Abstract
Background
Previous studies have reported that epidermal growth factor receptor (EGFR) mutation in tumor tissue and peripheral blood can predict the response to EGFR tyrosine kinase inhibitor (TKI) in non-small cell lung cancer (NSCLC). However, the heterogeneity of the sample sources makes it difficult to evaluate the detecting methodologies. The goal of this study is to compare different methods for analyzing EGFR mutation in blood and tumor tissue.
Materials and methods
Fifty-one advanced NSCLC patients treated with gefitinib were included in the study. The EGFR mutation status of each patients’ blood was analyzed by denaturing high-performance liquid chromatography (DHPLC), mutant-enriched liquidchip (ME-Liquidchip), and Scorpion Amplification Refractory Mutation System (Scorpion-ARMS) kits. EGFR mutation information in paired tumor samples detected by Scorpion-ARMS served as a reference. Comparative analyses were performed on mutation status results obtained from different methods and on the association between the clinical outcome of TKI treatment and EGFR mutation status.
Results
The response rate (RR) in the whole group was 33.3%. EGFR mutation rates were identified as 15.7%, 27.5%, and 29.4% by DHPLC, ME-Liquidchip, and Scorpion-ARMS in blood, respectively. In 34 cases that had paired tumor samples, the mutation rate in tissue was 41.2%. The RRs of patients with mutation detected by different methods were 71.4% (tumor), 62.5% (blood, DHPLC), 50.0% (blood, ME-Liquidchip), and 66.7% (blood, Scorpion-ARMS). EGFR mutation detected by Scorpion-ARMS in blood and tumor tissues had better prediction of RR to EGFR-TKI (P = 0.002 and P = 0.001) than mutation detected with DHPLC and ME-Liquidchip.
Conclusion
Tumor tissue sample is the best source for EGFR mutation analysis in NSCLC patients. Peripheral blood samples may be used as an alternative source only in special conditions. Scorpion-ARMS, DHPLC, or ME-Liquidchip methods are all optional for detecting tumor EGFR mutation from blood.
Keywords: non-small cell lung cancer, EGFR mutation, mutation detection methods, gefitinib
Introduction
The epidermal growth factor receptor (EGFR) is an interesting target for anticancer therapy as overexpression of EGFR was observed in a variety of tumors, including non-small cell lung cancer (NSCLC).1,2 Selective EGFR tyrosine kinase inhibitors (EGFR-TKI) such as gefitinib (Iressa, ZD1839; AstraZeneca, Wilmington, DE) and erlotinib (Tarceva, OSI-774; OSI Pharmaceuticals, Farmingdale, NY) have been used to treat NSCLC.3,4 Several reports have shown that EGFR mutation can predict the response to EGFR-TKI in patients with NSCLC.5,6 The most commonly found EGFR mutations are deletions in exon 19 (E 19dels) and a point mutation in exon 21 (L858R). Both mutations result in activation of the tyrosine kinase domain and both have been associated with sensitivity to the TKIs.5–11 Thus, the use of EGFR mutation in patient selection for EGFR-TKIs treatment has become the most important tool in clinical practice, especially in first-line use of these drugs.12–14
Most studies included mutation analyses of tumor tissue, surgical tissues, or biopsy specimens,15,16 which are limited by the fact that the rate of usable samples obtained from enrolled patients is very low, because it is often difficult to obtain sufficient amounts of tumor samples from advanced NSCLC patients. It is necessary to establish new methods for detecting EGFR mutation on surrogate sample types. Exploration on peripheral blood has been of interest recently.
In recent studies, several novel technologies, including Scorpion Amplified Refractory Mutation System technology (Scorpion-ARMS; DxS Ltd, Manchester, UK),17 length analysis of fluorescently labeled polymerase chain reaction (PCR), PCR Taqman assay,18,19 denaturing high-performance liquid chromatography (DHPLC),20 and mutant-enriched liquidchip (ME-Liquidchip)21 have been used to detect EGFR mutation in peripheral blood. The results demonstrated that EGFR mutations can be detected in serum DNA samples of patients with NSCLC and they were not only identical to those in the corresponding tumors but could also predict the response of EGFR-TKI.
While the feasibility and availability of serum DNA detection methods have been confirmed, a standard method with high sensitivity and specificity needs to be established, which may has strong impact on the setting of clinical strategy. However, most previous studies on serum EGFR mutation were carried out in a single cohort of a single center using different methods. Heterogeneity in patients could make it difficult to fairly evaluate the methodology. To our knowledge, no comparative assessment of these novel technologies has been available up until now. In the current study, we evaluated three different methods for determining EGFR mutation status in the peripheral blood of the same group of NSCLC patients treated with gefitinib. The EGFR mutation information detected by the Scorpion-ARMS method in paired tumor samples of the same group patients severed as a reference. Comparative analyses were performed on the EGFR mutation status results obtained from three different methods and on the association between clinical outcome of TKI treatment and EGFR mutation status.
