improving the immune response to subdominant epitopes following heterologous prime-boost vaccination of NHP (Vojnov et al., 2011). Therefore, new strategies to improve the breadth of the immune response might be developed in order to potentiate vaccine formulation.
TEM PHENOTYPE OF SPECIFIC LONG-LIVED CD8+ T CELLS
The current immunological paradigm divides antigen-experienced CD8+ T cells into three main types: (i) T effector (TE), (ii) T effector memory (TEM), and (iii) T central memory (TCM). These populations of T cells can be distinguished by the presence of activation markers, as well as by differences observed in their localization and recirculation patterns and their ability to proliferate and present certain effector functions/molecules (Angelosanto and Wherry, 2010; Cui and Kaech, 2010; Ahmed and Akondy, 2011; Sheridan and Lefrançois, 2011). More recently, other subpopulations of CD8+ T cells have also been described (Wakim et al., 2010; Sheridan and Lefrançois, 2011; Jiang et al., 2012). The concept of TE/TEM/TCM was primarily established by experimental infections with self-curing pathogens. In these cases, TE are short-lived effector cells (CD44High CD11aHigh CD62LLow CD127Low KLRG1High); TCM are long-lived memory cells (CD44High CD11aHigh CD62LHigh CD127High KLRG1High); and TEM are transitional cells that exist for a short period of time and have distinct surface marker expression (CD44High CD11aHigh CD62LLow CD127High KLRG1High). However, even by using models of self-curing infections, the existence of other memory T cell subpopulations that might be more rele-vant for long-term T cell immunity have been proposed (Hikono et al., 2007).
In addition, very recently, considerable interest has been placed on tissue-resident memory T (TRM) cells. These cells are potent long-lived effector cells present in a variety of peripheral tissues including the skin and sensory ganglia, gut, brain, lung, etc. (Wakim et al., 2008; Gebhardt et al., 2009; Masopust et al., 2010; Purwar et al., 2011). They mediate protective immunity against brain infection with vesicular stomatitis virus or skin infection with vaccinia or herpes simplex virus (Wakim et al., 2010; Jiang et al., 2012; Mackay et al., 2012). Their phenotype is CD44High, CD62LLow, CD103High, CD69High, CD127Low, and PD-1Low. The relationship of TRM cells with TEM or TCM is yet to be determin-ed. Some authors propose that these cells constitute an independ-ent lineage primed within the tissue (Wakim et al., 2010, 2012).
Very recently, the development of a methodology to perform transcriptional profiling at the single cell level has detected further differentiation profiles among TEM and TCM. The analysis of specific splenic CD8+ T cells from mice immunized with distinct vaccination protocols yields significant differences among these subpopulations (Flatz et al., 2011). For example, while 74% of the specific TEM elicited by the heterologous DNA prime-recombinant AdHu5 boost vaccination were Klrk1– Klrg1– Ccr5–, only 20% of the cells generated by HuAd5-prime-recombinant LCMV boost had a similar phenotype (Flatz et al., 2011). This type of analysis further highlights the heterogeneity still being uncovered within these memory cells.
It has been observed on multiple occasions that after heterologous prime-boost vaccination, the boosting immunization drives not only an expansion of the T cells but also a different phenotype in the long-lived memory T cells. Different doses of the vaccine also caused a significant increase in the frequency of specific CD8+ T cells with a TEM phenotype (CD44High CD11aHigh CD62LLow CD127High KLRG1High; Masopust et al., 2006). TEM cells were described initially as highly protective against certain viral and bacterial infections (Bachmann et al., 2005a,b; Huster et al., 2006). Likewise, protective immunity afforded by the different heterologous prime-boost vaccination protocols has been associated with the presence of this type of T cell (Hansen et al., 2011; Reyes-Sandoval et al., 2011; Rigato et al., 2011; Xiao et al., 2011; Barouch et al., 2012; Yamamoto et al., 2012).
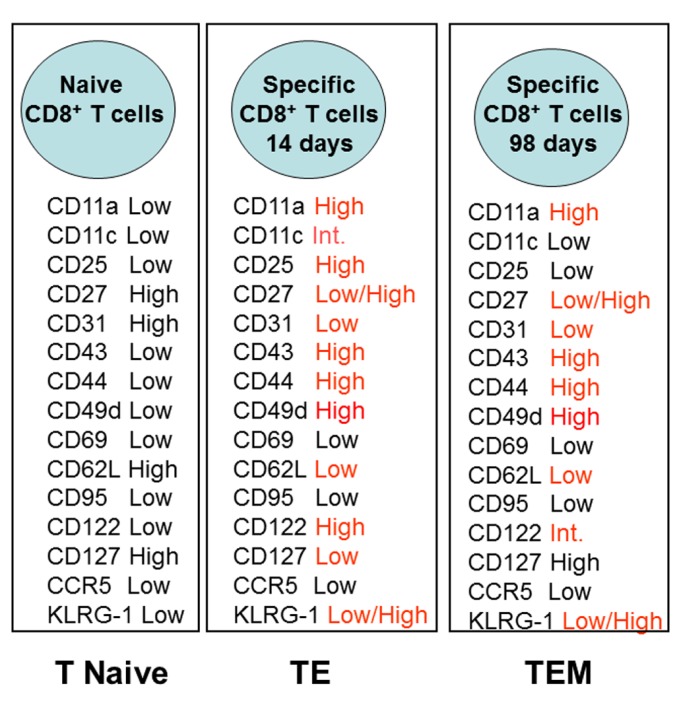
Based on the relatively poor knowledge of the surface activation markers present on long-lived specific TEM CD8+ T cells, we performed a detailed analysis of the different T cells markers following intramuscular DNA prime-adenovirus boost immunization. We identified transgenic epitope-specific T cells in the spleen of immunized mice 14 or 98 days after the boost vaccination. Figure 1 summarizes the surface marker phenotype of these epitope-specific T cells compared to the phenotype of the naïve cells.
FIGURE 1.
Phenotype of specific CD8+ T cells elicited by heterologous prime-boost vaccination using recombinant plasmid DNA and AdHu5. Prime-boost regimen was performed as detailed described by Rigato et al. (2011). Mice were primed i.m. with plasmid DNA (100 µg) and boosted 21 days later with AdHu5 (2 × 108) both expressing the gene encoding the amastigote surface protein-2 of T. cruzi. Expression of distinct adhesion/activation receptors on the surface of splenic CD8+ specific T cells is shown at day 14 or day 98 after the boost immunization.
