Abstract
A common process associated with oxidative stress and severe mitochondrial impairment is the opening of the mitochondrial permeability transition pore, as described in many neurodegenerative diseases. Thus, inhibition of mitochondrial permeability transition pore opening represents a potential target for inhibiting mitochondrial-driven cell death. Among the mitochondrial permeability transition pore components, cyclophilin D is the most studied and has been found increased under pathological conditions. Here, we have used in vitro and in vivo models of X-linked adrenoleukodystrophy to investigate the relationship between the mitochondrial permeability transition pore opening and redox homeostasis. X-linked adrenoleukodystrophy is a neurodegenerative condition caused by loss of function of the peroxisomal ABCD1 transporter, in which oxidative stress plays a pivotal role. In this study, we provide evidence of impaired mitochondrial metabolism in a peroxisomal disease, as fibroblasts in patients with X-linked adrenoleukodystrophy cannot survive when forced to rely on mitochondrial energy production, i.e. on incubation in galactose. Oxidative stress induced under galactose conditions leads to mitochondrial damage in the form of mitochondrial inner membrane potential dissipation, ATP drop and necrotic cell death, together with increased levels of oxidative modifications in cyclophilin D protein. Moreover, we show increased expression levels of cyclophilin D in the affected zones of brains in patients with adrenomyeloneuropathy, in spinal cord of a mouse model of X-linked adrenoleukodystrophy (Abcd1-null mice) and in fibroblasts from patients with X-linked adrenoleukodystrophy. Notably, treatment with antioxidants rescues mitochondrial damage markers in fibroblasts from patients with X-linked adrenoleukodystrophy, including cyclophilin D oxidative modifications, and reverses cyclophilin D induction in vitro and in vivo. These findings provide mechanistic insight into the beneficial effects of antioxidants in neurodegenerative and non-neurodegenerative cyclophilin D-dependent disorders.
Keywords: Cyclophilin D, mitochondrial permeability transition pore, neurodegenerative disease, oxidative stress, X-linked adrenoleukodystrophy
Introduction
Oxidative stress has been shown to participate in the onset and/or progression of several human neurodegenerative diseases (Lin and Beal, 2006; Pratico, 2008; Stack et al., 2008; Zhou et al., 2008; Martinez et al., 2010). Compelling evidence indicates that excessive levels of radical oxygen species can modify or deactivate protein activity or even alter its conformation (Wang et al., 2012). Oxidative stress also provokes mutations in mitochondrial and nuclear DNA, causing mitochondrial dysfunction and cell death (Pamplona and Barja, 2007).
Mitochondria are organelles playing a pivotal role in orchestrating cell survival and death, and their optimal functioning is required to preserve cell and organism viability (Lin and Beal, 2006; Du and Yan, 2010; Fernandez-Checa et al., 2010; Blackstone and Chang, 2011). Nonetheless, mitochondria are main targets of oxidative stress. In pathological conditions, there are increasing probabilities of mitochondrial permeability transition pore formation, triggering an irrevocable necrotic cell death (Petronilli et al., 1994; Baines et al., 2005; Halestrap, 2005; Nakagawa et al., 2005). It is well established that once the mitochondrial permeability transition pore is formed, a non-selective, high conductance pore allowing the flux of solutes up to 1.5 kDa across the inner mitochondrial membrane is opened. This results in the dissipation of mitochondrial membrane potential and damage to the mitochondrial respiratory chain, thereby reducing ATP production and exacerbating mitochondrial dysfunction. Moreover, mitochondrial osmotic swelling leads to ruptures in outer mitochondrial membrane triggering irreversible cell death (Baines et al., 2005; Bernardi et al., 2006; Du and Yan, 2010). Although the protein composition of mitochondrial permeability transition pore remains elusive, different components have been proposed, including the voltage-dependent anion channel, the adenine nucleotide translocator, the mitochondrial phosphate carrier and the cyclosporin A target cyclophilin D (Halestrap, 2009). Genetic ablation studies have confirmed that cyclophilin D is an essential component of mitochondrial permeability transition pore (Baines et al., 2005; Nakagawa et al., 2005; Schinzel et al., 2005), although formal proof is lacking for the rest of the above-mentioned candidates. In this regard, blockade of cyclophilin D by genetic abrogation or pharmacological inhibition protects mitochondria in many age-related neurodegenerative disease models, such as Alzheimer’s disease (Du et al., 2008), Parkinson’s disease (Gandhi et al., 2009; Thomas et al., 2011), amyotrophic lateral sclerosis (Karlsson et al., 2004; Martin et al., 2009; Martin, 2010) and multiple sclerosis (Forte et al., 2007).
Oxidative stress is a well-established inducer of mitochondrial permeability transition pore formation (Baines et al., 2005; Halestrap, 2005; Du et al., 2008; Martin et al., 2009), but little is known about the molecular mechanisms involved in this process. For this reason, we chose to study cellular and in vivo models of X-linked adrenoleukodystrophy (McKusick No.300100), a rare neurometabolic disease in which oxidative stress is the main early contributing pathogenic factor (Fourcade et al., 2008). X-linked adrenoleukodystrophy is the most frequent peroxisomal disorder (minimum incidence 1:17 000 males) characterized by progressive demyelination within the CNS, adrenal insufficiency and a pathognomonic accumulation of very long-chain fatty acids (C ≥ 22:0) in plasma and tissues, in particular hexacosanoic acid, C26:0 (Powers et al., 2000; Moser, 2001; Ferrer et al., 2010). This inheritable disorder is caused by mutations in the ABCD1 gene encoding the homonymous ATP-binding cassette peroxisomal transporter, which is involved in the import of very long-chain fatty acids, and very long-chain fatty acids–coenzyme A esters into the peroxisome for degradation (Hettema et al., 1996; van Roermund et al., 2008).
Classical inactivation of Abcd1 in the mouse results in late onset neurodegeneration with axonopathy in spinal cord, in the absence of inflammatory demyelination in the brain, resembling the most frequent X-linked adrenoleukodystrophy phenotype or adrenomyeloneuropathy (Forss-Petter et al., 1997; Lu et al., 1997; Pujol et al., 2002, 2004). Oxidative damage has been shown in post-mortem brain samples from individuals with cerebral X-linked adrenoleukodystrophy (Gilg et al., 2000) and in mouse spinal cords well before disease onset (Fourcade et al., 2008). Further, we recently reported that a combination of antioxidants halts clinical progression and reverses axonal damage in the X-linked adrenoleukodystrophy mouse model, thereby providing formal conceptual proof that oxidative injury is a major aetiopathogenic factor in this disease (Singh and Pujol, 2010; Lopez-Erauskin et al., 2011; Galea et al., 2012). These findings are currently being translated in a clinical trial for patients with adrenomyeloneuropathy (NCT01495260).
Our present study uncovers a functional impairment of mitochondria in X-linked adrenoleukodystrophy fibroblasts, underscoring the essential role of mitochondrial permeability transition pore in cell death. Further, we present novel molecular events underlying oxidative stress-induced mitochondrial permeability transition pore formation in X-linked adrenoleukodystrophy and molecular actions of antioxidants on cyclophilin D. This highlights the rationale for using these types of agents as a therapeutic option against mitochondrial permeability transition pore opening, a cardinal event observed in most prevalent neurodegenerative diseases.
Materials and methods
Chemicals
The following chemicals were used: 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate diacetoxymethyl-ester (H2-DCFDA; Invitrogen), L-Buthionine-sulfoximine (Sigma), cyclosporin A (Calbiochem), FK506 (LC Laboratories), Hoechst 33342 (Sigma), monochlorobimane (Molecular Probes), N-acetylcysteine (Sigma), staurosporine (Sigma), tetramethylrhodamine ethyl ester (TMRE; Invitrogen) and Z-VAD, (Sigma).
Antibodies
The following antibodies were used for western blots: anti-caspase 3, dilution 1:1000, incubated overnight at 4°C (Cell Signaling catalogue number 9661 and 9662); cyclophilin D antibody (MitoScience) 1:1000 dilution; anti-gamma-tubulin [T6557, clone GTU-88 (Sigma)], dilution: 1:5000, incubated 1 h at room temperature; goat anti-rabbit Immunoglobulin G linked to horseradish peroxidase, dilution: 1:10 000 [81–6520 (Invitrogen)]; and goat anti-mouse Immunoglobulin G linked to horseradish peroxidase, dilution: 1:10 000 [81–6120 (Invitrogen)] used as secondary antibodies and incubated 1 h at room temperature.
Cell cultures and treatments
Human control and X-linked adrenoleukodystrophy fibroblasts were obtained after informed consent at the Bellvitge University Hospital. Cells were grown in Dulbecco’s modified Eagle medium containing 10% foetal bovine serum, 100 U/ml penicillin and 100 mg streptomycin, at 37°C in humidified 95% air/5% CO2, to 80–90% confluence. To perform our experiments, Dulbecco’s modified Eagle medium without d-glucose, pyruvate or l-glutamine was used. Cells were cultured in this medium supplemented with 1 g/l of glucose or 1 g/l of galactose and 10% foetal bovine serum. In these conditions, different drug effects were tested: general caspase inhibitor Z-VAD (1 µM), cyclosporin A (5 µM), FK506 (5 µM), N-acetylcysteine (1 mM), L-Buthionine-sulfoximine (500 µM) and staurosporine (1 µM for 14 h).