Materials and methods
Clinical material
Patients with pathologically confirmed advanced NSCLC were recruited in our study between July 2007 and April 2009 at the Sun Yat-sen University Cancer Center. Three patients were treated with gefitinib as initial therapy. The remaining patients had received prior chemotherapy. The diagnosis of NSCLC was based on the histological or cytological findings, and the histological type was determined according to World Health Organization criteria.22 Smoking status was based on records at patients’ first clinic visit and having smoked greater than 100 cigarettes in a lifetime was used to define smokers. The study was reviewed and approved by the Institutional Ethics Committee at Sun Yat-sen University Cancer Center. All the patients signed informed consent to participate in this study and gave permission for the use of their plasma and tumor tissues.
The response to gefitinib was evaluated in accordance with the Response Evaluation Criteria in Solid Tumours (RECIST) guidelines.23 Patients’ blood and tumor samples were collected before EGFR-TKI treatment. We analyzed the EGFR mutation of blood samples by three different methods: DHPLC, ME-Liquidchip, and Scorpion-ARMS kit. EGFR mutation status in the corresponding tumor was analyzed using Scorpion ARMS.
Samples collection and DNA extraction
Blood samples from all 51 patients were collected before the initiation of gefitinib administration. Separated serum was stocked at −80°C until use. Plasma DNA was extracted and purified by using a Qiamp Blood Kit (Qiagen, Hilden, Germany) according to the protocol described in the manufacturer’s instructions. The extracted DNA was stored at −20°C until used.
Thirty-four paired tumor specimens were collected and underwent histologic examination to confirm the diagnosis of NSCLC. The tumor specimens obtained were fixed in formalin and embedded in paraffin wax. Serial sections containing representative malignant cells were deparaffinized in xylene washes and dehydrated in 100% ethanol. DNA was extracted from five serial 10 μm thick sections by using the Qiamp DNA Mini kit (Qiagen) according to the protocol described in the manufacturer’s instructions. The extracted DNA was stored at −20°C until used.
DHPLC
DHPLC was performed by using the Transgenomic Wave Nucleic Acid Fragment Analysis System with a DNASep column (Transgenomic, Omaha, NE). The mobile phases comprised 0.05% acetonitrile in 0.1 M triethylammonium acetate ([TEAA] eluent A) and 25% acetonitrile in 0.1 M TEAA (eluent B). The PCR products of exons 18, 20, and 21 were denatured at 95°C for 5 minutes and were cooled to 35°C at a rate of 1°C per minute to allow formation of heterozygote DNA. The product of exon 19 did not need to be denatured. The flow rate was 0.9 mL/minute and an ultraviolet detector was set at 260 nm. We identified the heterozygous profiles by visual inspection of the chromatograms on the basis of the appearance of additional earlier eluting peaks. Corresponding homozygous profiles showed only one peak. To determine the detection limit of DHPLC, we used four plasmids that contained the deletion mutation (delE746-A751) in exon 19, L858R mutation in exon 21, and wild-type exon 19 and 21 sequences. Serial dilutions (50%, 25%, 12.5%, 6.25%, 3.125%, and 1.6% of mutant alleles) were made for the DHPLC analysis.
ME-Liquidchip
To enrich mutant EGFR, a unique restriction site was introduced into the mutation alleles so that the wild-type sequence could be selectively removed by restriction digestion, and the undigested mutated DNA was amplified by PCR. The product was then hybridized to complementary probes (including: Del E746_A750(1); Del E746_A750(2); Del L747_E749 insP; Del L747_A750 insP; Del L747_T751; Del L747_T751 insA; Del L747_S752; Del L747_S752 insV; Del L747_S752 insD; Del L747_S752 insQ; Del L747_S752 insS; Del E749_S752 insD; Del E746_T751 insT; Del E746_P753 insVS; and Del E746_T751 insY in exon 19 and L858R mutation in exon 21), which had been conjugated to beads coded with different fluorescent dyes, followed by measurement with the Luminex 200 system (Luminex Corporation, Austin, TX). A plasmid DNA mixture with different EGFR genotypes was applied to determine the sensitivity and accuracy of MEL.
Scorpion-ARMS
The EGFR Scorpion-ARMS kit (DxS Ltd, Manchester, UK) was used to detect mutations by real-time PCR following the user manual. The most prevalent 29 mutations so far described in the EGFR gene were covered and classified into eight single or multiplex assays (Deletions, T790M, L858R, L861Q, G719X, S768I, and Insertion assays). This kit enabled us to detect the low-level mutant DNA in the background of wild-type DNA based on the allele-specific and real-time PCR technologies. Two nanograms of DNA were added to each 25 μL assay reaction in a 96-well plate. The plate was sealed and loaded into a Stratagene MX3005P real-time PCR system (Agilent Technologies, Santa Clara, CA). Cycling parameters from the user manual were carried out and the fluorescent signal was collected from FAM and HEX channels. The data were analyzed using MxPro v4.0 software (Agilent Technologies).