PROLIFERATIVE CAPACITY OF SPECIFIC CD8+ T CELLS
The proliferative capacity of specific T cells elicited by heterologous prime-boost vaccination has not been thoroughly studied to date. In general, after resolution of experimental self-curing infections, the frequency of total CD8+ T cells declines to less than 10% of the maximal number of specific T cells observed during the peak of the immune response; this is known as the contraction phase. The decrease in the number of specific T cells occurs mainly among the short-lived effector cells (Angelosanto and Wherry, 2010; Cui and Kaech, 2010; Ahmed and Akondy, 2011; Sheridan and Lefrançois, 2011). However, this does not seem to be the case for the TE cells elicited by the heterologous prime-boost vaccination regimen (Jameson and Masopust, 2009). In this case, the cells expand and are maintained at a high frequency in the spleen for a long period of time (Masopust et al., 2006; Vezys et al., 2009; Rigato et al., 2011). This is a somewhat unexpected finding and might be of great relevance for the success of this vaccination protocol. Because this expansion and maintenance contrasts with the retraction observed after self-curing infections, it opens a number of questions that should be studied in depth: (i) How can cells expand out of control to reach frequencies higher than the primary immune response? (ii) How do T cells avoid the apoptotic process observed for the short-lived effector cells during the contraction phase? (iii) How are T cells maintained for these long periods? These questions are keys to understanding the physiology of CD8+ T cells and developing better T cell vaccines.
After an infectious challenge, specific T cells proliferate. However, it is still unknown whether this proliferative response occurs among the antigen-primed TE(M) cells, rare TCM or naïve T cells. To address this question, it is now possible to use the gzmBCreERT2/ROSA26EYFP transgenic mouse line (Bannard et al., 2009). Specific TE CD8+ T lymphocytes can be indelibly labeled with enhanced yellow fluorescent protein (EYFP). Following an infectious challenge, it will be possible to follow the expansion of EYFP-labeled TE cells. This result will confirm or not whether the cells that expand after the infectious challenge are indeed mainly antigen-experienced TE. Also, by the adoptive transference of green-fluorescent naïve specific T cells prior to the infectious challenge it will be possible to determine whether these cells proliferate or not together with the specific TE cells. Whether this is true for all different protocols of heterologous prime-boost vaccination strategies is still unknown and might be relevant for the development of vaccines to be used in individuals primed with conventional vaccines such as Bacillus Calmette–Guérin (BCG; Hoft et al., 2012).
REFRACTORINESS TO APOPTOSIS OF SPECIFIC CD8+ T CELLS
As mentioned above, after an intense immune response and pathogen elimination, the number of specific CD8+ T cell is drastically reduced during a period called the contraction phase. In that period, TE or short-lived effector cells are eliminated by mechanisms that involve apoptosis mediated by BCL-2-interacting mediator of cell death (Bim) and CD95 (also known as FAS; Green, 2008; Hughes et al., 2008; Weant et al., 2008; Bouillet and O’Reilly, 2009). We observed that following prime-boost immunization with recombinant plasmid DNA and AdHu5, TEM cells do not upregulate surface CD95 expression and are refractory to anti-CD95-induced apoptosis in vitro (Rigato et al., 2011). Because CD95 is an important initiator of the intrinsic pathway of apoptosis in T lymphocytes, low levels of expression may protect T cells from apoptotic death.
Decreased levels of expression or resistance to activation of other receptors that control T cell death, such as TNF receptor and TRAIL, might also play a role during the survival of specific TEM cells. In contrast, increased expression of other receptors may act to protect these specific T cells. Recently, expression of PDL-1 (B7-H1) on antigen-activated CD8+ T cells made these cells more resistant to Ca2+-dependent and Fas ligand-dependent killing by cytotoxic T lymphocytes (Pulko et al., 2011). However, more details are necessary to understand the expression levels of many of these molecules controlling T cell survival.
It is plausible that Bim is poorly activated, or not activated at all, on these cells. Because Bim is activated in response to the lack of certain external stimuli, these activation signals might be maintained for long-term. One such mechanism is antigen presentation by dendritic cells. Using a replication-deficient adenoviral vector, Tatsis et al. (2007) demonstrated that the transgene product remains available for antigen presentation for as long as 16 weeks after a single immunizing dose. The importance of continuous transgene expression in the maintenance of the specific CD8+ T cells after AdHu5 vaccination was also demonstrated by a causal relationship between both using an inducible system to turn off the transgene expression (Finn et al., 2009). Bassett et al. (2011) showed that both hematopoietic and non-hematopoietic antigen-presenting cells are necessary for the maintenance of CD8+ T cells following immunization with recombinant adenovirus. However, it is not clear whether antigen persistence is also observed with other vectors.
We tested whether the IFN-γ or IL12/IL23 signaling pathways might be important for maintaining these long-lived CD8+ TEM cells. The absence of either pathway individually made little difference in the generation of specific CD8+ T cells. The absence of the IL12/IL23 pathway, but not the IFN-γ pathway, was important for the long-term survival of these cells (Rigato et al., 2011). Further details regarding the relevance of these signaling pathways in maintaining a high frequency of CD8+ T cells are unknown. In addition, little is known about the impact of the lack of the IL12/IL23 pathway in the maintenance of specific CD8+ T cells after other heterologous prime-boost vaccination regimens. We consider this area of critical importance in understanding how T cells can be maintained at high frequencies for long periods of time. A possible explanation that remains to be tested is whether the lack of contraction could be due to the fact that many specific long-lived CD8+ T cells express low levels of the chemokine receptors CXCR3 and CCR5 (Flatz et al., 2011). This possibility is supported by recent observations that genetically deficient CD8+ T cells that do not express both these receptors were refractory to contraction and accumulated in higher numbers in the spleen (Kohlmeier et al., 2011).