Evaluation of intracellular radical oxygen species
Intracellular radical oxygen species levels were estimated using the radical oxygen species-sensitive H2-DCFDA probe as described (Fourcade et al., 2008). Following incubation with 10 µM H2-DCFDA for 30 min, cells were washed twice with PBS and lysated with 1% Triton™. The fluorescence of H2-DCFDA-stained cells was measured with a spectrofluorimeter (excitation wavelength 493 nm, emission wavelength 527 nm).
Adenosine triphosphate measurement
At different time points (12, 24, 36 and 40 h) after changing the medium to galactose, ATP levels were measured by a chemiluminescence system using ATPlite 1step (PerkinElmer), according to the manufacturer’s protocol. ATP levels were normalized by total protein concentration.
Cell death measurements
After the indicated treatments, human fibroblasts viability was quantified using CytoTox-ONETM Homogeneous Membrane Integrity Assay (Promega), according to the manufacturer’s instructions.
Nuclear chromatin condensation
X-linked adrenoleukodystrophy fibroblasts nuclei were stained with Hoechst 33342 (10 µg/ml) as described (Lizard et al., 1995), and the nuclei were observed using confocal microscopy.
Evaluation of reduced glutathione
Reduced glutathione was estimated using monochlorobimane (excitation wave length 360 nm, emission wave-length 460 nm) as described (Fourcade et al., 2008).
Inner mitochondrial membrane potential quantification by flow cytometry
Treated cells were washed with PBS and incubated with 50 nM of TMRE (Molecular Probes) in pre-warmed PBS for 30 min at 37°C. Cells were trypzinized, centrifuged at 1000 g for 5 min and resuspended in pre-warmed PBS. All samples were captured in a FACSCantoTM recording 20 000 cells for each condition and genotype tested. Histograms showing the percentage of depolarized cells were obtained after gating live cells. Data were analysed with FlowJo Tree Star software.
Mono-dimensional electrophoresis and western blotting
After the required treatments, control and X-linked adrenoleukodystrophy fibroblast samples were trypsinized and washed twice with PBS. Tissues samples were removed from euthanized mice and flash frozen in liquid nitrogen. Frozen tissue samples were homogenized in radioimmunoprecipitation assay buffer using a motor-driven grinder (Sigma-Aldrich) and then sonicated for 2 min at 4°C in an ultrasonic processor UP50H (Hielscher-Ultrasound Technology). Fibroblast samples were also sonicated for 2 min at 4°C in the ultrasonic processor UP50H. Both kinds of samples were centrifuged at 1000g for 10 min at 4°C, and supernatant protein concentration was measured with BCA Protein Assay Reagent (Pierce/Thermo). Samples (10–100 µg) were loaded onto each lane of 10–15% polyacrylamide gels for 70 min at 120 mV. Resolved proteins were transferred to nitrocellulose. Secondary antibody for protein detection was conjugated with horseradish peroxidase, detected with chemiluminescence enhancer Luminol and followed by exposure to CL-XPosure™ Film (Thermo Scientific). Autoradiographs were scanned and quantified using GS800 densitometer (Bio-Rad).
Electron microscopy
The medium and the cells cultured in the required conditions were collected and centrifuged at 1500g for 10 min to obtain the pellet of all the cells in the culture. The pellets were washed with PBS and centrifuged again at 1500g for 10 min. Supernatants were discarded and pellets were fixed with 2% paraformaldehyde/2% glutaraldehyde in 0.1 M PBS for 1 h at room temperature. The pellets were washed three times with 0.1 M PBS and maintained in paraformaldehyde 2% in 0.1 M PBS and then post-fixed by immersion in 1% osmium tetroxide, embedded in EPON-812 and cut with an ultramicrotome. Ultrathin sections were stained with toluidine blue, and finally selected sections were collected on copper grids and stained with uranyl acetate and Reynold’s lead citrate. Sections were directly visualized with a Jem-1011 transmission electron microscope (Jeol).
Bidimensional electrophoresis and western blotting
Redox proteomics for the identification of oxidized cyclophilin D in human X-linked adrenoleukodystrophy fibroblasts were performed as previously described (Galino et al., 2011) with some modifications. After the first dimension steps, the equilibrated strips were loaded onto 15% SDS-PAGE gels (20 × 20 cm) and run at 250 V for 4.5 h at room temperature. Bidimensional gels were performed in parallel, one for Coomassie staining and the other to be transferred to nitrocellulose membrane. To detect oxidized proteins (oxyblot), the membrane was incubated with antibody against 2,4-dinitrophenylhydrazine (D9659, Sigma, dilution 1:500) (Robinson et al., 1999). For immunodetection of cyclophilin D, the same membrane was incubated with antibody against cyclophilin D (Molecular Probes). For Coomassie staining, bidimensional gels were fixed for 1 h in a 30% ethanol/70% glacial acetic acid solution. Then, the fixing solution was replaced with Coomassie solution (Brilliant Blue R, B0149, Sigma) and incubated overnight at room temperature. After gel washing, the cut spots were digested, and peptides were analysed with mass spectrometry as previously reported (Galino et al., 2011). The Swiss-Prot database was used to identify proteins from a spectrum generated by liquid chromatography coupled to tandem mass spectrometry (Supplementary Table S1). Briefly, we applied a taxonomy filter (Homo sapiens) and obtained 20 407 sequences that were used to match with our experimental data using a significance threshold P < 0.05. We obtained an identity score of 34, which lies under the score provided by the liquid chromatography coupled to tandem mass spectrometry (46.5), thus providing high reliability to the identification.
Human brain samples
Brain tissues from patients with cerebral adrenomyeloneuropathy and healthy age matched male control subjects were obtained from the Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, as described (Schluter et al., 2012). Informed written consent was obtained from all patients or their legal representatives, and the local ethics committee approved the studies.
Mouse breeding
The generation and genotyping of Abcd1- mice has previously been described (Lu et al., 1997; Pujol et al., 2002, 2004). Mice used for experiments were of a pure C57BL/6 J background and were all male. Animals were sacrificed, and tissues were recovered and conserved at –80°C. All methods used in this study were in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publications No. 85–23, revised 1996) and with the ethical committee of The Bellvitge Institute of Biomedical Research and the government of Catalonia.
Treatment of mice
α-Lipoic acid (0.5% w/w) was mixed into AIN-76A chow from Dyets. N-acetylcysteine (1%) was dissolved in water (pH 3.5) (Lopez-Erauskin et al., 2011). Eight-month-old animals were randomly assigned to one of the following dietary groups for 4 months. Group I: wild-type mice (n = 8) received only normal AIN-76A chow; Group II: Abcd1− mice (n = 8) received only normal AIN-76A chow; and Group III: Abcd1− + antioxidant mice (n = 6) were treated with chow containing α-lipoic acid and with N-acetylcysteine in drinking water (Lopez-Erauskin et al., 2011).
Statistical analysis
Data are given as mean ± standard deviation. Significant differences were determined by one-way ANOVA followed by Tukey’s Honestly Significant Difference post-test after verifying normality (*P< 0.05, **P< 0.01, ***P< 0.001) or Student’s t-test (*P< 0.05, **P< 0.01, ***P< 0.001). Statistical analyses were performed using SPSS 12.0 program (SPSS Inc.).
Results
X-linked adrenoleukodystrophy fibroblasts display mitochondrial impairment
Mitochondrial dysfunction has been reported in a wide array of diseases characterized by oxidative stress, energy failure, inner mitochondrial membrane depolarization and cell death (Lin and Beal, 2006; Du et al., 2008; Thomas et al., 2011). Recent evidence obtained in our laboratory suggested energetic failure with reduced NADH and ATP levels, as a result of oxidation of key components of tricarboxylic acid and glycolysis in Abcd1− spinal cords (Galino et al., 2011).
As a means to directly assess mitochondria function within intact cells, we cultured human X-linked adrenoleukodystrophy fibroblasts, usually grown in 1 g/l of glucose medium, in 1 g/l galactose medium. This is a common practice applied in molecular genetics laboratories as a diagnostic tool when a mitochondriopathy is suspected. Cells grown in glucose produce their ATP by glycolysis, largely bypassing the mitochondria. However, when the same cells are grown in media supplemented with galactose as opposed to glucose, they are forced to produce ATP through oxidative phosphorylation, as galactose is used in the glycolytic pathway at a much slower rate than glucose (Robinson et al., 1992; Hofhaus et al., 1996; Ghelli et al., 2003; Acin-Perez et al., 2009). If mitochondrial damage exists, cell viability is compromised.
