Abstract
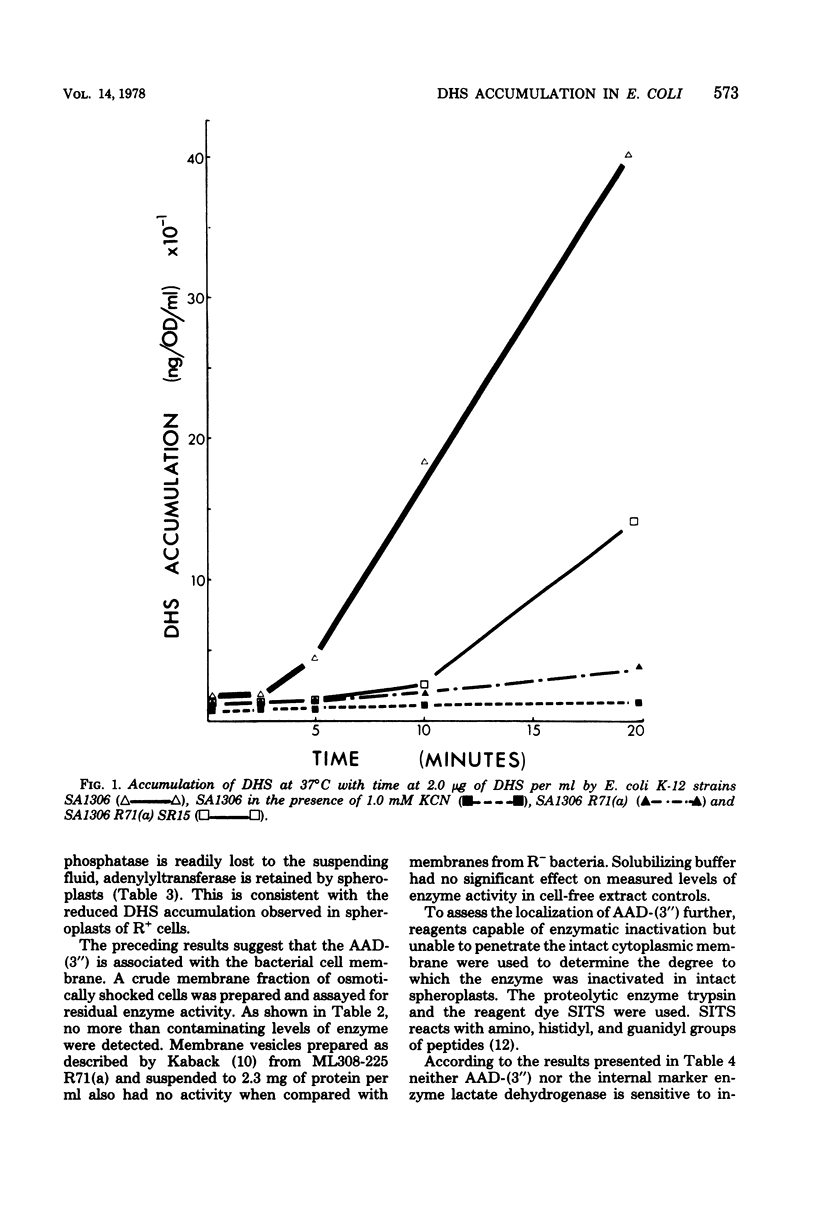
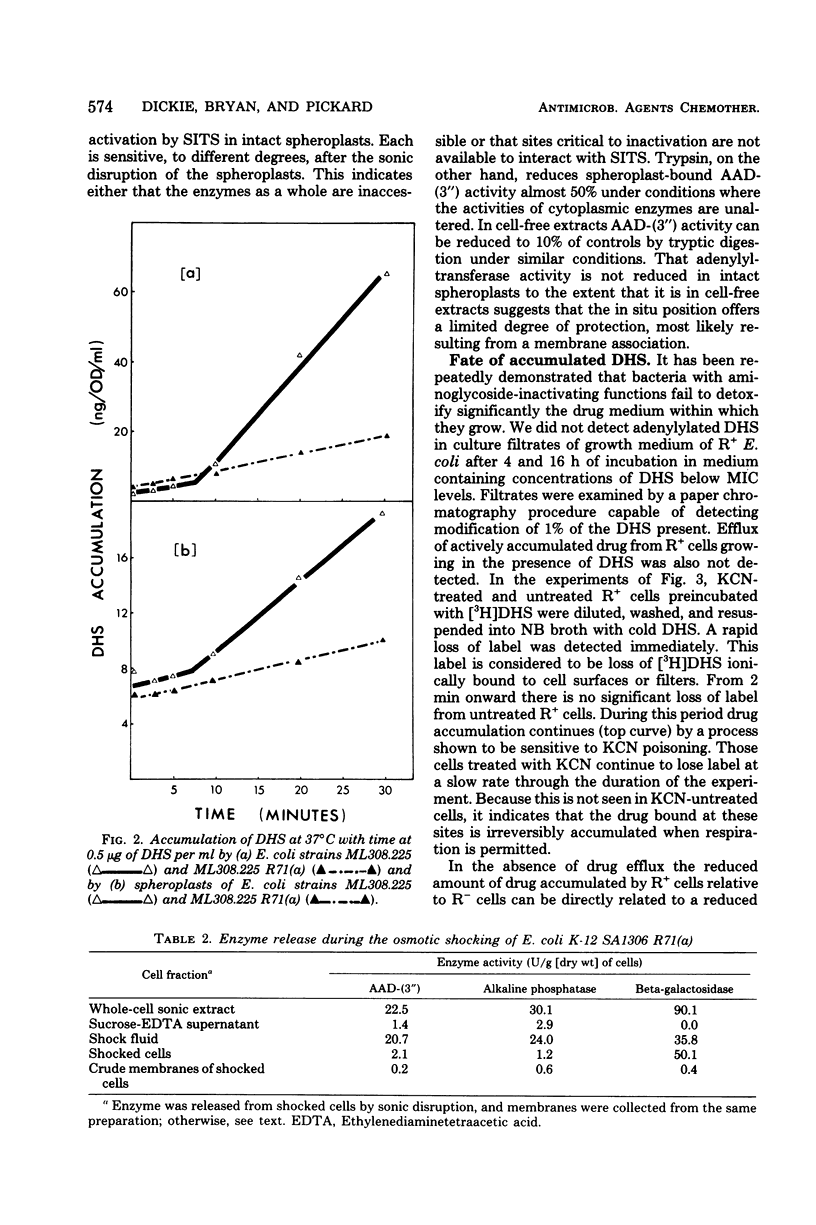
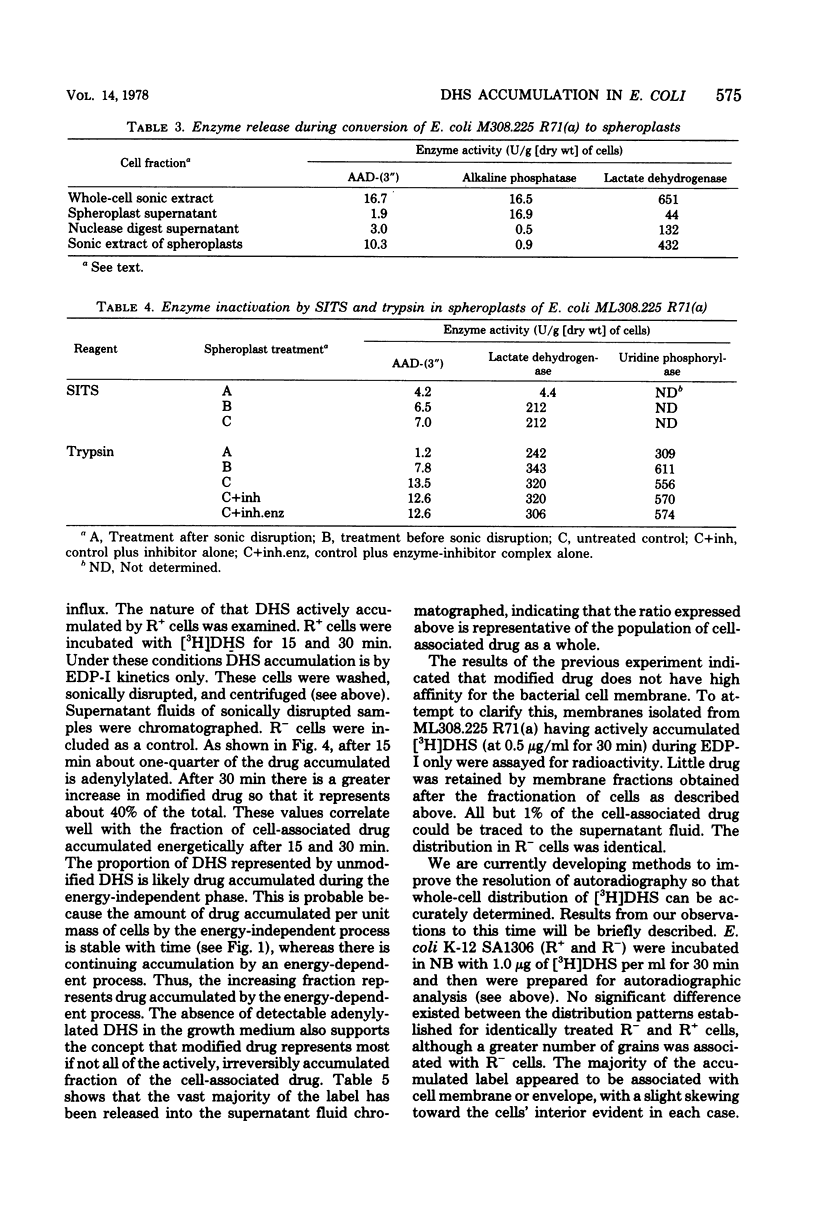
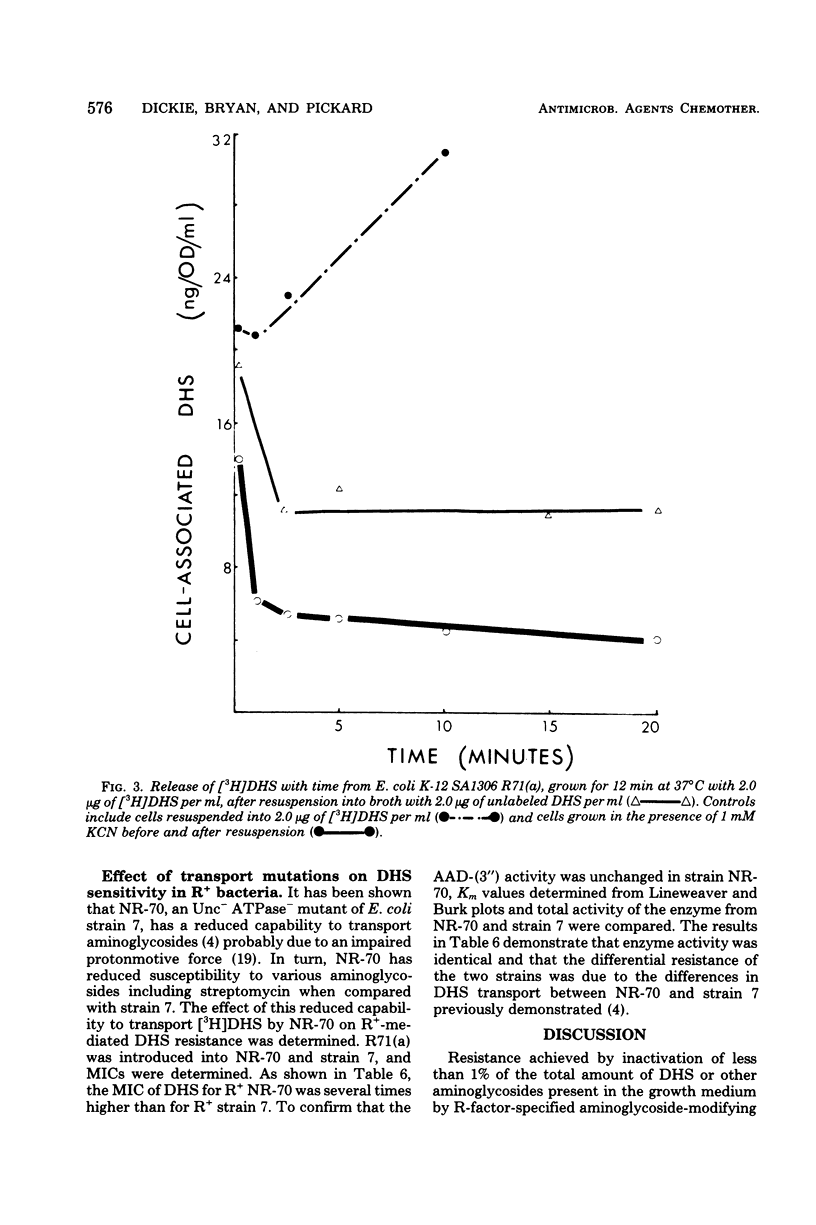
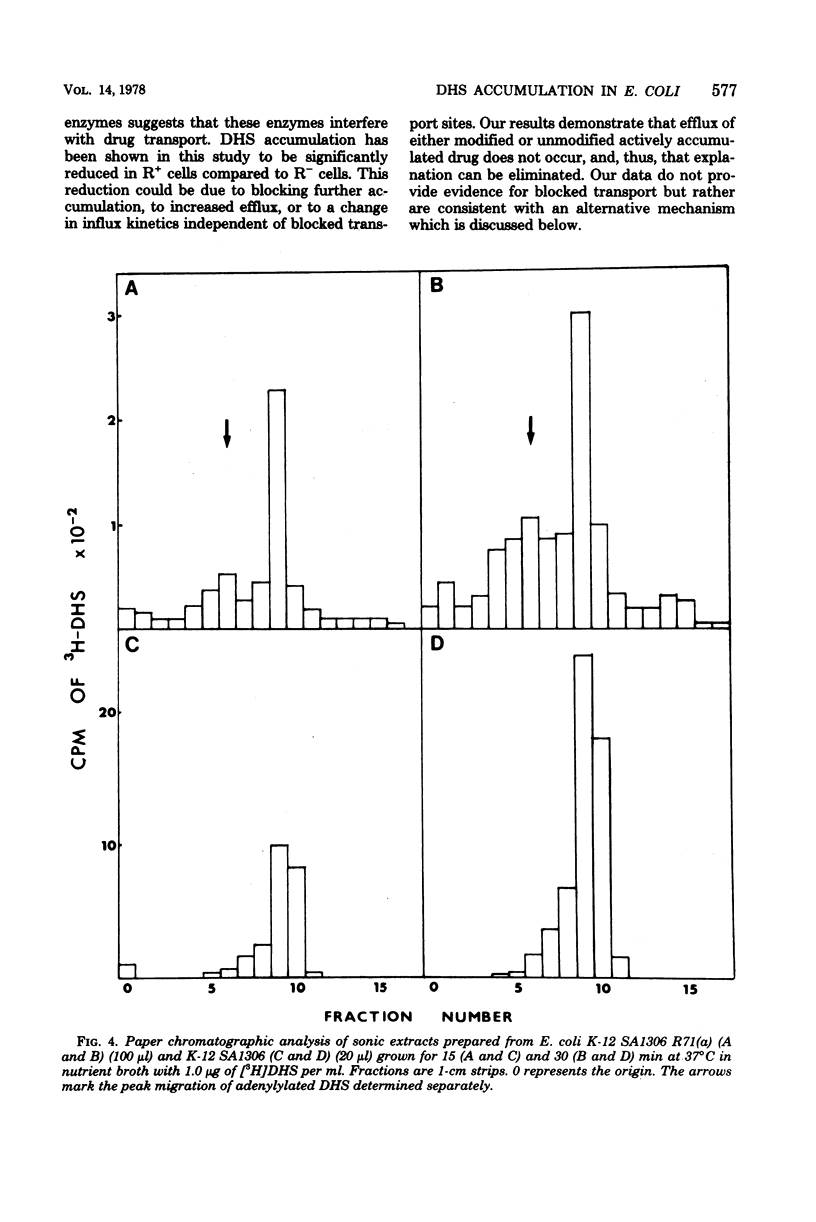
Strains of Escherichia coli carrying R-factor R71(a), which codes for a streptomycin-spectinomycin adenylyltransferase, have elevated levels of resistance to dihydrostreptomycin (DHS) compared with isogenic R− bacteria. DHS accumulated by whole cells and spheroplasts of R+ bacteria is lower than that observed for R− strains, a result of the absence of the second and more rapid of the two energy-dependent phases of DHS uptake seen in susceptible E. coli. A mutant of R+E. coli with reduced DHS resistance has been shown to have reduced levels of streptomycin-spectinomycin adenylyltransferase activity as well as enhanced drug accumulation. Actively accumulated DHS was recovered from R+ cells as the adenylylated derivative. Neither was inactivated antibiotic detected in culture filtrates, nor was actively accumulated drug lost from R+ cells under normal conditions. The cellular distribution of actively accumulated DHS in R+ and R− cells was found to be the same. Membranes isolated from these cells retained only a small fraction (≃1%) of the total cell-associated drug. The R+ derivative of a mutant with defective energy transduction (E. coli NR-70) and