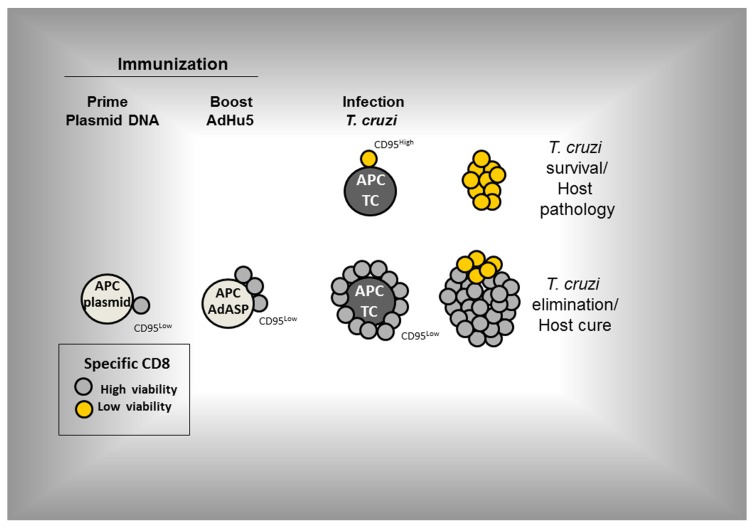
Recently, we described a new and potentially very important aspect of CD8+ TE cells. After recombinant AdHu5 vaccination, CD8+ TE cells do not undergo apoptosis, as well as prevent the development of a pro-apoptotic phenotype that occurs during experimental infection with the protozoan parasite T. cruzi. This phenomenon was observed when the administration of the recombinant AdHu5 vaccine was provided before (as part of a prime-boost regimen or alone) or at the time of the infectious challenge. AdHu5 treatment modulated specifically the CD8+ TE cells to express lower levels of CD95 (FAS) and become resistant to CD95-induced apoptosis. The determination of the distinct adhesion/activation receptors on the surface of the CD8+-specific T cells elicited by either infection or recombinant AdHu5 immunization showed very limited differences that were almost exclusively confined to the apoptotic receptor CD95 (Figure 2).
FIGURE 2.
Expression of distinct adhesion/activation receptors on the surface of specific CD8+ T cells elicited by either T. cruzi infection or recombinant AdHu5 immunization (Vasconcelos et al., 2012). Mice were infected s.c. with T. cruzi (150 blood stream trypomastigotes) or immunized i.m. with AdHu5 (2 × 108 pfu) expressing the gene encoding the amastigote surface protein-2 of T. cruzi 19 days earlier.
Despite the above results, we have not ruled out that other pro-apoptotic signaling pathways might also be altered. In these immunized and challenged mice, the CD8+ T cell population expanded largely and protected mice against an otherwise lethal infection (Vasconcelos et al., 2012). An important mechanism that controls the expression of CD95 on the surface of specific CD8+ T cells has just been described during development of immune responses to viral infections. TCR activation led to an increase of the RIG-I-like receptor LGP2 expression which down-regulated CD95 expression. In KO mice, the absence of this molecule significantly increased the apoptosis reducing the effectiveness of the immune response and resistance to the viral infection (Suthar et al., 2012). We consider this a promising area of study because it suggests that perhaps the control of T cell survival might be the key element for the development of new or improved vaccines. The proposed pathway of specific CD8+ T cell activation following prime-boost vaccination and infection is shown in Figure 3 (based on Vasconcelos et al., 2012).
FIGURE 3.
The proposed pathway of activation of specific CD8+ T cells following prime-boost vaccination and infection (based on Vasconcelos et al., 2012). Prime-boost regimen was performed as detailed described by Rigato et al. (2011). Mice were primed i.m. with plasmid DNA (100 µg) and boosted 21 days later with AdHu5 (2 × 108) both expressing the gene encoding the amastigote surface protein-2 of T. cruzi. Mice were infected s.c. with T. cruzi (150 blood stream trypomastigotes).
REDUCED IMMUNOLOGICAL EROSION OF SPECIFIC CD8+ T CELLS
Memory CD8+ T cells are not a stable pool; subsequent infections may cause attrition and erosion of the memory T cell pool (Selin et al., 1996, 1999; Dudani et al., 2008; Huster et al., 2009; Schmidt and Harty, 2011). However, different heterologous prime-boost regimens generated a pool of CD8+ T cells that was not eroded by subsequent viral infections (Vezys et al., 2009; Rigato et al., 2011). After a vaccination regimen consisting of recombinant plasmid DNA prime-AdHu5 boost, we observed that viral infections had limited impact on the number or quality of the TEM cells as measured by different functional immunological assays. Most importantly, protective immunity mediated by these CD8+ TEM cells was not altered in these mice (Rigato et al., 2011).
In contrast, using a different prime-boost vaccination protocol consisting of dendritic cells coated with circumsporozoite protein peptide and a booster immunization with recombinant actA–inlB-deficient Listeria monocytogenes expressing the same epitope elicited an immune response that could be eroded by multiple subsequent infections (Schmidt and Harty, 2011). The discrepant results highlight the importance of determining the characteristic of each TEM elicited by the distinct regimen of vaccination, as suggested earlier (Flatz et al., 2011).
The resistance to immunological erosion is one more interesting characteristic of these long-lived TEM cells that has been poorly explored and may have an important impact in the development of efficient vaccines. As mentioned above, this high-resistance immunological erosion can be linked to the differential expression of surface molecules (death receptors such as CD95/FAS) or apoptosis mediators (such as Bim).
UNKNOWN FATE OF THE SPECIFIC CD8+ TEM CELLS
Although some studies described that theses TEM can be long-lived in some mouse models, it is not clear what will be the fate of these cells on extended periods of time. Will they become TCM by upregulating CD62L? Will they simple die? Can they be boosted? Perhaps these questions can be addressed by using the gzmBCreERT2/ROSA26EYFP transgenic mouse line (Bannard et al., 2009). Labeling specific TE CD8+ T lymphocytes will allow us to follow theses cells for longer periods of time.
Also, their long-term fate in NHP or humans is even more important for the purpose of development of practical vaccines. Due to the obvious constrains, few studies so far have addressed this issue.
CONCLUDING REMARKS AND PERSPECTIVES
Genetic vaccination using heterologous prime-boost regimen protocols has the potential to serve as the basis for the development of new vaccines against many pathogens. In spite of the progress over the past 20 years, much work is required to improve the relevance of vaccine research, considering that human immune response is still at least one order of magnitude lower than that observed in mouse or even NHP models.
Several areas can be pursued for this matter. So far, protective immunity is clearly associated with the presence of a high number of long-lived specific polyfunctional TEM cells. Nevertheless, several points should be considered. First, TEM may vary from one protocol to the other. It is also important to improve the number of specific TCM cells, for example, by using pharmacological modulators (Li et al., 2012; Takai et al., 2012). However, it will be a challenge to increase the number of TCM cells without reducing the number of TEM cells. Fine tuning the TEM subpopulations and increasing the number of TCM cells could further improve and prolong protective immunity beyond the levels currently achieved. Furthermore, new strategies to broaden the epitopes recognized by protective T cells would also improve both the quantity and the quality of the immune response. Finally, the circulation of these cells should be further studied for the purpose of personalizing the vaccination regimen for different types of infections, considering that the site of entry of each pathogen will vary, as will the localization of the T cells at the time of the infection.