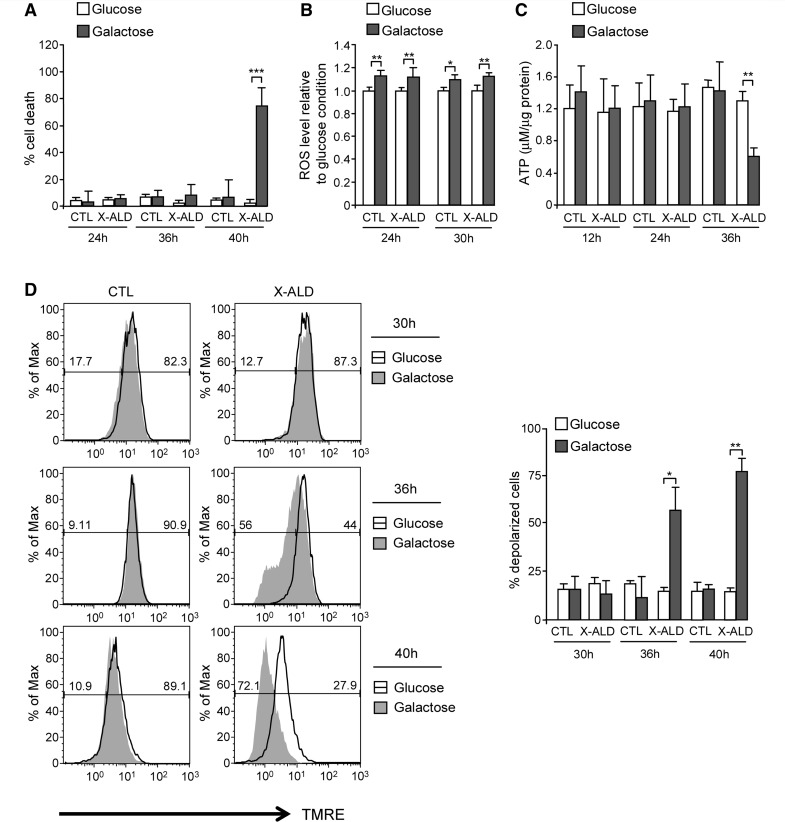
Indeed, we observed that galactose induced X-linked adrenoleukodystrophy fibroblast cell death at 40 h, but not at earlier times (e.g. 24 and 36 h), indicating mitochondrial dysfunction in these cells (Fig. 1A).
Figure 1.
Mitochondrial dysfunction in X-linked adrenoleukodystrophy fibroblasts. Cell death (A), intracellular radical oxygen species levels (B), ATP content (C) and inner mitochondrial membrane potential (D) were quantified in control and X-linked adrenoleukodystrophy fibroblasts cultured in presence of 1 g/l glucose or glucose-free medium containing 1 g/l galactose (*P < 0.05; **P < 0.01; ***P < 0.001; n = 4/genotype and condition). In D, the numeric values above the line correspond to the percentage of polarized cells on the right-hand and depolarized cells on the left-hand of each panel. CTL = control; ROS = radical oxygen species; X-ALD = X-linked adrenoleukodystrophy.
To dissect the events occurring before cell death, we measured the level of radical oxygen species and ATP content as described (Fourcade et al., 2008; Galino et al., 2011; Lopez-Erauskin et al., 2011). Also, we measured inner mitochondrial membrane potential with TMRE and analysed the results by flow cytometry. TMRE is a voltage-sensitive red–orange fluorescent indicator for mitochondrial transmembrane potential (Zhou et al., 2010). Mitochondrial depolarization (disrupting or decreasing membrane potential) results in a loss of dye from the mitochondria and a decrease in fluorescence intensity.
We observed that culture in galactose provoked (i) an increase in intracellular radical oxygen species level compared with glucose conditions, both in control and X-linked adrenoleukodystrophy fibroblasts (Fig. 1B); (ii) energetic failure in X-linked adrenoleukodystrophy fibroblasts as shown by lowered levels of ATP (Fig. 1C); and (iii) depolarization in X-linked adrenoleukodystrophy fibroblasts (Fig. 1D). All these events preceded cell death. However, once cell death was observed (40 h), reactive oxygen species and ATP levels dramatically dropped (Supplementary Fig. 1A and B), and the inner mitochondrial membrane potential was completely disrupted (Fig. 1D), coinciding with a shift of the whole cell population to a smaller size region (Supplementary Fig. 1C).
Galactose-induced cell death occurs independently of caspase activity
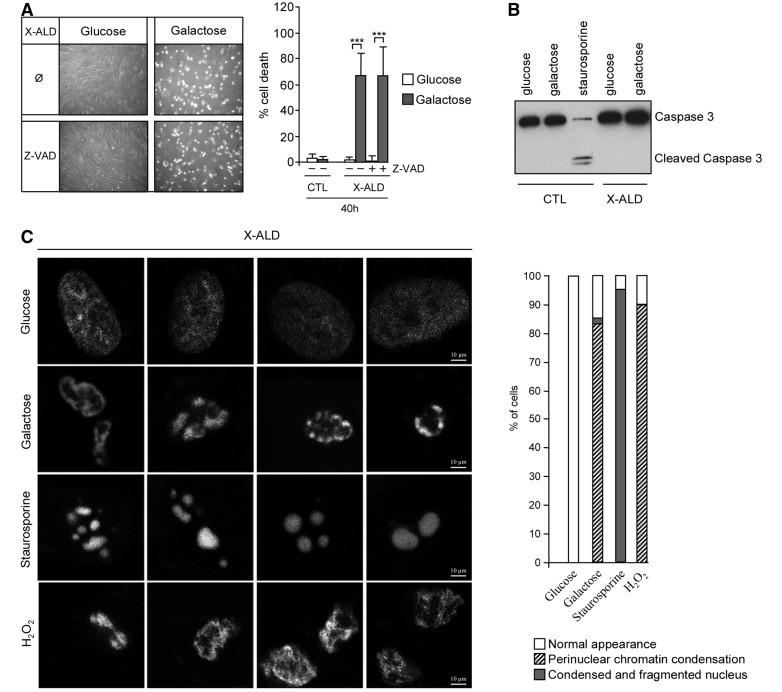
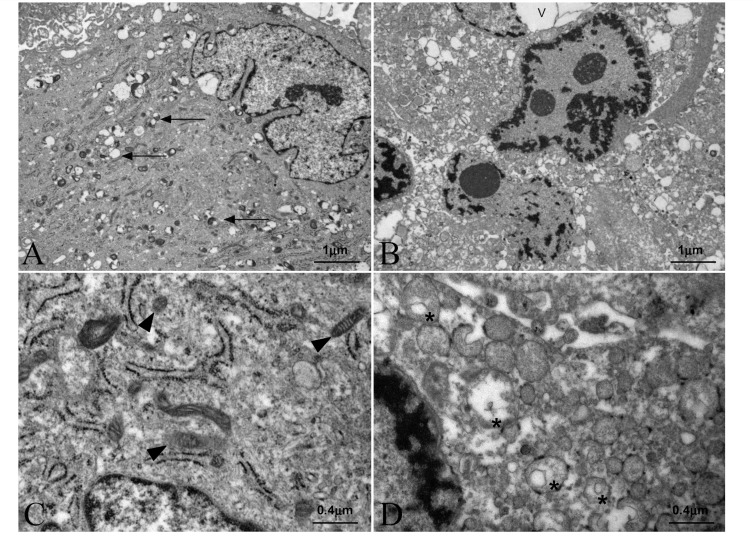
We next evaluated the type of cell death induced by galactose in X-linked adrenoleukodystrophy fibroblasts. To discriminate between types of cell demise, we inhibited caspase activation using the general caspase inhibitor Z-VAD. Interestingly, we observed no prevention of galactose-induced cell death (Fig. 2A). Moreover, galactose treatment did not generate cleavage of caspase 3, the caspase active form, in contrast with cleavage observed with staurosporine, a classical apoptosis inducer and caspase 3 activator (Fig. 2B). Further, we examined nuclear chromatin condensation using the Hoechst 33342 dye as described (Lizard et al., 1995). We thus compared galactose-induced chromatin condensation pattern with (i) H2O2-treated cells as positive control for necrotic cell death (Lee and Shacter, 2000) and (ii) staurosporine-treated cells as positive control for apoptotic cell death (Seo and Seo, 2009) (Fig. 2C). Galactose-induced nuclear morphology presented more similarities with H2O2-treated cells than with the classical apoptotic chromatin condensation phenomenon observed in staurosporine-treated cells. In addition, electron microscopy analysis of X-linked adrenoleukodystrophy fibroblasts treated with galactose showed that these cells had features of necrosis, such as vacuolization, and non-apoptotic chromatin condensation, instead of the classical apoptotic morphology (Fig. 3A–D). Collectively, these data support the notion that galactose induces necrotic cell death in X-linked adrenoleukodystrophy fibroblasts.
Figure 2.
Galactose induces necrotic cell death in X-linked adrenoleukodystrophy fibroblasts. Control and X-linked adrenoleukodystrophy fibroblasts cultured in glucose or galactose were treated with general caspase inhibitor Z-VAD, and cell death was measured (A). Cleaved caspase 3 was analysed in different conditions by western blot (B). Staurosporine (1 microM during 14 h) was used as a positive control of caspase 3 activator (***P < 0.001; n = 4/genotype and condition). The pattern of chromatin condensation induced by galactose was analysed using Hoescht 33342 staining (C). H2O2 (10 mM for 3 h) and staurosporine (2 µM for 6 h) were used as positive control for necrotic and apoptotic cell death, respectively. X-linked adrenoleukodystrophy fibroblasts of six different patients were analysed, and 25 cells per condition were scored. Representative pictures and quantification are shown. CTL = control; X-ALD = X-linked adrenoleukodystrophy.