Statistical analysis
Fisher’s exact test was used to test the relationship between the presence of EGFR mutations and treatment response. Overall survival (OS) and progression-free survival (PFS) according to EGFR mutation status were estimated by the Kaplan–Meier method and compared using the two-sided log rank test.
OS was defined as the interval between the start of gefitinib therapy and death from any cause; patients known to be still alive at the time of the analysis were censored at the time of their last follow-up. PFS was defined as the interval between the start of gefitinib therapy and the first manifestation of progressive disease (PD) or death from any cause; patients known to be alive and without PD at the time of analysis were censored at the time of their last follow-up.
Results
Patient characteristics
From July 2007 to April 2009, 51 pathologically confirmed advanced NSCLC patients were enrolled into the study at Sun Yat-sen University Cancer Center. There were 31 males and 20 females, with a median age of 54 years (range 25–77). The most common histological subtype was adenocarcinoma (43/51, 84.3%). A total of 19 patients were smokers and 32 were never-smokers. All patients were initially diagnosed with stages IIIB to IV NSCLC; 48 patients (94.1%) had received prior chemotherapy, whereas 3 (5.9%) were treatment naive at the time of sample collection. The patients’ clinical and disease characteristics are listed in Table 1.
Table 1.
Patient characteristics
| Characteristic | No of patients (n = 51) | % |
|---|---|---|
| Sex | ||
| Male | 31 | 60.8 |
| Female | 20 | 39.2 |
| Stage | ||
| IIIB | 6 | 11.8 |
| IV | 45 | 88.2 |
| Histology | ||
| Adenocarcinoma | 43 | 84.3 |
| Non-adenocarcinoma | 8 | 15.7 |
| Smoking history | ||
| Yes | 19 | 37.3 |
| No | 32 | 62.7 |
| Previous chemotherapy | ||
| 0 regimen | 3 | 5.9 |
| 1 regimen | 33 | 64.7 |
| 2 regimens | 9 | 17.6 |
| ≥3 regimens | 6 | 11.8 |
Note: Median age of all patients: 54 years; range 25–77 years.
Response and survival
According to RECIST, we observed 17 partial responses (33.3%), 16 stable diseases (31.4%), and 18 PDs (35.3%). In the whole study population, the median PFS was 91 days (95% CI, 80.3 to 101.7 days) and the median survival time was 408 days (95% CI, 200.1 to 615.9 days).
EGFR mutation in peripheral blood and paired tumor samples
EGFR mutation rates were identified 15.7% (8/51), 27.5% (14/51), and 29.4% (15/51) by DHPLC, ME-Liquidchip, and Scorpion-ARMS in peripheral blood, respectively; 34 cases were available for paired tumor sample EGFR mutation detection. In these patients, EGFR mutation rates were 11.8% (4/34), 26.5% (9/34), and 29.4% (10/34) by DHPLC, ME-Liquidchip, and Scorpion-ARMS in blood samples and 41.2% (14/34) in paired tissues (Tables 2 and 3).
Table 2.
Comparison between EGFR mutation (19Del) status analysis by DHPLC, ME-Liquidchip, and Scorpion-ARMS in peripheral blood and Scorpion-ARMS in tumor tissue
| No | Scorpion-ARMS in T | Total | |
|---|---|---|---|
|
|
|||
| (+) | (−) | ||
| DHPLC in B | |||
| (+) | 0 | 1 | 1 |
| (−) | 7 | 26 | 33 |
| Total | 7 | 27 | 34 |
| Scorpion-ARMS in B | |||
| (+) | 3 | 4 | 7 |
| (−) | 4 | 23 | 27 |
| Total | 7 | 27 | 34 |
| ME-Liquidchip in B | |||
| (+) | 2 | 5 | 7 |
| (−) | 5 | 22 | 27 |
| Total | 7 | 27 | 34 |
Abbreviations: EGFR, epidermal growth factor receptor; 19Del, exon 19 mutation of EGFR; DHPLC, denaturing high-performance liquid chromatography; ME-Liquidchip, mutant-enriched liquidchip; Scorpion-ARMS, Scorpion Amplification Refractory Mutation System; T, tumor tissue; B, peripheral blood.
Table 3.
Comparison between EGFR mutation (L858R) status analysis by DHPLC, ME-Liquidchip, and Scorpion-ARMS in peripheral blood and Scorpion-ARMS in tumor tissue
| No | Scorpion-ARMS in T | Total | |
|---|---|---|---|
|
|
|||
| (+) | (−) | ||
| DHPLC in B | |||
| (+) | 2 | 2 | 4 |
| (−) | 6 | 24 | 30 |
| Total | 8 | 26 | 34 |
| Scorpion-ARMS in B | |||
| (+) | 4 | 0 | 4 |
| (−) | 4 | 26 | 30 |
| Total | 8 | 26 | 34 |
| ME-Liquidchip in B | |||
| (+) | 2 | 1 | 3 |
| (−) | 6 | 25 | 31 |
| Total | 8 | 26 | 34 |
Abbreviations: EGFR, epidermal growth factor receptor; L858R, exon 21 mutation of EGFR; DHPLC, denaturing high-performance liquid chromatography; ME-Liquidchip, mutant-enriched liquidchip; Scorpion-ARMS, Scorpion Amplification Refractory Mutation System; T, tumor tissue; B, peripheral blood; M+, mutation positive; M-, mutation negative.