reduced ability to transport aminoglycosides has a significantly higher minimal inhibitory concentration of DHS than its R+ parent (strain 7). Streptomycin-spectinomycin adenylyltransferase activity, from comparisons of Km values and total activities of enzyme, was the same in both strains. The enzyme has been localized to the exterior surface of the bacterial inner membrane, although isolated membranes lacked detectable enzyme activity. The preceding observations are consistent with the proposal that the level of R71(a)-mediated DHS resistance is the outcome of competition between the rate of adenylylation and the rate of the first energy-dependent phase of DHS transport. When the rate of adenylylation exceeds the first energy-dependent phase, adenylylated DHS is accumulated, apparently in a manner identical to the accumulation of DHS. Unlike DHS, adenylylated DHS does not interact with ribosomes, and, consequently, there is a failure to initiate ribosomally dependent sequelae such as the second energy-dependent phase of accumulation, inhibition of protein synthesis, and/or misreading of mRNA.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Yamada T., Davies J. Enzymatic Adenylylation of Streptomycin and Spectinomycin by R-Factor-Resistant Escherichia coli. Infect Immun. 1970 Jan;1(1):109–119. doi: 10.1128/iai.1.1.109-119.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddlecome S., Haas M., Davies J., Miller G. H., Rane D. F., Daniels P. J. Enzymatic modification of aminoglycoside antibiotics: a new 3-N-acetylating enzyme from a Pseudomonas aeruginosa isolate. Antimicrob Agents Chemother. 1976 Jun;9(6):951–955. doi: 10.1128/aac.9.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Effects of membrane-energy mutations and cations on streptomycin and gentamicin accumulation by bacteria: a model for entry of streptomycin and gentamicin in susceptible and resistant bacteria. Antimicrob Agents Chemother. 1977 Aug;12(2):163–177. doi: 10.1128/aac.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van den Elzen H. M. Streptomycin accumulation in susceptible and resistant strains of Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Jun;9(6):928–938. doi: 10.1128/aac.9.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert Y. A., Scavizzi M. R., Witchitz J. L., Gerbaud G. R., Bouanchaud D. H. Incompatibility groups and the classification of fi - resistance factors. J Bacteriol. 1972 Nov;112(2):666–675. doi: 10.1128/jb.112.2.666-675.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F. N., Flaks J. G. Binding of dihydrostreptomycin to Escherichia coli ribosomes: characteristics and equilibrium of the reaction. Antimicrob Agents Chemother. 1972 Oct;2(4):294–307. doi: 10.1128/aac.2.