Figure 3.
X-linked adrenoleukodystrophy fibroblasts show necrotic features during galactose-induced cell death. X-linked adrenoleukodystrophy fibroblasts were cultured in glucose (A) (shown at larger magnification in C); or in glucose-free medium containing galactose (B) (shown at larger magnification in D). After 40 h, cells were collected and analysed by electron microscopy. V = vacuole; arrows indicate lipid inclusions; arrowheads indicate morphologically normal appearing mitochondria; asterisks indicate swollen mitochondria (n = 4/condition).
Galactose-induced cell death is oxidative stress dependent
We have previously shown that a cocktail of antioxidants, including N-acetylcysteine, α-lipoic acid and vitamin E, or N-acetylcysteine and α-lipoic acid alone, reversed oxidative damage to proteins and DNA, mitochondrial energetic metabolism impairment, immunohistological signs of axonal degeneration and associated locomotor disability in an X-linked adrenoleukodystrophy mouse model (Galino et al., 2011; Lopez-Erauskin et al., 2011).
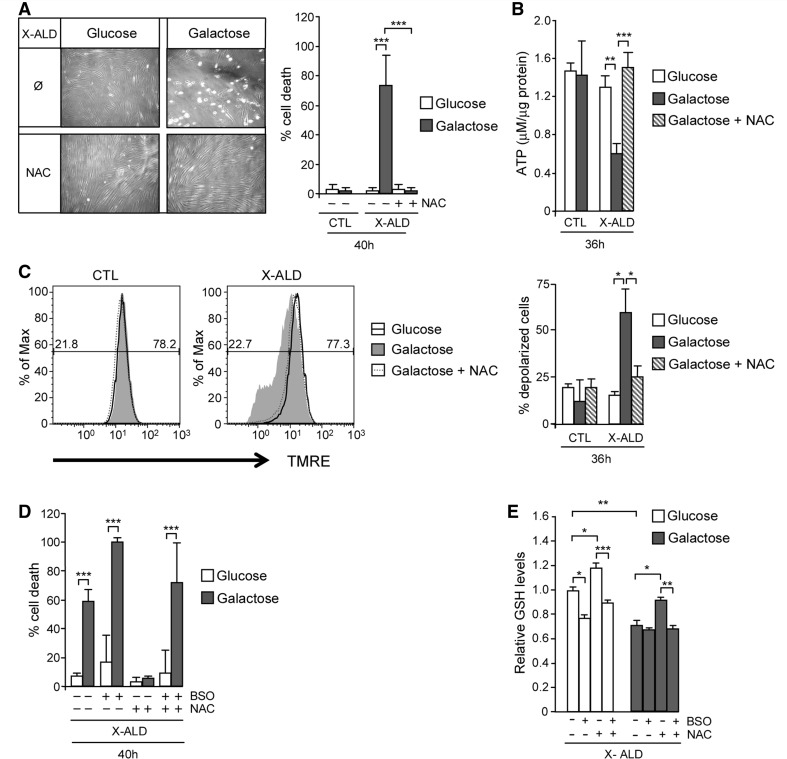
To evaluate whether oxidative stress plays a role in galactose-induced cell death, we cultured X-linked adrenoleukodystrophy fibroblasts in the presence of galactose and the antioxidant N-acetylcysteine. The treatment induced cell survival (Fig. 4A), led to the recovery of normal levels of ATP (Fig. 4B), and reversed mitochondrial depolarization (Fig. 4C), demonstrating that oxidative stress plays a central role in the mitochondrial dysfunction observed under these conditions.
Figure 4.
X-linked adrenoleukodystrophy fibroblast cell death and metabolic failure in galactose is oxidative stress dependent. Control and X-linked adrenoleukodystrophy fibroblasts cultured in glucose or galactose were treated with 1 mM of N-acetylcysteine. Cell death (A), ATP content (B) and inner mitochondrial membrane potential (C) were measured. In C, the numeric values above the line correspond to the percentage of polarized cells on the right-hand and depolarized cells on the left-hand of each panel. Fibroblasts cell death was quantified under the same conditions with L-buthionine-sulfoximine (500 µM) treatment (D). Reduced glutathione amount was quantified fluorimetrically using monochlorobimane in X-linked adrenoleukodystrophy fibroblasts cultured in glucose or in galactose under N-acetylcysteine and/or L-buthionine-sulfoximine treatments (E) (*P < 0.05; **P < 0.01; ***P < 0.001; n = 4/condition). BSO = L-buthionine-sulfoximine; CTL = control; GSH = reduced glutathione; NAC = N-acetylcysteine; X-ALD = X-linked adrenoleukodystrophy.
We and others have previously reported that C26:0 excess provokes decrease of the reduced glutathione (Fourcade et al., 2008; Baarine et al., 2012b), the major antioxidant barrier of the cell (Harvey et al., 2008). Considering that N-acetylcysteine is the precursor of reduced glutathione, we wondered whether the positive effect observed with N-acetylcysteine was due to regeneration of reduced glutathione, or alternatively, due to other effects (anti-oxidant or other) of N-acetylcysteine. To answer this question, we used L-buthionine-sulfoximine, a specific inhibitor of the key enzyme of reduced glutathione synthesis, the γ-gluthamyl-cysteine-syntetase enzyme, as described (Fourcade et al., 2008). Galactose-induced cell death of X-linked adrenoleukodystrophy fibroblasts was not prevented when L-buthionine-sulfoximine was applied together with N-acetylcysteine (Fig. 4D). Further, we measured reduced glutathione using monochlorobimane and confirmed that cell survival was correlated with reduced glutathione amount (Fig. 4E). Taken together, our data indicate that reduced glutathione synthesis is required to prevent cell death, and, thus, oxidative stress plays a paramount role in this process.
Mitochondrial permeability transition pore opening underlies galactose-induced cell death in X-linked adrenoleukodystrophy fibroblasts
Mitochondria play a critical role in initiating both apoptotic and necrotic cell death. A major player in necrosis is the mitochondrial permeability transition pore, a non-specific pore that opens in the inner mitochondrial membrane promoting massive swelling of mitochondria, drop of membrane potential, rupture of the outer membrane and cell death (Halestrap et al., 2002; Baines et al., 2005; Bernardi et al., 2006; Du and Yan, 2010). When mitochondria become depolarized, inhibition of oxidative phosphorylation and stimulation of ATP hydrolysis occur (Du et al., 2008; Halestrap, 2009).
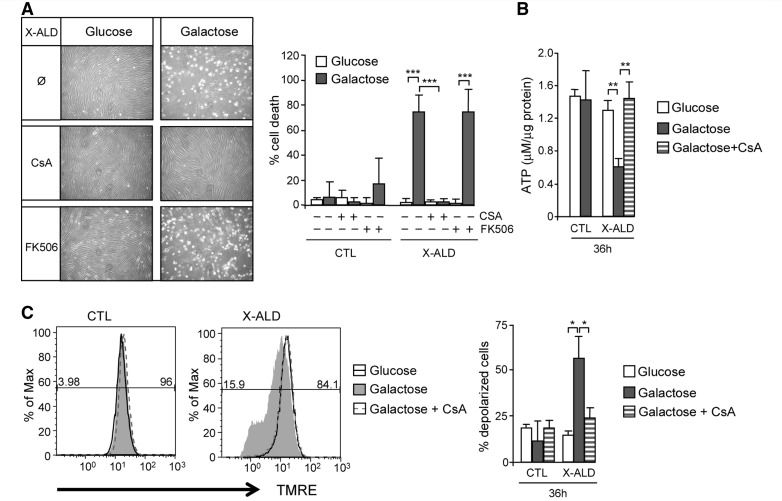
To study a potential implication of mitochondrial permeability transition pore opening in galactose-induced cell death in X-linked adrenoleukodystrophy fibroblasts, we used cyclosporin A, a clinically used immunosuppressive agent, which among other properties, acts to inhibit mitochondrial permeability transition pore opening through its binding to mitochondrial matrix protein cyclophilin D (Crompton et al., 1988; Halestrap and Davidson, 1990).
Cyclophilin D is the only existent mitochondrial peptidyl-prolyl cis-trans isomerase that under pathological conditions is translocated to the inner mitochondrial membrane, where it is perceived to interact with the adenine nucleotide translocator inducing pore formation (Connern and Halestrap, 1994; Crompton et al., 2002; Halestrap et al., 2002; Zamzami et al., 2005; Halestrap, 2006).
We observed that cyclosporin A prevented galactose-induced X-linked adrenoleukodystrophy fibroblasts cell death (Fig. 5A). However, considering that cyclosporin A can also inhibit calcineurin, we used the compound FK506 that inhibits calcineurin activity, but shows no binding to cyclophilin D, as a negative control (Friberg et al., 1998). The treatment of X-linked adrenoleukodystrophy fibroblasts with FK506 compound did not prevent galactose-induced cell death, indicating that induction of cell death occurs through mitochondrial permeability transition pore formation (Fig. 5A).
Figure 5.