In summary, the study of the control of memory T cell generation, maintenance, quality, and recirculation after distinct heterologous prime-boost vaccination regimens will provide important clues regarding the physiology of lymphocytes and the immune system, with potential applications in public health.
Conflict of Interest Statement
Mauricio M. Rodrigues and Oscar Bruna Romero are named inventors on patent applications covering Trypanosoma cruzi vectored vaccines and immunization regimens. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (2009/06820-4), Instituto Nacional de Ciência e Tecnologia em Vacinas (INCTV-CNPq), The Millennium Institute for Vaccine Development and Technology (CNPq - 420067/2005-1), The Millennium Institute for Gene Therapy (Brazil), Malaria-PRONEX and PNPD. JoséR. Vasconcelos, Adriano F. Araújo, Mariana R. Dominguez, and Cibele A. Tararam are recipients of fellowship from FAPESP. JoséR. Vasconcelos, Oscar Bruna-Romero, and Mauricio M. Rodrigues are recipients of fellowships from CNPq.
REFERENCES
- Acierno P. M., Schmitz J. E., Gorgone D. A., Sun Y., Santra S., Seaman M. S., et al. (2006). Preservation of functional virus-specific memory CD8+ T lymphocytes in vaccinated, simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 176 5338–5345 [DOI] [PubMed] [Google Scholar]
- Ahmed R., Akondy R. S. (2011). Insights into human CD8+ cell memory using the yellow fever and smallpox vaccines. Immunol. Cell Biol. 89 340–345 [DOI] [PubMed] [Google Scholar]
- Aidoo M., Otten R. A., Rodriguez V., Sariol C. A., Martinez M., Kraiselburd E., et al. (2007). Absence of SHIV infection in gut and lymph node tissues in rhesus monkeys after repeated rectal challenges following HIV-1 DNA/MVA immunizations. Vaccine 25 6474–6481 [DOI] [PubMed] [Google Scholar]
- Akram A., Inman R. D. (2012). Immunodominance: a pivotal principle in host response to viral infections. Clin. Immunol. 143 99–115 [DOI] [PubMed] [Google Scholar]
- Amara R. R., Villinger F., Altman J. D., Lydy S. L., O’Neil S. P., Staprans S. L., et al. (2001). Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292 69–74 [DOI] [PubMed] [Google Scholar]
- Angelosanto J. M., Wherry E. J. (2010). Transcription factor regulation of CD8+ T-cell memory and exhaustion. Immunol. Rev. 236 167–175 [DOI] [PubMed] [Google Scholar]
- Bachmann M. F., Wolint P., Schwarz K., Jäger P., Oxenius A. (2005a). Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J. Immunol. 175 4686–4696 [DOI] [PubMed] [Google Scholar]
- Bachmann M. F., Wolint P., Schwarz K., Oxenius A. (2005b). Recall proliferation potential of memory CD8+ T cells and antiviral protection. J. Immunol. 175 4677–4685 [DOI] [PubMed] [Google Scholar]
- Bannard O., Kraman M., Fearon D. T. (2009). Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science 323 505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch D. H. (2010). Novel adenovirus vector-based vaccines for HIV-1. Curr. Opin. HIV AIDS 5 386–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch D. H., Liu J., Li H., Maxfield L. F., Abbink P., Lynch D. M., et al. (2012). Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 7383 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch D. H., McKay P. F., Sumida S. M., Santra S., Jackson S. S., Gorgone D. A., et al. (2003). Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J. Virol. 77 8729–8735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett J. D., Yang T. C., Bernard D., Millar J. B., Swift S. L., McGray A. J., et al. (2011). CD8+ T-cell expansion and maintenance after recombinant adenovirus immunization rely upon cooperation between hematopoietic and nonhematopoietic antigen-presenting cells. Blood 117 1146–1155 [DOI] [PubMed] [Google Scholar]
- Beveridge N. E., Price D. A., Casazza J. P., Pathan A. A., Sander C. R., Asher T. E., et al. (2007). Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur. J. Immunol. 37 3089–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, O’Reilly L. A. (2009). CD95, BIM and T cell homeostasis. Nat. Rev. Immunol. 9 514–519 [DOI] [PubMed] [Google Scholar]
- Bruña-Romero O., González-Aseguinolaza G., Hafalla J. C., Tsuji M., Nussenzweig R. S. (2001). Complete, long-lasting protection against malaria of mice primed and boosted with two distinct viral vectors expressing the same plasmodial antigen. Proc. Natl. Acad. Sci. U.S.A. 98 11491–11496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey K. A., Fraser K. A., Schenkel J. M., Moran A., Abt M. C., Beura L. K., et al. (2012). Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 188 4866–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro D. R., Chen L., Fu T. M., Evans R. K., Caulfield M. J., Davies M. E., et al. (2003). Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77 6305–6313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro D. R., Wang F., Schleif W. A., Liang X., Zhang Z. Q., Tobery T. W., et al. (2005). Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 79 15547–15555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayabyab M. J., Korioth-Schmitz B., Sun Y., Carville A., Balachandran H., Miura A., et al. (2009). Recombinant Mycobacterium bovis BCG prime-recombinant adenovirus boost vaccination in rhesus monkeys elicits robust polyfunctional simian immunodeficiency virus-specific T-cell responses. J. Virol. 83 5505–5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebere I., Dorrell L., McShane H., Simmons A., McCormack S., Schmidt C., et al. (2006). Phase I clinical trial safety of DNA- and modified virus Ankara-vectored human immunodeficiency virus type 1 (HIV-1) vaccines administered alone and in a prime-boost regime to healthy HIV-1-uninfected volunteers. Vaccine 24 417–425 [DOI] [PubMed] [Google Scholar]
- Chopera D. R., Wright J. K., Brockman M. A., Brumme Z. L. (2011). Immune-mediated attenuation of HIV-1. Future Virol. 6 917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchyard G. J., Morgan C., Adams E., Hural J., Graham B. S., Moodie Z., et al. (2011). A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204). PLoS ONE 6 e21225 10.1371/journal.pone.0021225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn I. A., Chakravarty S., Overstreet M. G., García-Sastre A., Zavala F. (2008). Memory CD8+ T cell responses expand when antigen presentation overcomes T cell self-regulation. J. Immunol. 180 64–71 [DOI] [PubMed] [Google Scholar]