In the analysis of methods used in peripheral blood, Scorpion-ARMS showed the highest sensitivity (42.9%/50.0%, 19Del/L858R), positive predictive value (42.9%/100.0%), negative predictive value (85.2%/86.7%), and concordance (76.5%/88.2%) in the detection of both mutations. It also demonstrated the best specificity for L858R (100%). But when detecting 19Del, the specificity of DHPCL was higher (96.3%). The results of L858R detected by Scorpion-ARMS in peripheral blood seem more consistent with tumor tissue (Kappa coefficient 0.605) than other assessments results (Tables 4 and 5).
Table 4.
Sensitivity, specificity, positive predictive value, negative predictive value, concordance, and Kappa coefficient of EGFR mutation (19Del) status analysis by DHPLC, ME-Liquidchip, and Scorpion-ARMS in peripheral blood
| Method | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Concordance (%) | Kappa coefficient |
|---|---|---|---|---|---|---|
| DHPLC | 0.0% | 96.3%* | 0.0% | 78.8% | 76.5% | −0.054 |
| Scorpion-ARMS | 42.9%* | 85.2% | 42.9%* | 85.2%* | 76.5%* | 0.280* |
| ME-Liquidchip | 28.6% | 81.5% | 28.6% | 81.5% | 70.6% | 0.101 |
Notes: Sensitivity = (M+ in both T and B)/(M+ in T) × 100%; specificity = (M− in both T and B)/(M− in T) × 100%; positive predictive value = (M+ in both T and B)/ (M+ in B) × 100%; negative predictive value = (M− in both T and B)/(M− in B) × 100%; concordance = ([M+ in both T and B] + [M− in both T and B])/34 paired samples × 100%.
The best result compared to other measures.
Abbreviations: EGFR, epidermal growth factor receptor; 19Del, exon 19 mutation of EGFR; DHPLC, denaturing high-performance liquid chromatography; ME-Liquidchip, mutant-enriched liquidchip; Scorpion-ARMS, Scorpion Amplification Refractory Mutation System; T, tumor tissue; B, peripheral blood; M+, mutation positive; M−, mutation negative.
Table 5.
Sensitivity, specificity, positive predictive value, negative predictive value, concordance, and Kappa coefficient of EGFR mutation (L858R) status analysis by DHPLC, ME-Liquidchip, and Scorpion-ARMS in peripheral blood
| Method | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Concordance (%) | Kappa coefficient |
|---|---|---|---|---|---|---|
|
|
|
|
||||
| DHPLC | 25.0% | 92.3% | 50.0% | 80.0% | 76.5% | 0.209 |
| Scorpion-ARMS | 50.0%* | 100.0%* | 100.0%* | 86.7%* | 88.2%* | 0.605* |
| ME-Liquidchip | 25.0% | 96.2% | 66.7% | 80.6% | 79.4% | 0.270 |
Notes: Sensitivity = (M+ in both T and B)/(M+ in T) × 100%; specificity = (M− in both T and B)/(M– in T) × 100%; positive predictive value = (M+ in both T and B)/ (M+ in B) × 100%; negative predictive value = (M− in both T and B)/(M− in B) × 100%; concordance = ([M+ in both T and B] + [M− in both T and B])/34 paired samples × 100%.
The best result compared to other measures.
Abbreviations: EGFR, epidermal growth factor receptor; L858R, exon 21 mutation of EGFR; DHPLC, denaturing high-performance liquid chromatography; ME-Liquidchip, mutant-enriched liquidchip; Scorpion-ARMS, Scorpion Amplification Refractory Mutation System; T, tumor tissue; B, peripheral blood; M+, mutation positive; M−, mutation negative.
Correlation between EGFR mutation and response
The response rate of patients with EGFR mutation versus wild-type DNA in tumor tissue by Scorpion-ARMS was 64.3% vs 10%. In blood, the response rate of patients with EGFR mutation versus wild-type DNA in blood by Scorpion-ARMS was 66.7% vs 19.4%, by DHPLC was 62.5% vs 27.9%, and by ME-Liquidchip was 50.0% versus 27.0%. Only EGFR mutation detected by Scorpion-ARMS both in tumor tissue and in blood significantly correlated with the response of gefitinib (P = 0.002 for tumor tissue and P = 0.001 for blood; Table 6).
Table 6.