Galactose-induced mitochondrial dysfunction and cell death in X-linked adrenoleukodystrophy fibroblasts is caused by mitochondrial permeability transition pore opening. Control and X-linked adrenoleukodystrophy fibroblasts cultured in glucose or galactose were treated with 5 µM of cyclosporin A or 5 µM of FK506, and cell death was measured (A). In the same conditions with cyclosporin A treatment, ATP content (B) and inner mitochondrial membrane potential (C) were quantified (*P < 0.05; **P < 0.01; ***P < 0.001; n = 4/genotype and condition). In C, the numeric values above the line correspond to the percentage of polarized cells on the right-hand and depolarized cells on the left-hand of each panel. CsA = cyclosporin A; CTL = control; X-ALD = X-linked adrenoleukodystrophy.
In addition, the presence of cyclosporin A counteracts reduction in ATP (Fig. 5B) and inner mitochondrial membrane potential dissipation (Fig. 5C) induced by galactose in X-linked adrenoleukodystrophy fibroblasts. Moreover, we observed mitochondrial swelling in galactose-treated X-linked adrenoleukodystrophy fibroblasts by electron microscopy preparations (Fig. 3B and D). Taken together, these data indicate that mitochondrial permeability transition pore opening underlies the cell death of X-linked adrenoleukodystrophy fibroblasts on galactose.
Cyclophilin D expression is modulated by oxidative stress in X-linked adrenoleukodystrophy in vitro and in vivo
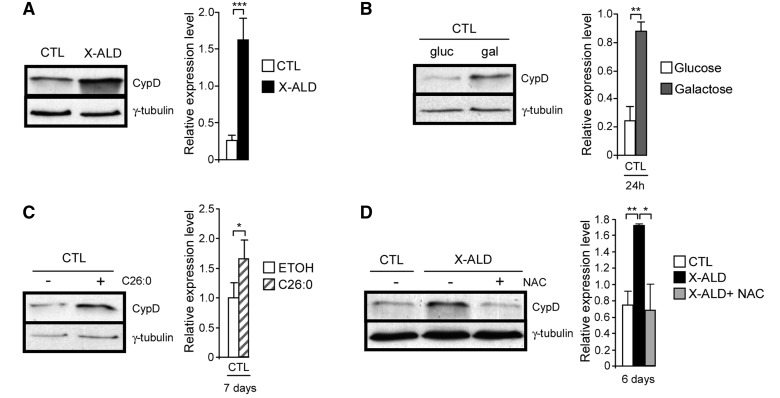
Cyclophilin D is the only firmly established mitochondrial permeability transition pore component and plays an essential role in its formation (Baines et al., 2005; Nakagawa et al., 2005; Schinzel et al., 2005). As accumulation of cyclophilin D under pathological conditions in several disorders and in ageing has been described (Du et al., 2008; Gandhi et al., 2009; Martin, 2010), we decided to study cyclophilin D expression in X-linked adrenoleukodystrophy fibroblasts. We found more marked cyclophilin D expression in X-linked adrenoleukodystrophy compared with control fibroblasts at baseline levels, when cells were cultured in glucose (Fig. 6A). Galactose incubation (Fig. 6B) and incubation with the very long-chain fatty acid C26:0 (Fig. 6C) induced cyclophilin D overexpression in control fibroblasts. Treatment with the antioxidant N-acetylcysteine reduced cyclophilin D to control levels in X-linked adrenoleukodystrophy fibroblasts (Fig. 6D).
Figure 6.
Cyclophilin D expression is modulated by oxidative stress. Cyclophilin D levels were quantified in control and X-linked adrenoleukodystrophy fibroblasts at basal levels (A), in control fibroblasts after culture in galactose for 24 h (B) and with C26:0 (50 µM) for 7 days (C), and after treating X-linked adrenoleukodystrophy fibroblasts with N-acetylcysteine (0.5 mM) for 6 days (D) (*P < 0.05; **P < 0.01; ***P < 0.001; n = 5/genotype and condition). CTL = control; CypD = cyclophilin D; ETOH = ethanol; NAC = N-acetylcysteine; X-ALD = X-linked adrenoleukodystrophy.
Cyclophilin D is oxidized in X-linked adrenoleukodystrophy human fibroblasts, and N-acetylcysteine counteracts this effect
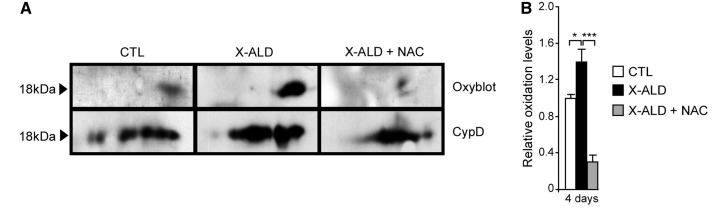
We earlier reported an increase in oxidized proteins in Abcd1- mice spinal cord and in X-linked adrenoleukodystrophy fibroblasts (Fourcade et al., 2008; Galino et al., 2011; Lopez-Erauskin et al., 2011). Wondering whether cyclophilin D could also be oxidized in X-linked adrenoleukodystrophy, we performed redox proteomic experiments with human fibroblasts. We observed a significant increase in cyclophilin D oxidation in X-linked adrenoleukodystrophy fibroblasts (Fig. 7A and B). Interestingly, antioxidant treatment with N-acetylcysteine reversed cyclophilin D oxidation (Fig. 7A and B). These data suggest a plausible molecular mechanism underlying the beneficial action of antioxidants in mitochondrial permeability transition pore opening, which deserves further testing in relevant models (Petrosillo et al., 2009; Greco et al., 2011; Loor et al., 2011).
Figure 7.
Antioxidant treatment halts cyclophilin D oxidation in X-linked adrenoleukodystrophy fibroblasts. Oxyblot and western blot with specific antibody against cyclophilin D were performed to compare control and X-linked adrenoleukodystrophy fibroblasts treated or not with 1 mM of N-acetylcysteine. Three independent experiments were performed for each condition. Representative blots are shown (A). Relative protein oxidation levels were quantified and expressed as a percentage of control and referred to cyclophilin D expression (B) (*P < 0.05; ***P < 0.001; n = 3/genotype and condition). CTL = control; CypD = cyclophilin D; NAC = N-acetylcysteine; X-ALD = X-linked adrenoleukodystrophy.
Cyclophilin D expression is increased in patients with X-linked adrenoleukodystrophy and in a mouse model of X-linked adrenoleukodystrophy, and its expression is reduced by antioxidants in vivo
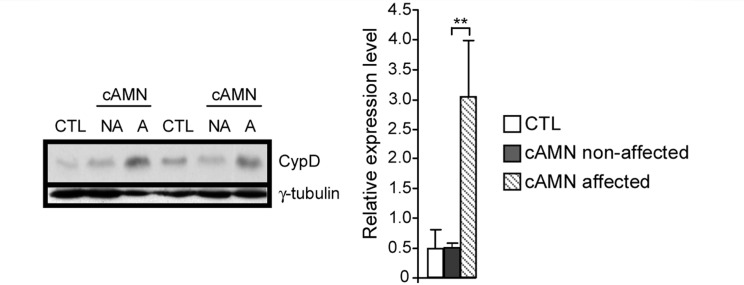
Evidence for oxidative damage underlying neurodegeneration in X-linked adrenoleukodystrophy patient brains abounds (Gilg et al., 2000; Powers, 2005; Fourcade et al., 2008; Ferrer et al., 2010; Galea et al., 2012). We therefore assessed cyclophilin D expression in the brains of patients with cerebral adrenomyeloneuropathy. We found accumulation of cyclophilin D in the affected white matter areas (Fig. 8), although no significant differences in the non-demyelinated areas were found.
Figure 8.
Cyclophilin D is accumulated in affected white matter brain zones of cerebral adrenomyeloneuropathy patients. Cyclophilin D levels were analysed in control and in different areas of brains in patients with cerebral adrenomyeloneuropathy (**P < 0.01; n = 4/genotype and condition). A = affected; cAMN = cerebral adrenomyeloneuropathy; CTL = control; CypD = cyclophilin D; NA = non-affected.
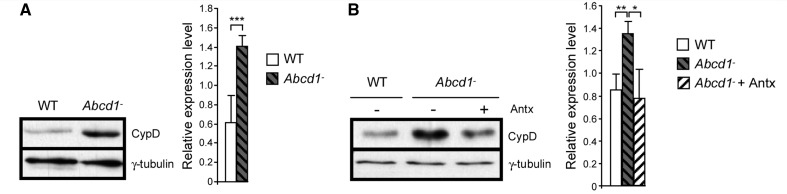
The mouse model of X-linked adrenoleukodystrophy presents long tract axonopathy in spinal cords, along with microgliosis and astrocytosis, accompanied by peripheral nerve conduction impairment and motor disability ∼20 months of age, thus allowing for a wide window for monitoring the pathogenic cascade and for therapeutic intervention (Pujol et al., 2002, 2004). However, oxidative damage to proteins appears early, ∼3 months of age (Fourcade et al., 2008). No changes in cyclophilin D levels were observed at this early age in wild-type and Abcd1- mice spinal cord (Supplementary Fig. 2A). However, later in time, but before disease onset, at 8 months of age, we observed increased cyclophilin D levels when compared with wild-type littermates (Fig. 9A). In addition, cyclophilin D levels were unchanged in brain cortex and liver of 8-month-old mice (Supplementary Fig. 2B and C), suggesting that cyclophilin D expression induction is target-organ specific and precedes axonal degeneration.