- Cox K. S., Clair J. H., Prokop M. T., Sykes K. J., Dubey S. A., Shiver J. H., et al. (2008). DNA gag/adenovirus type 5 (Ad5) gag and Ad5 gag/Ad5 gag vaccines induce distinct T-cell response profiles. J. Virol. 82 8161–8171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W., Kaech S. M. (2010). Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol. Rev. 236 151–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alencar B. C. G., Persechini P. M., Haolla F. A., De Oliveira G., Silverio J. C., Lannes-Vieira J., et al. (2009). Perforin and gamma interferon expression are required for CD4+ and CD8+ T-Cell-dependent protective immunity against a human parasite, Trypanosoma cruzi, elicited by heterologous plasmid DNA prime-recombinant adenovirus 5 boost vaccination. Infect. Immun. 77 4383–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa S. C., Thomas E. P., Bui J., Huang Y., deCamp A., Morgan C., et al. (2011). National institute of allergy and infectious diseases HIV vaccine trials network. V-DNA priming alters T cell responses to HIV-adenovirus vaccine even when responses to DNA are undetectable. J. Immunol. 187 3391–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez M. R., Ersching J., Lemos R., Machado A. V., Bruna-Romero O., Rodrigues M. M., et al. (2012). Re-circulation of lymphocytes mediated by sphingosine-1-phosphate receptor-1 contributes to resistance against experimental infection with the protozoan parasite Trypanosoma cruzi. Vaccine 30 2882–2891 [DOI] [PubMed] [Google Scholar]
- Dominguez M. R., Silveira E. L., de Vasconcelos J. R., de Alencar B. C., Machado A. V., Bruna-Romero O., et al. (2011). Subdominant/cryptic CD8 T cell epitopes contribute to resistance against experimental infection with a human protozoan parasite. PLoS ONE 6 e22011 10.1371/journal.pone.0022011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudani R., Murali-Krishna K., Krishnan L., Sad S. (2008). IFN-gamma induces the erosion of preexisting CD8 T cell memory during infection with a heterologous intracellular bacterium. J. Immunol. 181 1700–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke C. M., Maguire C. A., Keefer M. C., Federoff H. J., Bowers W. J., Dewhurst S. (2007). HSV-1 amplicon vectors elicit polyfunctional T cell responses to HIV-1 Env, and strongly boost responses to an adenovirus prime. Vaccine 25 7410–7421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunachie S. J., Walther M., Epstein J. E., Keating S., Berthoud T., Andrews L., et al. (2006). DNA prime-modified vaccinia virus Ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect. Immun. 74 5933–5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger D., Otten R. A., Li B., Aidoo M., Rodriguez I. V., Sariol C. A., et al. (2006). HIV-1 DNA/MVA vaccination reduces the per exposure probability of infection during repeated mucosal SHIV challenges. Virology 352 216–225 [DOI] [PubMed] [Google Scholar]
- Elvang T., Christensen J. P., Billeskov R., Thi Kim Thanh Hoang T., Holst P., Thomsen A. R., et al. (2009). CD4 and CD8 T cell responses to the M. tuberculosis Ag85B-TB10.4 promoted by adjuvanted subunit, adenovector or heterologous prime boost vaccination. PLoS ONE 4 e5139 10.1371/journal.pone.0005139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn J. D., Bassett J., Millar J. B., Grinshtein N., Yang T. C., Parsons R., et al. (2009). Persistence of transgene expression influences CD8+ T-cell expansion and maintenance following immunization with recombinant adenovirus. J. Virol. 83 12027–12036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatz L., Roychoudhuri R., Honda M., Filali-Mouhim A., Goulet J. P., Kettaf N., et al. (2011). Single-cell gene-expression profiling reveals qualitatively distinct CD8 T cells elicited by different gene-based vaccines. Proc. Natl. Acad. Sci. U.S.A. 108 5724–5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm N., DeCamp A. C., Friedrich D. P., Carter D. K., Defawe O. D., Kublin J. G., et al. (2012). Human adenovirus-specific T cells modulate HIV-specific T cell responses to an Ad5-vectored HIV-1 vaccine. J. Clin. Invest. 122 359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freel S. A., Lamoreaux L., Chattopadhyay P. K., Saunders K., Zarkowsky D., Overman R. G., et al. (2010). Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination. J. Virol. 84 4998–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T., Wakim L. M., Eidsmo L., Reading P. C., Heath W. R., Carbone F. R. (2009). Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 10 524–530 [DOI] [PubMed] [Google Scholar]
- Geisbert T. W., Bailey M., Geisbert J. B., Asiedu C., Roederer M., Grazia-Pau M., et al. (2010). Vector choice determines immunogenicity and potency of genetic vaccines against Angola Marburg virus in nonhuman primates. J. Virol. 84 10386–10394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. C., Moorthy V. S., Andrews L., Pathan A. A., McConkey S. J., Vuola J. M., et al. (2006). Synergistic DNA-MVA prime-boost vaccination regimes for malaria and tuberculosis. Vaccine 24 4554–4561 [DOI] [PubMed] [Google Scholar]
- Gilbert S. C., Schneider J., Hannan C. M., Hu J. T., Plebanski M., Sinden R., et al. (2002). Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine 20 1039–1045 [DOI] [PubMed] [Google Scholar]
- Gómez C. E., Nájera J. L., Krupa M., Perdiguero B., Esteban M. (2011). MVA and NYVAC as vaccines against emergent infectious diseases and cancer. Curr. Gene Ther. 11 189–217 [DOI] [PubMed] [Google Scholar]
- Goonetilleke N., Moore S., Dally L., Winstone N., Cebere I., Mahmoud A., et al. (2006). Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 80 4717–4728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R. (2008). Fas Bim boom! Immunity 28 141–143 [DOI] [PubMed] [Google Scholar]
- Hansen S. G., Ford J. C., Lewis M. S., Ventura A. B., Hughes C. M., Coyne-Johnson L., et al. (2011). Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473 523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari A., Bart P. A., Stöhr W., Tapia G., Garcia M., Medjitna-Rais E., et al. (2008). An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J. Exp. Med. 205 63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut L. H., Ratcliffe S., Pinto A. R., Ertl H. (2011). Effect of preexisting immunity to adenovirus on transgene product-specific genital T cell responses on vaccination of mice with a homologous vector. J. Infect. Dis. 203 1073–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig C., Gentek R., Backer R. A., de Souza Y., Derks I. A., Eldering E., et al. (2012). Notch controls the magnitude of T helper cell responses by promoting cellular longevity. Proc. Natl. Acad. Sci. U.S.A. 109 9041–9046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley L. E., Mulangu S., Asiedu C., Johnson J., Honko A. N., Stanley D., et al. (2010). Demonstration of cross-protective vaccine immunity against an emerging pathogenic Ebolavirus species. PLoS Pathog. 6 e1000904 10.1371/journal.ppat.1000904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikono H., Kohlmeier J. E., Takamura S., Wittmer S. T., Roberts A. D., Woodland D. L. (2007). Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J. Exp. Med. 204 1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft D. F., Blazevic A., Stanley J., Landry B., Sizemore D., Kpamegan E., et al. (2012). A recombinant adenovirus expressing immunodominant TB antigens can significantly enhance BCG-induced human immunity. Vaccine 30 2098–2108 [DOI] [PubMed] [Google Scholar]