Correlation between EGFR mutation and response
| No (%) | EGFR mutation | PR | SD + PD | Total |
|---|---|---|---|---|
| Scorpion-ARMS in T | (+) | 9 (26.5) | 5 (14.7) | 14 (41.2) |
| (−) | 2 (5.9) | 18 (52.9) | 20 (58.8) | |
| Total | 11 (32.4) | 23 (67.6) | 34 (100.0) | |
| P-value | 0.002 | |||
| DHPLC in B | (+) | 5 (9.8) | 3 (5.9) | 8 (15.7) |
| (−) | 12 (23.5) | 31 (60.8) | 43 (84.3) | |
| Total | 17 (33.3) | 34 (66.7) | 51 (100.0) | |
| P-value | 0.134 | |||
| Scorpion-ARMS in B | (+) | 10 (19.6) | 5 (9.8) | 15 (29.4) |
| (−) | 7 (13.7) | 29 (56.9) | 36 (70.6) | |
| Total | 17 (33.3) | 34 (66.7) | 51 (100.0) | |
| P-value | 0.001 | |||
| ME-Liquidchip in B | (+) | 7 (13.7) | 7 (13.7) | 14 (27.5) |
| (−) | 10 (19.6) | 27 (52.9) | 37 (72.5) | |
| Total | 17 (33.3) | 34 (66.7) | 51 (100.0) | |
| P-value | 0.120 |
Abbreviations: EGFR, epidermal growth factor receptor; PR, partial response; SD, stable disease; PD, progressive disease; Scorpion-ARMS, Scorpion Amplification Refractory Mutation System; T, tumor tissue; DHPLC, denaturing high-performance liquid chromatography; B, peripheral blood; ME-Liquidchip, mutant-enriched liquidchip.
Correlation between EGFR mutation and survival
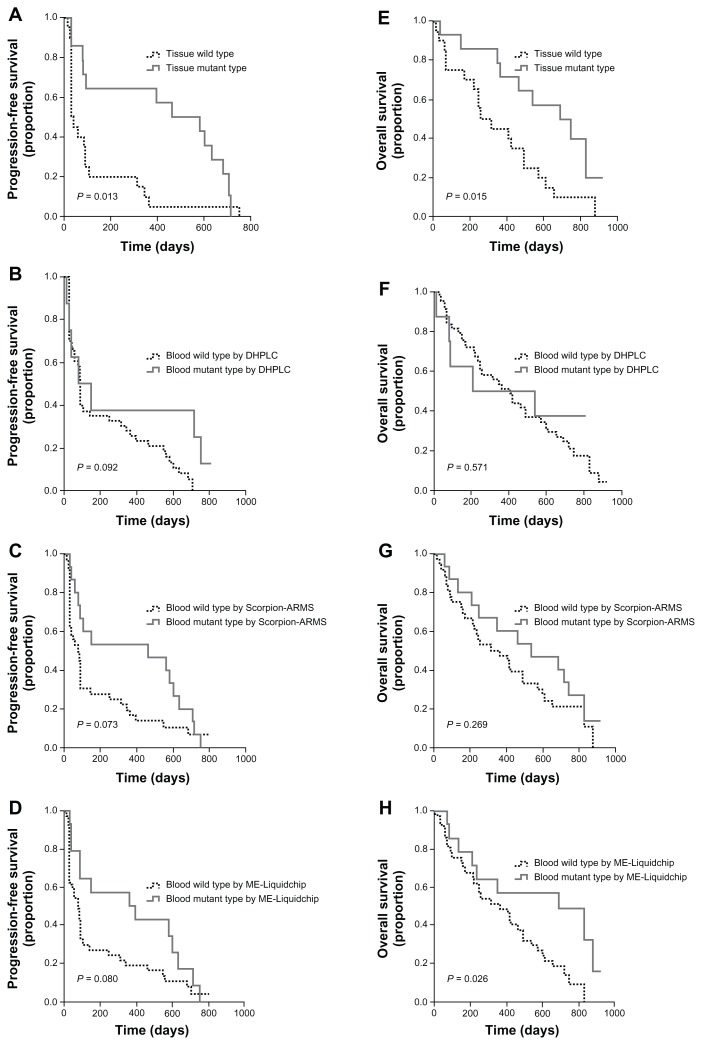
The median PFS and OS of patients with EGFR mutations detected by Scorpion-ARMS in tumor tissue were significantly longer than those of patients with wild-type EGFR. PFS was 463 days (95% confidence interval [CI]: 123.8 to 802.2 days) versus 32 days (95% CI: 7.9 to 56.1 days, P = 0.013), and OS was 688 days (95% CI: 370.2 to 1005.8 days) versus 255 days (95% CI: 112.6 to 397.4 days, P = 0.015) for EGFR mutation patients versus wild-type EGFR patients, respectively.
Association between EGFR mutation status and PFS or OS was not observed in blood DNA analyses, except OS and mutation status detected by ME-Liquidchip in plasma. In that group of patients with EGFR mutation, median OS was significantly different from patients without the mutation (688 days, 95% CI: 163.5 to 1212.5 days vs 364 days, 95% CI: 161.4 to 566.6 days, P = 0.026; Figure 1).