Figure 9.
Antioxidant treatment prevented cyclophilin D increase in Abcd1− mice spinal cord. Cyclophilin D expression levels were quantified in wild-type and Abcd1− mice spinal cord at 8 months (A) and at 12 months after an antioxidant cocktail treatment (B) (*P < 0.05; **P < 0.01; ** P < 0.001; n = 5/genotype and condition). Antx = antioxidants; CypD = cyclophilin D; WT = wild-type.
We have previously shown that a combination of antioxidants, including N-acetylcysteine and α-lipoic acid, reversed metabolic failure and axonal degeneration in an X-linked adrenoleukodystrophy mouse model (Galino et al., 2011; Lopez-Erauskin et al., 2011). To investigate whether these antioxidants were effective counteracting cyclophilin D overexpression, we treated 8-month-old Abcd1− mice with a combination of N-acetylcysteine and α-lipoic acid for 4 months and found that the levels of cyclophilin D were normalized by antioxidants (Fig. 9B).
Discussion
Neurodegenerative disorders are often linked to oxidative stress and mitochondrial dysfunction, including pathological mitochondrial permeability transition pore opening (Lin and Beal, 2006; Su et al., 2009). In the present report, we have uncovered novel links between oxidative stress, mitochondrial depolarization, ATP loss and ensuing cell death in X-linked adrenoleukodystrophy. We found that enforcement of mitochondrial metabolism by culturing X-linked adrenoleukodystrophy fibroblasts in galactose was sufficient to promote cell death. Although control and X-linked adrenoleukodystrophy fibroblasts underwent oxidative stress under galactose conditions, only X-linked adrenoleukodystrophy fibroblasts displayed the loss of ATP and mitochondrial depolarization that preceded cell death, in an oxidative stress-dependent manner. These data suggest that X-linked adrenoleukodystrophy cells are particularly sensitive to oxidative stress, and that an excess of radical oxygen species production is responsible for the opening of the mitochondrial permeability transition pore and resulting cell death. We propose that high levels and/or increased oxidation of cyclophilin D mediate or contribute to this sensitivity. Moreover, we conclude that the oxidative stress that emerges from the accumulation of very long-chain fatty acids (Fourcade et al., 2008; Baarine et al., 2012a) triggers necrotic cell death in X-linked adrenoleukodystrophy fibroblasts. We show that this is associated with non-apoptotic chromatin condensation (absence of cells with totally condensed and/or fragmented nuclei), ATP drop and mitochondrial swelling, and it does not involve caspase activation. Along the same lines, and although formal proof for necrosis in neural tissue is lacking, we had formerly shown lack of caspase-3 activation in spinal cords of both Abcd1 null and Abcd1/Abcd2 null mice (Pujol et al., 2004). Interestingly, very long-chain fatty acids, such as C24:0 or C26:0, also provoke necrotic cell death in cultured oligodendrocytes (Kahn et al., 2011). These results offer the opportunity of future studies aiming to delve deeper into characterization of specific forms of necrosis, such as necroptosis. This is a form of cell death frequently associated with bioenergetic stress and which requires RIPK1 or RIPK3 proteins (Degterev et al., 2008).
The vulnerability of X-linked adrenoleukodystrophy fibroblasts to mitochondria-dependent energy production invites the speculation that specific neural cell types, which largely depend on mitochondria for their energy supply, may be preferentially exposed and jeopardized in vivo. Indeed, axons have high metabolic demands and unusual length that renders them susceptible to oxidative damage, ischaemia or mitochondrial defects, as seen in multiple sclerosis (Lassmann, 2011) or amyotrophic lateral sclerosis (Shi et al., 2010). It follows that malfunctioning mitochondria could be an important factor accounting for the long-tract axonopathy of spinal cords observed in patients with adrenomyeloneuropathy and the X-linked adrenoleukodystrophy mouse models.
Although the consequences of the mitochondrial permeability transition pore opening have been extensively studied in many laboratories, its exact molecular identity remains unknown. Knockout-based studies have confirmed that cyclophilin D plays an essential role regulating mitochondrial permeability transition pore opening and necrotic cell death, although cyclophilin D is neither a structural component of the pore nor a transmembrane protein (Baines et al., 2005; Nakagawa et al., 2005; Schinzel et al., 2005). Cyclophilin D, located in the mitochondrial matrix, has been suggested as helping in the correct folding of proteins (Matouschek et al., 1995). However, in response to pathological insults, such as calcium overload and/or oxidative stress, cyclophilin D is translocated to the inner mitochondrial membrane to induce mitochondrial permeability transition pore formation (Connern and Halestrap, 1994).
One major consequence of mitochondrial permeability transition pore opening is that the inner mitochondrial membrane no longer maintains a barrier to protons, which leads to dissipation of the proton motive force. The resulting uncoupling of oxidative phosphorylation not only prevents mitochondria from making ATP, and produces cytotoxic radical oxygen species, but also the proton-translocating ATP synthase goes into reverse, consuming ATP, due to futile cycles in an effort to restore the inner mitochondrial membrane potential, thereby further contributing to cellular damage (Halestrap, 2009; Zorov et al., 2009). In this study, we have demonstrated that oxidative stress leads to energetic failure and necrotic cell death mediated by opening of mitochondrial permeability transition pore, concomitant with cyclophilin D overexpression and oxidation, in fibroblasts of patients with X-linked adrenoleukodystrophy. Mitochondrial dysfunction characterized by ATP drop, inner mitochondrial membrane potential dissipation and cell death is prevented with both cyclosporin A (inhibitor of cyclophilin D) and the antioxidant N-acetylcysteine in this system. Taken together, these results show that (i) mitochondrial permeability transition pore opening is implicated in cell death and (ii) oxidative stress is the main inducer of mitochondrial permeability transition pore opening-induced cell death in X-linked adrenoleukodystrophy fibroblasts. Additionally, we have demonstrated that cyclophilin D is more markedly carbonylated in X-linked adrenoleukodystrophy fibroblasts, and that antioxidants reverse these oxidative modifications. Recently, cyclophilin D has been suggested as a redox sensor where oxidation would change its conformation, translocating it to the inner mitochondrial membrane and forming the pore (Linard et al., 2009; Nguyen et al., 2011). We therefore propose cyclophilin D as a key molecule in X-linked adrenoleukodystrophy physiopathology and a prime novel therapeutic target for intervention.
Given the positive correlation of cyclophilin D expression with mitochondrial permeability transition pore opening (Karlsson et al., 2004; Baines et al., 2005; Brown et al., 2006; Du et al., 2008), we predict that cells with increased expression of cyclophilin D may be primed for mitochondrial permeability transition pore formation and the ensuing consequences. Interestingly, we observed increased expression of cyclophilin D in X-linked adrenoleukodystrophy mouse model spinal cord and an induced cyclophilin D expression in control human fibroblasts after placing them on galactose or excess of C26:0, in an oxidative stress-dependent manner. Also, we found elevated cyclophilin D expression levels in affected zones in brains of patients with cerebral adrenomieloneuropathy, in a similar manner to the increased cyclophilin D levels that were observed in affected brain regions of patients with Alzheimer’s (Du et al., 2008) and Huntington’s disease (Shirendeb et al., 2011). Moreover, higher levels of cyclophilin D have been found preceding axonal degeneration of the Wallerian type (Barrientos et al., 2011).
Further, antioxidant treatment decreases cyclophilin D expression levels in both in vitro and in vivo models of X-linked adrenoleukodystrophy, which confirms that oxidative stress regulates cyclophilin D expression. In agreement with this, it has recently been reported that mitochondria-targeted antioxidants, such as MitoQ, and resveratrol significantly decrease cyclophilin D expression levels in primary hippocampal neuron cultures from amyloid-β precursor protein transgenic mice (Tg2576 line), protecting cells against amyloid-β toxicity (Manczak et al., 2010). Of note, we show here for the first time that chronic oral treatment with a combination of classical antioxidants is able to lower both cyclophilin D expression and oxidation levels in the CNS in vivo.
Our findings emphasize the importance of cyclophilin D as target for neurodegenerative diseases. Indeed, blockade of cyclophilin D by genetic abrogation or pharmacological inhibition protects mitochondria in many age-related chronic neurological disease models, such as Alzheimer’s disease (Du et al., 2008, 2011), Parkinson’s disease (Gandhi et al., 2009; Thomas et al., 2011), amyotrophic lateral sclerosis (Karlsson et al., 2004; Martin et al., 2009; Martin, 2010) and multiple sclerosis (Forte et al., 2007; Martin, 2010). The latter studies suggest that cyclophilin D-dependent mechanisms are critical in the neurodegenerative aspects of demyelinating and motor neuron diseases. Thus, molecules crossing blood–brain barrier able to target cyclophilin D expression or activity hold great promise in the treatment of cyclophilin D-dependent diseases of the nervous system, including X-linked adrenoleukodystrophy. As cyclosporin A and non-immunosuppressive derivatives show poor CNS penetration (Begley et al., 1990), drug modification and use of barrier-permeable delivery vehicles may need to be taken into account, as well as drug targeting to mitochondria (Camara et al., 2010).