- Hughes P. D., Belz G. T., Fortner K. A., Budd R. C., Strasser A., Bouillet P. (2008). Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity 28 197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster K. M., Koffler M., Stemberger C., Schiemann M., Wagner H., Busch D. H. (2006). Unidirectional development of CD8+ central memory T cells into protective Listeria-specific effector memory T cells. Eur. J. Immunol. 36 1453–1464 [DOI] [PubMed] [Google Scholar]
- Huster K. M., Stemberger C., Gasteiger G., Kastenmüller W., Drexler I., Busch D. H. (2009). Cutting edge: memory CD8 T cell compartment grows in size with immunological experience but nevertheless can lose function. J. Immunol. 183 6898–6902 [DOI] [PubMed] [Google Scholar]
- Hutnick N. A., Myles D. J., Hirao L., Scott V. L., Ferraro B., Khan A. S., et al. (2012). An optimized SIV DNA vaccine can serve as a boost for Ad5 and provide partial protection from a high-dose SIVmac251 challenge. Vaccine 30 3202–3208 [DOI] [PubMed] [Google Scholar]
- Irvine K. R., Chamberlain R. S., Shulman E. P., Surman D. R., Rosenberg S. A., Restifo N. P. (1997). Enhancing efficacy of recombinant anticancer vaccines with prime/boost regimens that use two different vectors. J. Natl. Cancer Inst. 89 1595–1601 [DOI] [PubMed] [Google Scholar]
- Jameson S. C., Masopust D. (2009). Diversity in T cell memory: an embarrassment of riches. Immunity 31 859–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaoko W., Karita E., Kayitenkore K., Omosa-Manyonyi G., Allen S., Than S., et al. (2010). Safety and immunogenicity study of multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS ONE 5 e12873 10.1371/journal.pone.0012873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Clark R. A., Liu L., Wagers A. J., Fuhlbrigge R. C., Kupper T. S. (2012). Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483 227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. R., Bivas-Benita M., Simmons N. L., Miller D., Barouch D. H. (2010). Route of adenovirus-based HIV-1 vaccine delivery impacts the phenotype and trafficking of vaccine-elicited CD8+ T lymphocytes. J. Virol. 84 5986–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating S. M., Bejon P., Berthoud T., Vuola J. M., Todryk S., Webster D. P., et al. (2005). Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J. Immunol. 175 5675–5680 [DOI] [PubMed] [Google Scholar]
- Kohlmeier J. E., Reiley W. W., Perona-Wright G., Freeman M. L., Yager E. J., Connor L. M., et al. (2011). Inflammatory chemokine receptors regulate CD8+ T cell contraction and memory generation following infection. J. Exp. Med. 208 1621–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup R. A., Roederer M., Lamoreaux L., Fischer J., Novik L., Nason M. C., et al. (2010). Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS ONE 5 e9015 10.1371/journal.pone.0009015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursar M., Jnner N., Pfeffer K., Brinkmann V., Kaufmann S. H, Mittrücker H. W. (2008). Requirement of secondary lymphoid tissues for the induction of primary and secondary T cell responses against Listeria monocytogenes. Eur. J. Immunol. 38 127–138 [DOI] [PubMed] [Google Scholar]
- Lai L., Kwa S., Kozlowski P. A., Montefiori D. C., Ferrari G., Johnson W. E., et al. (2011). Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J. Infect. Dis. 204 164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhashe S. K., Velu V., Sciaranghella G., Siddappa N. B., Dipasquale J. M., Hemashettar G., et al. (2011). Prime-boost vaccination with heterologous live vectors encoding SIV gag and multimeric HIV-1 gp160 protein: efficacy against repeated mucosal R5 clade C SHIV challenges. Vaccine 29 5611–5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasaro M. O., Ertl H. C. (2009). New insights on adenovirus as vaccine vectors. Mol. Ther. 17 1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin N. L., Mascola J. R., Sun Y., Gorgone D. A., Buzby A. P., Xu L., et al. (2006). Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312 1530–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Rao R., Vazzana J., Goedegebuure P., Odunsi K., Gillanders W., et al. (2012). Regulating mammalian target of rapamycin to tune vaccination-induced CD8+ T cell responses for tumor immunity. J. Immunol. 188 3080–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Rodrigues M., Rodriguez D., Rodriguez J. R., Esteban M., Palese P., et al. (1993). Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc. Natl. Acad. Sci. U.S.A. 90 5214–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Ewald B. A., Lynch D. M., Denholtz M., Abbink P., Lemckert A. A., et al. (2008). Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 82 4844–4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay L. K., Stock A. T., Ma J. Z., Jones C. M., Kent S. J., Mueller S. N., et al. (2012). Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. U.S.A. 109 7037–7042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes I., Sizemore D. R., Ahmed R. K., Mueller S., Wehlin L., Scanga C., et al. (2008). rBCG induces strong antigen-specific T cell responses in rhesus macaques in a prime-boost setting with an adenovirus 35 tuberculosis vaccine vector. PLoS ONE 3 e3790 10.1371/journal.pone.0003790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F., Brochard P., Ripaux M., Delache B., Aurégan G., Vaslin B., et al. (2008). Improved protection against simian immunodeficiency virus mucosal challenge in macaques primed with a DNA vaccine and boosted with the recombinant modified vaccinia virus Ankara and recombinant Semliki Forest virus. Vaccine 26 532–545 [DOI] [PubMed] [Google Scholar]
- Martins M. A., Wilson N. A., Reed J. S., Ahn C. D., Klimentidis Y. C., Allison D. B., et al. (2010). T-cell correlates of vaccine efficacy after a heterologous simian immunodeficiency virus challenge. J. Virol. 84 4352–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D., Choo D., Vezys V., Wherry E. J., Duraiswamy J., Akondy R., et al. (2010). Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D., Ha S. J., Vezys V., Ahmed R. (2006). Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J. Immunol. 177 831–839 [DOI] [PubMed] [Google Scholar]