Figure 1.
PFS and OS curves for patients treated with gefitinib. (A) PFS by EGFR mutation status measured in tumor tissue by Scorpion-ARMS. (B) PFS by EGFR mutation status measured in peripheral blood DNA by DHPLC. (C) PFS by EGFR mutation status measured in peripheral blood DNA by Scorpion-ARMS. (D) PFS by EGFR mutation status measured in peripheral blood DNA by ME-Liquidchip. (E) OS by EGFR mutation status measured in tumor tissue by Scorpion-ARMS. (F) OS by EGFR mutation status measured in peripheral blood DNA by DHPLC. (G) OS by EGFR mutation status measured in peripheral blood DNA by Scorpion-ARMS. (H) OS by EGFR mutation status measured in peripheral blood DNA by ME-Liquidchip.
Abbreviations: PFS, progression-free survival; OS, overall survival; EGFR, epidermal growth factor receptor; Scorpion-ARMS, Scorpion Amplification Refractory Mutation System; DHPLC, denaturing high-performance liquid chromatography; ME-Liquidchip, mutant-enriched liquidchip.
Discussion
EGFR mutation is now widely used in clinical practice to predict the treatment benefit from EGFR-TKIs in NSCLC. In the detection of EGFR mutation, tumor tissue is the most common sample source. A blood sample can be obtained safely, with the option of repeat sampling from all NSCLC patients regardless of their characteristics; blood sampling was used in EGFR mutation detection recently.17–21 The primary objective of the current study was to compare the performance of three commonly used EGFR mutation detection methods for the assessment of EGFR mutation status in the peripheral blood of NSCLC patients.
The Scorpion-ARMS method has been proved to be a stable, specific, and sensitive way for a tumor tissue sample to be assessed for EGFR mutation status.17,24,25 The DHPLC and ME-Liquidchip methods utilized in this study are technically easier and cheaper and have a quicker turnaround time than sequencing analysis. They have been used for EGFR mutation analysis by some investigators.20,21,26 In our study, we chose the Scorpion-ARMS method (instead of a DNA sequencing method) to evaluate paired tumor samples. Then, the EGFR mutation status obtained from the tumor sample served as a reference to compare with the results obtain from the peripheral blood samples in our study.
A previous study showed that DHPLC was able to detect mutations in samples containing as little as 1% to 6.25% mutated DNA, whereas direct sequencing required at least 20%–30%.27,28 DHPLC is not only an efficient method for screening for genomic alterations in exon 19 and 21 of the EGFR gene as compared with direct sequence analysis, but is also 56% less expensive and 39% faster than direct sequencing.27 A study conducted by Bai et al recently showed a high correlation between the mutations detected in plasma DNA and the mutations detected in the corresponding tumor DNA by DHPLC (P = 0.001; correlation index, 0.74).20
ME-Liquidchip is a novel technology, which integrates the sensitive mutant enriched PCR and quantitative high-throughput liquidchip (suspension array), to detect DNA somatic mutations in EGFR, KRAS, BRAF, and PIK3CA genes from tissue or serum samples. It has been reported that ME-Liquidchip is capable of detecting as few as 20 copies of mutant EGFR alleles with a sensitivity limit of at least mutant:wild-type ratio of 0.1%.21,26,29 Previous research also shows that ME-Liquidchip can not only confirm EGFR mutations status in tissue specimens already known by direct sequencing, but also detect mutations in some of those showing wild-type by sequencing.21 Our research is the first time that ME-Liquidchip has been used to test EGFR mutations noninvasively for predicting the response in advanced NSCLC patients treated by targeted therapy.
In our study, the EGFR mutation status of blood samples tested by DHPLC and ME-Liquidchip did not show strong coincidence with tumor tissues (Kappa coefficient = −0.054 in E19Dels and 0.209 in L858R by DHPLC, Kappa coefficient = 0.101 in E19Dels and 0.270 in L858R by ME-Liquidchip). The results of the tests also could not predict the response to the treatment of gefitinib (P = 0.134 by DHPLC, P = 0.120 by ME-Liquidchip). Our DHPLC testing did not even confirm the results of previous research.20 This inconsistency might be due to the instability of DHPLC and ME-Liquidchip. DHPLC and ME-Liquidchip are still lab-based technologies usually used for detecting mutation in tumor tissues; their stability and the optimized procedure for blood samples might need to be verified by more research. Their sensitivity and specificity are still in discussion.
EGFR mutation detected by the Scorpion-ARMS method in either blood or tumor tissue had better correlation with the response of gefitinib, which is consistent with previously reported data.17,24 Scorpion-ARMS showed the highest sensitivity, positive predictive value, negative predictive value, and concordance in the detection of both mutations. The results of L858R detected by Scorpion-ARMS in peripheral blood seem more consistent with tumor tissue (Kappa coefficient = 0.605) than other assessments results.