In summary, the present study unveils cyclophilin D as a plausible effecter preventing axonal degeneration in X-linked adrenoleukodystrophy and as a molecular target for antioxidant actions. This emphasizes the rationale for mitochondria-targeted antioxidant strategies in other neurodegenerative and non-neurodegenerative cyclophilin D-dependent disorders.
Funding
The European Commission (FP7-241622), the European Leukodystrophy Association (ELA2009-036C5 and ELA2008-040C4 to A.P.; ELA 2010-020F1 to S.F. and A.P.), the COST action (BM0604 to A.P.), the Spanish Institute for Health Carlos III (FIS PI080991 and FIS PI11/01043 to A.P., FIS PI10/00104 to C.M.-P.), the Autonomous Government of Catalonia (2009SGR85 to A.P.), the Oliver's Army (to A.P.), the Spanish Institute for Health Carlos III (Miguel Servet program CP11/00080 to S.F.), the Department of Education, Universities, and Research of the Basque Country Government (BFI07.126 to J. L.-E.), the Bellvitge Biomedical Research Institute program of PhD-student fellowships (IDIBELL to J.G. and P.B.) and Fundació La Marató de TV3 (Marató TV3 111630 to C.M.-P.). The CIBER on Rare Diseases (CIBERER), and CIBER on Neurodegenerative Diseases (CIBERNED) are initiatives of the Spanish Institute for Health Carlos III.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors express their gratitude to Dr José C. Fernandez-Checa for helpful discussions; to the Brain and Tissue Bank for Developmental Disorders at the University of Maryland for supplying the case material used in the human studies and to Dr Joan Josep Bech at the Laboratory of Proteomics of the Institut de Recerca, Hospital Universitari Vall d'Hebron in Barcelona, led by Dr Francesc Canals, for the Mass Spectrometry analysis.
Glossary
Abbreviations
- H2-DCFDA
6-carboxy-2’,7’-dichlorodihydrofluorescein diacetate diacetoxymethyl-ester
- TMRE
tetramethylrhodamine ethyl ester
References
- Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–76. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarine M, Andreoletti P, Athias A, Nury T, Zarrouk A, Ragot K, et al. Evidence of oxidative stress in very long chain fatty acid - Treated oligodendrocytes and potentialization of ROS production using RNA interference-directed knockdown of ABCD1 and ACOX1 peroxisomal proteins. Neuroscience. 2012a;213:1–18. doi: 10.1016/j.neuroscience.2012.03.058. [DOI] [PubMed] [Google Scholar]
- Baarine M, Ragot K, Athias A, Nury T, Kattan Z, Genin EC, et al. Incidence of Abcd1 level on the induction of cell death and organelle dysfunctions triggered by very long chain fatty acids and TNF-alpha on oligodendrocytes and astrocytes. Neurotoxicology. 2012b;33:212–28. doi: 10.1016/j.neuro.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–62. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Barrientos SA, Martinez NW, Yoo S, Jara JS, Zamorano S, Hetz C, et al. Axonal degeneration is mediated by the mitochondrial permeability transition pore. J Neurosci. 2011;31:966–78. doi: 10.1523/JNEUROSCI.4065-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley DJ, Squires LK, Zlokovic BV, Mitrovic DM, Hughes CC, Revest PA, et al. Permeability of the blood-brain barrier to the immunosuppressive cyclic peptide cyclosporin A. J Neurochem. 1990;55:1222–30. doi: 10.1111/j.1471-4159.1990.tb03128.x. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–99. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- Blackstone C, Chang CR. Mitochondria unite to survive. Nat Cell Biol. 2011;13:521–2. doi: 10.1038/ncb0511-521. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–68. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Camara AK, Lesnefsky EJ, Stowe DF. Potential therapeutic benefits of strategies directed to mitochondria. Antioxid Redox Signal. 2010;13:279–347. doi: 10.1089/ars.2009.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem J. 1994;302 (Pt 2):321–4. doi: 10.1042/bj3020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M, Barksby E, Johnson N, Capano M. Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie. 2002;84:143–52. doi: 10.1016/s0300-9084(02)01368-8. [DOI] [PubMed] [Google Scholar]
- Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–60. [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–21. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol Aging. 2011;32:398–406. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Yan SS. Mitochondrial permeability transition pore in Alzheimer’s disease: cyclophilin D and amyloid beta. Biochim Biophys Acta. 2010;1802:198–204. doi: 10.1016/j.bbadis.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Fernandez A, Morales A, Mari M, Garcia-Ruiz C, Colell A. Oxidative stress and altered mitochondrial function in neurodegenerative diseases: lessons from mouse models. CNS Neurol Disord Drug Targets. 2010;9:439–54. doi: 10.2174/187152710791556113. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Aubourg P, Pujol A. General aspects and neuropathology of X-linked adrenoleukodystrophy. Brain Pathol. 2010;20:817–30. doi: 10.1111/j.1750-3639.2010.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forss-Petter S, Werner H, Berger J, Lassmann H, Molzer B, Schwab MH, et al. Targeted inactivation of the X-linked adrenoleukodystrophy gene in mice. J.Neurosci Res. 1997;50:829–43. doi: 10.1002/(SICI)1097-4547(19971201)50:5<829::AID-JNR19>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Forte M, Gold BG, Marracci G, Chaudhary P, Basso E, Johnsen D, et al. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc Natl Acad Sci USA. 2007;104:7558–63. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade S, Lopez-Erauskin J, Galino J, Duval C, Naudi A, Jove M, et al. Early oxidative damage underlying neurodegeneration in X-adrenoleukodystrophy. Hum Mol Genet. 2008;17:1762–73. doi: 10.1093/hmg/ddn085. [DOI] [PubMed] [Google Scholar]
- Friberg H, Ferrand-Drake M, Bengtsson F, Halestrap AP, Wieloch T. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J Neurosci. 1998;18:5151–9. doi: 10.1523/JNEUROSCI.18-14-05151.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea E, Launay N, Portero-Otin M, Ruiz M, Pamplona R, Aubourg P, et al. Oxidative stress underlying axonal degeneration in adrenoleukodystrophy: a paradigm for multifactorial neurodegenerative diseases? Biochim Biophys Acta. 2012;1822:1475–88. doi: 10.1016/j.bbadis.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Galino J, Ruiz M, Fourcade S, Schluter A, Lopez-Erauskin J, Guilera C, et al. Oxidative damage compromises energy metabolism in the axonal degeneration mouse model of x-adrenoleukodystrophy. Antioxid Redox Signal. 2011;15:2095–107. doi: 10.1089/ars.2010.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, et al. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33:627–38. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelli A, Zanna C, Porcelli AM, Schapira AH, Martinuzzi A, Carelli V, et al. Leber’s hereditary optic neuropathy (LHON) pathogenic mutations induce mitochondrial-dependent apoptotic death in transmitochondrial cells incubated with galactose medium. J Biol Chem. 2003;278:4145–50. doi: 10.1074/jbc.M210285200. [DOI] [PubMed] [Google Scholar]
- Gilg AG, Singh AK, Singh I. Inducible nitric oxide synthase in the central nervous system of patients with X-adrenoleukodystrophy. J Neuropathol Exp Neurol. 2000;59:1063–9. doi: 10.1093/jnen/59.12.1063. [DOI] [PubMed] [Google Scholar]
- Greco T, Shafer J, Fiskum G. Sulforaphane inhibits mitochondrial permeability transition and oxidative stress. Free Radic Biol Med. 2011;51:2164–71. doi: 10.1016/j.freeradbiomed.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. Biochemistry: a pore way to die. Nature. 2005;434:578–9. doi: 10.1038/434578a. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–7. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–31. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990;268:153–60. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84:153–66. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Joubert C, du Preez JL, Berk M. Effect of chronic N-acetyl cysteine administration on oxidative status in the presence and absence of induced oxidative stress in rat striatum. Neurochem Res. 2008;33:508–17. doi: 10.1007/s11064-007-9466-y. [DOI] [PubMed] [Google Scholar]
- Hettema EH, van Roermund CW, Distel B, van den Berg M, Vilela C, Rodrigues-Pousada C, et al. The ABC transporter proteins Pat1 and Pat2 are required for import of long-chain fatty acids into peroxisomes of Saccharomyces cerevisiae. Embo J. 1996;15:3813–22. [PMC free article] [PubMed] [Google Scholar]
- Hofhaus G, Johns DR, Hurko O, Attardi G, Chomyn A. Respiration and growth defects in transmitochondrial cell lines carrying the 11778 mutation associated with Leber’s hereditary optic neuropathy. J Biol Chem. 1996;271:13155–61. doi: 10.1074/jbc.271.22.13155. [DOI] [PubMed] [Google Scholar]