- Mattapallil J. J., Douek D. C., Buckler-White A., Montefiori D., Letvin N. L., Nabel G. J., et al. (2006). Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J. Exp. Med. 203 1533–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey S. J., Reece W. H., Moorthy V. S., Webster D., Dunachie S., Butcher G., et al. (2003). Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat. Med. 9 729–735 [DOI] [PubMed] [Google Scholar]
- McShane H., Brookes R., Gilbert S. C., Hill A. V. (2001). Enhanced immunogenicity of CD4+ T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect. Immun. 69 681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. C., Hill A. V. (2004). Progress in DNA-based heterologous prime-boost immunization strategies for malaria. Immunol. Rev. 199 126–143 [DOI] [PubMed] [Google Scholar]
- Moorthy V. S., Imoukhuede E. B., Milligan P., Bojang K., Keating S., Kaye P., et al. (2004). A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med. 1 e33 10.1371/journal.pmed.0010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd P. A., Watkins D. I. (2011). Understanding animal models of elite control: windows on effective immune responses against immunodeficiency viruses. Curr. Opin. HIV AIDS 6 197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K., García-Sastre A., Tsuji M., Rodrigues M., Rodriguez D., Rodriguez J. R., et al. (1996). Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia viruses. Cell. Immunol. 173 96–107 [DOI] [PubMed] [Google Scholar]
- Nigam P., Earl P. L., Americo J. L., Sharma S., Wyatt L. S., Edghill-Spano Y., et al. (2007). DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology 151 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz J. C., Harty J. T. (2011). Strategies and implications for prime-boost vaccination to generate memory CD8 T cells. Adv. Exp. Med. Biol. 780 69–83 [DOI] [PubMed] [Google Scholar]
- Peiperl L., Morgan C., Moodie Z., Li H., Russell N., Graham B. S., et al. (2010). Safety and immunogenicity of a replication-defective adenovirus type 5 HIV vaccine in Ad5-seronegative persons: a randomized clinical trial (HVTN 054). PLoS ONE 5 e13579 10.1371/journal.pone.0013579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinschewer D. D., Ochsenbein A. F., Odermatt B., Brinkmann V., Hengartner H., Zinkernagel R. M. (2000). FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J. Immunol. 164 5761–5770 [DOI] [PubMed] [Google Scholar]
- Pinto A. R., Fitzgerald J. C., Giles-Davis W., Gao G. P., Wilson J. M., Ertl H. C. (2003). Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J. Immunol. 171 6774–6779 [DOI] [PubMed] [Google Scholar]
- Porter D. W., Thompson F. M., Berthoud T. K., Hutchings C. L., Andrews L., Biswas S., et al. (2011). A human phase I/IIa malaria challenge trial of a polyprotein malaria vaccine. Vaccine 29 7514–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulko V., Harris K. J., Liu X., Gibbons R. M., Harrington S. M., Krco C. J., et al. (2011). B7-h1 expressed by activated CD8 T cells is essential for their survival. J. Immunol. 187 5606–5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwar R., Campbell J., Murphy G., Richards W. G., Clark R. A., Kupper T. S. (2011). Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS ONE 6 e16245 10.1371/journal.pone.0016245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Sandoval A., Berthoud T., Alder N., Siani L., Gilbert S. C., Nicosia A., et al. (2010). Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect. Immun. 78 145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Sandoval A., Wyllie D. H., Bauza K., Milicic A., Forbes E. K., Rollier C. S., et al. (2011). CD8+ T effector memory cells protect against liver-stage malaria. J. Immunol. 187 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice J., Ottensmeier C. H., Stevenson F. K. (2008). DNA vaccines: precision tools for activating effective immunity against cancer. Nat. Rev. Cancer 8 108–120 [DOI] [PubMed] [Google Scholar]
- Rigato P. O., De Alencar B. C., De Vasconcelos J. R. C., Dominguez M. R., Araujo A. F., Machado A. V., et al. (2011). Heterologous plasmid DNA prime-recombinant human adenovirus 5 boost vaccination generates a stable pool of protective long-lived CD8+ T effector memory cells specific for a human parasite, Trypanosoma cruzi. Infect. Immun. 79 2120–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez D., González-Aseguinolaza G., Rodríguez J. R., Vijayan A., Gherardi M., Rueda P., et al. (2012). Vaccine efficacy against malaria by the combination of porcine parvovirus-like particles and vaccinia virus vectors expressing CS of Plasmodium. PLoS ONE 7 e34445 10.1371/journal.pone.0034445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M., Li S., Murata K., Rodriguez D., Rodriguez J. R., Bacik I., et al. (1994). Influenza and vaccinia viruses expressing malaria CD8+ T and B cell epitopes. Comparison of their immunogenicity and capacity to induce protective immunity. J. Immunol. 153 4636–4648 [PubMed] [Google Scholar]
- Saade F., Petrovsky N. (2012). Technologies for enhanced efficacy of DNA vaccines. Expert Rev. Vaccines 11 189–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagopal S., Amara R. R., Kannanganat S., Sharma S., Chennareddi L., Robinson H. L. (2008). Expansion and exhaustion of T-cell responses during mutational escape from long-term viral control in two DNA/modified vaccinia virus Ankara-vaccinated and simian-human immunodeficiency virus SHIV-89.6P-challenged macaques. J. Virol. 82 4149–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S., Seaman M. S., Xu L., Barouch D. H., Lord C. I., Lifton M. A., et al. (2005). Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J. Virol. 79 6516–6522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck R., Reimann J., Kochanek S., Kreppel F. (2008). The immunogenicity of adenovirus vectors limits the multispecificity of CD8 T-cell responses to vector-encoded transgenic antigens. Mol. Ther. 16 1609–1616 [DOI] [PubMed] [Google Scholar]
- Schmidt N. W., Harty J. T. (2011). Cutting edge: attrition of Plasmodium-specific memory CD8 T cells results in decreased protection that is rescued by booster immunization. J. Immunol. 186 3836–3840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Gilbert S. C., Blanchard T. J., Hanke T., Robson K. J., Hannan C. M., et al. (1998). Enhanced immunogenicity for CD8 T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4 397–402 [DOI] [PubMed] [Google Scholar]