According to the current study, the different methods provide different EGFR mutation test results, such as the appearance of EGFR mutations in the blood that was not detected in the tumor in a considerable number of patients. This might be due to the false positive of blood or false negative of tumor tissues. The instability of DHPLC and ME-Liquidchip may cause false positives. There could be several explanations for the potential false negative of EGFR mutation in tumor tissue. First, it could be due to the difference in timing between tumor tissue collection and blood sample collection. In our study, the tumor samples were acquired before first line chemotherapy treatment, but blood samples were acquired before the initiation of gefitinib administration. Of our patients, 94.1% had previously received two or more treatments of chemotherapy. As the diseases progressed, the tumor burdens must have become more severe; this might have increased the amount of circulating tumor DNA and led to the positive rate. In some patients, new mutations may have been generated. Second, intra-tumor heterogeneity could also be an explanation. If the tumor tissues and circulating DNA embraced different EGFR status (such as tissue mutation negative, but circulating DNA mutation positive); the test results could also be different. Third, the mechanism of DHPLC does not detect the mutant DNA directly. It detects heterozygote DNA. So when used for 19Del, DHPLC could detect some mutant types that were not detected by probes of ME-Liquidchip and Scorpion-ARMS.
In our study, no matter what methods were used, though the EGFR mutation in blood tended to predict longer PFS, the difference was not significant. But the PFS of patients with EGFR mutation detected from tumor tissue was significantly longer than those without the mutation. No difference in OS was seen between patients with or without EGFR mutation when we tested samples by Scorpion-ARMS (blood and tumor tissues) and DHPLC (blood). In previous studies, the correlation between EGFR mutation and survival was discussed repeatedly, but the results were not totally consistent: though the researchers tend to agree that EGFR mutation in either tumor tissue or blood will prolong the PFS, they are still uncertain if EGFR mutation can predict longer OS of patients treated by gefitinib.12,17,30–32 Because of the heterogeneity of treatment before and after the failure of gefitinib, it is really difficult to estimate and compare the survival data. Besides, many studies are not specifically designed to test gefitinib treatment and many patients received other chemotherapeutic agents, which makes data interpretation difficult. Additional clinical studies with specifically defined treatment regimens and larger sample sizes are necessary.
There have been many studies to discuss the accuracy and predictive effect of EGFR mutation detected using a peripheral blood sample.17–21,24 However, to the best of our knowledge, the current study is the first one to compare different methodologies by using paired tumor samples as a reference. In our research, we chose Scorpion-ARMS but not DNA sequencing to test EGFR mutation in tumor tissue because Scorpion-ARMS was able to detect mutations in samples containing as little as 1% mutated DNA, whereas direct sequencing required at least 20%–30%. Besides, patients enrolled in our research all had advanced NSCLC; most samples were obtained from biopsy or puncture biopsy of tumor lesions. If DNA sequencing had been used, the amounts of mutant DNA might have been insufficient. Scorpion-ARMS has also been used as a reference for tumor tissue mutation detection in many other research studies.17,24,33,34 Our sample size, especially the paired sample, was still limited. Additionally, some other methods to test EGFR mutation such as length analysis of fluorescently labeled PCR and PCR Taqman assay18,19 were not included in our research. Further investigations with larger sample sizes and more methodologies to validate our results are warranted.
Conclusion
Notwithstanding its limitation, our study provides evidence to support that tumor tissue sample is still the best source for EGFR mutation analysis in NSCLC patients. A peripheral blood sample may only be used as an alternative source in special circumstances (for example, not enough tumor tissue sample available). The Scorpion-ARMS, DHPLC, or ME-Liquidchip methods are all optional for detecting EGFR mutation from peripheral blood samples. In our research, Scorpion-ARMS showed better sensitivity, positive predictive value, negative predictive value, and concordance rate. Our study is retrospective; the results still need to be further confirmed by more investigation.
Footnotes
Disclosure
Yachao Lu is the associate scientist of Innovation Centre China, R&D AstraZeneca. Guanshan Zhu is the team leader of tumor genetics of Innovation Centre China, R&D AstraZeneca. Jiasen Xu is the CEO of SurExam Bio-Tech Co, Ltd. The other authors report no conflicts of interest in this work.