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. E., Benveniste R. E. Enzymes that inactivate antibiotics in transit to their targets. Ann N Y Acad Sci. 1974 May 10;235(0):130–136. doi: 10.1111/j.1749-6632.1974.tb43262.x. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Höltje J. V. Streptomycin uptake via an inducible polyamine transport system in Escherichia coli. Eur J Biochem. 1978 May 16;86(2):345–351. doi: 10.1111/j.1432-1033.1978.tb12316.x. [DOI] [PubMed] [Google Scholar]
- Lundbäck A. K., Lundbäck A., Nordström K. Effect of R-factor-mediated drug-metabolizing enzymes on survival of Escherichia coli K-12 in presence of ampicillin, chloramphenicol, or streptomycin. Antimicrob Agents Chemother. 1974 May;5(5):492–499. doi: 10.1128/aac.5.5.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADDY A. H. A FLUORESCENT LABEL FOR THE OUTER COMPONENTS OF THE PLASMA MEMBRANE. Biochim Biophys Acta. 1964 Sep 25;88:390–399. doi: 10.1016/0926-6577(64)90194-9. [DOI] [PubMed] [Google Scholar]
- MATNEY T. S., ACHENBACH N. E. New uses for membrane filters III. Bacterial mating procedure. J Bacteriol. 1962 Oct;84:874–875. doi: 10.1128/jb.84.4.874-875.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Person S., Phillips S., Funk F. A determination of mutagen specificity in bacteria using nonsense mutants of bacteriophage T4. J Mol Biol. 1967 Jun 28;26(3):437–447. doi: 10.1016/0022-2836(67)90314-2. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Watanabe K. Location of sulfate-binding protein in Salmonella typhimurium. J Bacteriol. 1968 Oct;96(4):1049–1054. doi: 10.1128/jb.96.4.1049-1054.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P., Adler L. W. The maintenance of the energized membrane state and its relation to active transport in Escherichia coli. Biochim Biophys Acta. 1975 Apr 14;387(1):23–36. doi: 10.1016/0005-2728(75)90049-3. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Restoration of active transport in an Mg2+-adenosine triphosphatase-deficient mutant of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1124–1129. doi: 10.1128/jb.116.3.1124-1129.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrabadi M. S., Marusyk R. G., Crawford T. B. Electron-microscopic study of the development of an equine adenovirus in cultured fetal equine kidney cells. Can J Microbiol. 1977 May;23(5):497–509. doi: 10.1139/m77-074. [DOI] [PubMed] [Google Scholar]
- Weiss R. L. Protoplast formation in Escherichia coli. J Bacteriol. 1976 Nov;128(2):668–670. doi: 10.1128/jb.128.2.668-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Tipper D., Davies J. Enzymatic inactivation of streptomycin by R factor-resistant Escherichia coli. Nature. 1968 Jul 20;219(5151):288–291. doi: 10.1038/219288a0. [DOI] [PubMed] [Google Scholar]