- Schooley R. T., Spritzler J., Wang H., Lederman M. M., Havlir D., Kuritzkes D. R., et al. (2010). AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J. Infect. Dis. 202 705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedegah M., Jones T. R., Kaur M., Hedstrom R., Hobart P., Tine J. A., et al. (1998). Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. U.S.A. 95 7648–7653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin L. K., Lin M. Y., Kraemer K. A., Pardoll D. M., Schneck J. P., Varga S. M., et al. (1999). Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 11 733–742 [DOI] [PubMed] [Google Scholar]
- Selin L. K., Vergilis K., Welsh R. M., Nahill S. R. (1996). Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J. Exp. Med. 183 2489–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan B. S., Lefrançois L. (2011). Regional and mucosal memory T cells. Nat. Immunol. 12 485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck H., Nixon D. F. (2010). T cell immunity in acute HIV-1 infection. J. Infect. Dis. 202(Suppl. 2) S302–S308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Schmitz J. E., Buzby A. P., Barker B. R., Rao S. S., Xu L., et al. (2006). Virus-specific cellular immune correlates of survival in vaccinated monkeys after simian immunodeficiency virus challenge. J. Virol. 80 10950–10956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar M. S., Ramos H. J., Brassil M. M., Netland J., Chappell C. P., Blahnik G., et al. (2012). The RIG-I-like receptor LGP2 controls CD8+ T cell survival and fitness. Immunity 37 235–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S., Sabzevari H., Farsaci B., Schlom J., Greiner J. W. (2012). Distinct effects of saracatinib on memory CD8+ T cell differentiation. J. Immunol. 188 4323–4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao D., Barba-Spaeth G., Rai U., Nussenzweig V., Rice C. M., Nussenzweig R. S. (2005). Yellow fever 17D as a vaccine vector for microbial CTL epitopes: protection in a rodent malaria model. J. Exp. Med. 201 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsis N., Fitzgerald J. C., Reyes-Sandoval A., Harris-McCoy K. C., Hensley S. E., Zhou D., et al. (2007). Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood 110 1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos J. R., Bruña-Romero O., Araújo A. F., Dominguez M. R., Ersching J., de Alencar B. C., et al. (2012). Pathogen-induced proapoptotic phenotype and high CD95 (Fas) expression accompany a suboptimal CD8+ T-cell response: reversal by adenoviral vaccine. PLoS Pathog. 8 e1002699 10.1371/journal.ppat.1002699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezys V., Yates A., Casey K. A., Lanier G., Ahmed R., Antia R., et al. (2009). Memory CD8 T-cell compartment grows in size with immunological experience. Nature 457 196–199 [DOI] [PubMed] [Google Scholar]
- Vijayan A., Gómez C. E., Espinosa D. A., Goodman A. G., Sanchez-Sampedro L., Sorzano C. O., et al. (2012). Adjuvant-like effect of vaccinia virus 14K protein: a case study with malaria vaccine based on the circumsporozoite protein. J. Immunol. 188 6407–6417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojnov L., Bean A. T., Peterson E. J., Chiuchiolo M. J., Sacha J. B., Denes F. S., et al. (2011). DNA/Ad5 vaccination with SIV epitopes induced epitope-specific CD4+ T cells, but few subdominant epitope-specific CD8+ T cells. Vaccine 29 7483–7490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuola J. M., Keating S., Webster D. P., Berthoud T., Dunachie S., Gilbert S. C., et al. (2005). Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J. Immunol. 174 449–455 [DOI] [PubMed] [Google Scholar]
- Wakim L. M., Waithman J., van Rooijen N., Heath W. R., Carbone F. R. (2008). Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319 198–202 [DOI] [PubMed] [Google Scholar]
- Wakim L. M., Woodward-Davis A., Bevan M. J. (2010). Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. U.S.A. 107 17872–17879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim L. M., Woodward-Davis A., Liu R., Hu Y., Villadangos J., Smyth G., et al. (2012). The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J. Immunol. 189 3462–3471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weant A. E., Michalek R. D., Khan I. U., Holbrook B. C., Willingham M. C., Grayson J. M. (2008). Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity 28 218–230 [DOI] [PubMed] [Google Scholar]
- Wiesel M., Walton S., Richter K., Oxenius A. (2009). Virus-specific CD8 T cells: activation, differentiation and memory formation. APMIS 117 356–381 [DOI] [PubMed] [Google Scholar]
- Wilks A. B., Christian E. C., Seaman M. S., Sircar P., Carville A., Gomez C. E., et al. (2010). Robust vaccine-elicited cellular immune responses in breast milk following systemic simian immunodeficiency virus DNA prime and live virus vector boost vaccination of lactating rhesus monkeys. J. Immunol. 185 7097–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N. A., Reed J., Napoe G. S., Piaskowski S., Szymanski A., Furlott J., et al. (2006). Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 80 5875–5885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstone N., Guimarães-Walker A., Roberts J., Brown D., Loach V., Goonetilleke N., et al. (2009). Increased detection of proliferating, polyfunctional, HIV-1-specific T cells in DNA-modified vaccinia virus Ankara-vaccinated human volunteers by cultured IFN-gamma ELISPOT assay. Eur. J. Immunol. 39 975–985 [DOI] [PubMed] [Google Scholar]
- Winstone N., Wilson A. J., Morrow G., Boggiano C., Chiuchiolo M. J., Lopez M., et al. (2011). Enhanced control of pathogenic simian immunodeficiency virus SIVmac239 replication in macaques immunized with an interleukin-12 plasmid and a DNA prime-viral vector boost vaccine regimen. J. Virol. 85 9578–9587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H., Peng Y., Hong Y., Liu Y., Guo Z. S., Bartlett D. L., et al. (2011). Lentivector prime and vaccinia virus vector boost generate high-quality CD8 memory T cells and prevent autochthonous mouse melanoma. J. Immunol. 187 1788–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Johnson M. J., Price D. A., Wolinsky D. I., Almeida J. R., Petrovas C., et al. (2012). Virus inhibition activity of effector memory CD8+ T cells determines simian immunodeficiency virus load in vaccinated monkeys after vaccine breakthrough infection. J. Virol. 86 5877–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala F., Rodrigues M., Rodriguez D., Rodriguez J. R., Nussenzweig R. S., Esteban M. (2001). A striking property of recombinant poxviruses: efficient inducers of in vivo expansion of primed CD8+ T cells. Virology 280 155–159 [DOI] [PubMed] [Google Scholar]