References
- 1.Rusch V, Baselga J, Cordon-Cardo C, et al. Differential expression of the epidermal growth factor receptor and its ligands in primary non-small cell lung cancers and adjacent benign lung. Cancer Res. 1993;53(10 Suppl):2379–2385. [PubMed] [Google Scholar]
- 2.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19(3):183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 3.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] J Clin Oncol. 2003;21(12):2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 7.Huang SF, Liu HP, Li LH, et al. High frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin Cancer Res. 2004;10(24):8195–8203. doi: 10.1158/1078-0432.CCR-04-1245. [DOI] [PubMed] [Google Scholar]
- 8.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 2004;64(24):8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 9.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 11.Tokumo M, Toyooka S, Kiura K, et al. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res. 2005;11(3):1167–1173. [PubMed] [Google Scholar]
- 12.Ettinger DS, Akerley W, Bepler G, et al. NCCN Non-Small Cell Lung Cancer Panel Members. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8(7):740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 13.D’Addario G, Früh M, Reck M, et al. ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v116–v119. doi: 10.1093/annonc/mdq189. [DOI] [PubMed] [Google Scholar]
- 14.Azzoli CG, Baker S, Jr, Temin S American Society of Clinical Oncology. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009;27(36):6251–6266. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell DW, Lynch TJ, Haserlat SM, et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. J Clin Oncol. 2005;23(31):8081–8092. doi: 10.1200/JCO.2005.02.7078. [DOI] [PubMed] [Google Scholar]
- 16.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer – molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 17.Kimura H, Suminoe M, Kasahara K, et al. Evaluation of epidermal growth factor receptor mutation status in serum DNA as a predictor of response to gefitinib (IRESSA) Br J Cancer. 2007;97(6):778–784. doi: 10.1038/sj.bjc.6603949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moran T, Pas-Ares L, Isla D, et al. High correspondence between EGFR mutations in tissue and in circulating DNA form non-small-cell lung cancer (NSCLC) patients (p) with poor performance status (PS). 2007 ASCO Annual Meeting Proceedings Part I. J Clin Oncol. 2007;25(18S, June 20 Supplement):7505. [Google Scholar]
- 19.Rosell R, Moran T, Queralt C, et al. Spanish Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 20.Bai H, Mao L, Wang HS, et al. Epidermal growth factor receptor mutations in plasma DNA samples predict tumor response in Chinese patients with stages IIIB to IV non-small-cell lung cancer. J Clin Oncol. 2009;27(16):2653–2659. doi: 10.1200/JCO.2008.17.3930. [DOI] [PubMed] [Google Scholar]
- 21.Lu S, Yang H, Ye X, et al. A novel mutant-enriched liquidchip for detection of circulating EGFR mutations in advanced non-small cell lung cancer patients. J Clin Oncol. 2009;27(Suppl) Abstr e14526. [Google Scholar]
- 22.Travis W, Colby TV, Corrin B. International Classification of Tumors. 3rd edition. New York, NY: Springer Verlag; 1999. Histologic Typing of Tumors of Lung and Pleura: World Health Organization. [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Kimura H, Kasahara K, Kawaishi M, et al. Detection of epidermal growth factor receptor mutations in serum as a predictor of the response to gefitinib in patients with non-small-cell lung cancer. Clin Cancer Res. 2006;12(13):3915–3921. doi: 10.1158/1078-0432.CCR-05-2324. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Liang ZY, Zeng X, Wu SF, Gao J, Liu TH. Detection of epidermal growth factor receptor gene mutations in non-small cell lung cancers by real-time polymerase chain reaction using scorpion amplification refractory mutation system. Zhonghua Bing Li Xue Za Zhi. 2008;37(5):294–299. Chinese. [PubMed] [Google Scholar]
- 26.Li G, Luo X, He J, et al. A novel liquidchip platform for simultaneous detection of 70 alleles of DNA somatic mutations on EGFR, KRAS, BRAF and PIK3CA from formalin-fixed and paraffin-embedded slides containing tumor tissue. Clin Chem Lab Med. 2011;49(2):191–195. doi: 10.1515/CCLM.2011.040. [DOI] [PubMed] [Google Scholar]
- 27.Chin TM, Anuar D, Soo R, et al. Detection of epidermal growth factor receptor variations by partially denaturing HPLC. Clin Chem. 2007;53(1):62–70. doi: 10.1373/clinchem.2006.074831. [DOI] [PubMed] [Google Scholar]
- 28.Cohen V, Agulnik JS, Jarry J, et al. Evaluation of denaturing high-performance liquid chromatography as a rapid detection method for identification of epidermal growth factor receptor mutations in nonsmall-cell lung cancer. Cancer. 2006;107(12):2858–2865. doi: 10.1002/cncr.22331. [DOI] [PubMed] [Google Scholar]
- 29.Wu S, Zhu Z, He J, Luo X, Xu J, Ren-Heidenreich L. A novel mutant-enriched liquidchip technology for the qualitative detection of somatic mutations in KRAS gene from both serum and tissue samples. Clin Chem Lab Med. 2010;48(8):1103–1106. doi: 10.1515/CCLM.2010.227. [DOI] [PubMed] [Google Scholar]
- 30.Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23(11):2513–2520. doi: 10.1200/JCO.2005.00.992. [DOI] [PubMed] [Google Scholar]
- 31.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 32.Maemondo M, Inoue A, Kobayashi K, et al. North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 33.Goto K, Ichinose Y, Ohe Y, et al. Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J Thorac Oncol. 2012;7(1):115–121. doi: 10.1097/JTO.0b013e3182307f98. [DOI] [PubMed] [Google Scholar]
- 34.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359(4):366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]