- Kahn E, Baarine M, Dauphin A, Ragot K, Tissot N, Seguin A, et al. Impact of 7-ketocholesterol and very long chain fatty acids on oligodendrocyte lipid membrane organization: evaluation via LAURDAN and FAMIS spectral image analysis. Cytometry A. 2011;79:293–305. doi: 10.1002/cyto.a.21017. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Fong KS, Hansson MJ, Elmer E, Csiszar K, Keep MF. Life span extension and reduced neuronal death after weekly intraventricular cyclosporin injections in the G93A transgenic mouse model of amyotrophic lateral sclerosis. J Neurosurg. 2004;101:128–37. doi: 10.3171/jns.2004.101.1.0128. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Mechanisms of neurodegeneration shared between multiple sclerosis and Alzheimer’s disease. J Neural Transm. 2011;118:747–52. doi: 10.1007/s00702-011-0607-8. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Shacter E. Hydrogen peroxide inhibits activation, not activity, of cellular caspase-3 in vivo. Free Radic Biol Med. 2000;29:684–92. doi: 10.1016/s0891-5849(00)00366-x. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Linard D, Kandlbinder A, Degand H, Morsomme P, Dietz KJ, Knoops B. Redox characterization of human cyclophilin D: identification of a new mammalian mitochondrial redox sensor? Arch Biochem Biophys. 2009;491:39–45. doi: 10.1016/j.abb.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Lizard G, Fournel S, Genestier L, Dhedin N, Chaput C, Flacher M, et al. Kinetics of plasma membrane and mitochondrial alterations in cells undergoing apoptosis. Cytometry. 1995;21:275–83. doi: 10.1002/cyto.990210308. [DOI] [PubMed] [Google Scholar]
- Loor G, Kondapalli J, Iwase H, Chandel NS, Waypa GB, Guzy RD, et al. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim Biophys Acta. 2011;1813:1382–94. doi: 10.1016/j.bbamcr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Erauskin J, Fourcade S, Galino J, Ruiz M, Schluter A, Naudi A, et al. Antioxidants halt axonal degeneration in a mouse model of X-adrenoleukodystrophy. Ann Neurol. 2011;70:84–92. doi: 10.1002/ana.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JF, Lawler AM, Watkins PA, Powers JM, Moser AB, Moser HW, et al. A mouse model for X-linked adrenoleukodystrophy. Proc Natl Acad Sci USA. 1997;94:9366–71. doi: 10.1073/pnas.94.17.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, et al. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis. 2010;20 (Suppl 2):S609–31. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ. The mitochondrial permeability transition pore: a molecular target for amyotrophic lateral sclerosis therapy. Biochim Biophys Acta. 2010;1802:186–97. doi: 10.1016/j.bbadis.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Gertz B, Pan Y, Price AC, Molkentin JD, Chang Q. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp Neurol. 2009;218:333–46. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Portero-Otin M, Pamplona R, Ferrer I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol. 2010;20:281–97. doi: 10.1111/j.1750-3639.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matouschek A, Rospert S, Schmid K, Glick BS, Schatz G. Cyclophilin catalyzes protein folding in yeast mitochondria. Proc Natl Acad Sci USA. 1995;92:6319–23. doi: 10.1073/pnas.92.14.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser H, Smith KD, Watkins PA, Powers J, Moser AB. X-linked adrenoleukodystrophy. In: Scriver C, editor. The metabolic and molecular bases of inherited disease. Vol. II. New-York: McGraw-Hill; 2001. pp. 3257–301. [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–8. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Stevens MV, Kohr M, Steenbergen C, Sack MN, Murphy E. Cysteine 203 of cyclophilin D is critical for cyclophilin D activation of the mitochondrial permeability transition pore. J Biol Chem. 2011;286:40184–92. doi: 10.1074/jbc.M111.243469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona R, Barja G. Highly resistant macromolecular components and low rate of generation of endogenous damage: two key traits of longevity. Ageing Res Rev. 2007;6:189–210. doi: 10.1016/j.arr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Petronilli V, Costantini P, Scorrano L, Colonna R, Passamonti S, Bernardi P. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J Biol Chem. 1994;269:16638–42. [PubMed] [Google Scholar]
- Petrosillo G, Colantuono G, Moro N, Ruggiero FM, Tiravanti E, Di Venosa N, et al. Melatonin protects against heart ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am J Physiol Heart Circ Physiol. 2009;297:H1487–93. doi: 10.1152/ajpheart.00163.2009. [DOI] [PubMed] [Google Scholar]
- Powers JM. Adreno-leukodystrophy: a personal historical note. Acta Neuropathol. 2005;109:124–7. doi: 10.1007/s00401-004-0961-9. [DOI] [PubMed] [Google Scholar]
- Powers JM, DeCiero DP, Ito M, Moser AB, Moser HW. Adrenomyeloneuropathy: a neuropathologic review featuring its noninflammatory myelopathy. J Neuropathol Exp Neurol. 2000;59:89–102. doi: 10.1093/jnen/59.2.89. [DOI] [PubMed] [Google Scholar]
- Pratico D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: lights and shadows. Ann N Y Acad Sci. 2008;1147:70–8. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- Pujol A, Ferrer I, Camps C, Metzger E, Hindelang C, Callizot N, et al. Functional overlap between ABCD1 (ALD) and ABCD2 (ALDR) transporters: a therapeutic target for X-adrenoleukodystrophy. Hum Mol Genet. 2004;13:2997–3006. doi: 10.1093/hmg/ddh323. [DOI] [PubMed] [Google Scholar]
- Pujol A, Hindelang C, Callizot N, Bartsch U, Schachner M, Mandel JL. Late onset neurological phenotype of the X-ALD gene inactivation in mice: a mouse model for adrenomyeloneuropathy. Hum Mol Genet. 2002;11:499–505. doi: 10.1093/hmg/11.5.499. [DOI] [PubMed] [Google Scholar]
- Robinson BH, Petrova-Benedict R, Buncic JR, Wallace DC. Nonviability of cells with oxidative defects in galactose medium: a screening test for affected patient fibroblasts. Biochem Med Metab Biol. 1992;48:122–6. doi: 10.1016/0885-4505(92)90056-5. [DOI] [PubMed] [Google Scholar]
- Robinson CE, Keshavarzian A, Pasco DS, Frommel TO, Winship DH, Holmes EW. Determination of protein carbonyl groups by immunoblotting. Anal Biochem. 1999;266:48–57. doi: 10.1006/abio.1998.2932. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA. 2005;102:12005–10. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter A, Espinosa L, Fourcade S, Galino J, Lopez E, Ilieva E, et al. Functional genomic analysis unravels a metabolic-inflammatory interplay in adrenoleukodystrophy. Hum Mol Genet. 2012;21:1062–77. doi: 10.1093/hmg/ddr536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SR, Seo JT. Calcium overload is essential for the acceleration of staurosporine-induced cell death following neuronal differentiation in PC12 cells. Exp Mol Med. 2009;41:269–76. doi: 10.3858/emm.2009.41.4.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Gal J, Kwinter DM, Liu X, Zhu H. Mitochondrial dysfunction in amyotrophic lateral sclerosis. Biochim Biophys Acta. 2010;1802:45–51. doi: 10.1016/j.bbadis.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, et al. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: implications for selective neuronal damage. Hum Mol Genet. 2011;20:1438–55. doi: 10.1093/hmg/ddr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I, Pujol A. Pathomechanisms underlying X-adrenoleukodystrophy: a three-hit hypothesis. Brain Pathol. 2010;20:838–44. doi: 10.1111/j.1750-3639.2010.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack EC, Matson WR, Ferrante RJ. Evidence of oxidant damage in Huntington’s disease: translational strategies using antioxidants. Ann N Y Acad Sci. 2008;1147:79–92. doi: 10.1196/annals.1427.008. [DOI] [PubMed] [Google Scholar]
- Su KG, Banker G, Bourdette D, Forte M. Axonal degeneration in multiple sclerosis: the mitochondrial hypothesis. Curr Neurol Neurosci Rep. 2009;9:411–17. doi: 10.1007/s11910-009-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B, Banerjee R, Starkova NN, Zhang SF, Calingasan NY, Yang L, et al. Mitochondrial permeability transition pore component cyclophilin D distinguishes nigrostriatal dopaminergic death paradigms in the MPTP mouse model of Parkinson’s disease. Antioxid Redox Signal. 2012;16:855–68. doi: 10.1089/ars.2010.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roermund CW, Visser WF, Ijlst L, van Cruchten A, Boek M, Kulik W, et al. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008;22:4201–8. doi: 10.1096/fj.08-110866. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yang J, Yi J. Redox sensing by proteins: oxidative modifications on cysteines and the consequent events. Antioxid Redox Signal. 2012;16:649–57. doi: 10.1089/ars.2011.4313. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Larochette N, Kroemer G. Mitochondrial permeability transition in apoptosis and necrosis. Cell Death Differ. 2005;12 (Suppl 2):1478–80. doi: 10.1038/sj.cdd.4401682. [DOI] [PubMed] [Google Scholar]
- Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson’s disease: a mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci. 2008;1147:93–104. doi: 10.1196/annals.1427.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Yi J, Fu R, Liu E, Siddique T, Rios E, et al. Hyperactive intracellular calcium signaling associated with localized mitochondrial defects in skeletal muscle of an animal model of amyotrophic lateral sclerosis. J Biol Chem. 2010;285:705–12. doi: 10.1074/jbc.M109.041319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res. 2009;83:213–25. doi: 10.1093/cvr/cvp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.