Abstract
Resin-based composite dental restoration materials may release bisphenol-A, an endocrine-disrupting chemical. Using secondary analysis of a randomized clinical safety trial of amalgam vs. composites, we tested the hypothesis that dental restoration materials affect children’s growth. Children (N = 218 boys, N = 256 girls) aged 6 to 10 yrs at baseline with ≥ 2 decayed posterior teeth were randomized to amalgam or composites (bisphenol-A-diglycidyl-dimethacrylate composite for permanent teeth, urethane-dimethacrylate compomer for primary teeth) for treatment of posterior caries throughout follow-up. Primary outcomes for this analysis were 5-year changes in BMI-for-age z-scores, body fat percentage (BF%), and height velocity; exploratory analyses (n = 113) examined age at menarche. Results showed no significant differences between treatment assignment and changes in physical development in boys [(composites vs. amalgam) BF%, 4.9 vs. 5.7, p = 0.49; (BMI-z-score) 0.13 vs. 0.25, p = 0.36] or girls (8.8 vs. 7.7, p = 0.95; 0.36 vs. 0.21, p = 0.49). Children with more treatment on primary teeth had greater increases in BF% regardless of material type. Girls assigned to composites had lower risk of menarche during follow-up (hazard ratio = 0.57, 95% CI 0.35-0.95). Overall, there were no significant differences in physical development over 5 years in children treated with composites or amalgam. Additional studies examining these restoration materials in relation to age at menarche are warranted (clinicaltrials.gov number NCT00065988).
Keywords: composite resins, bisphenol A-glycidyl methacrylate, dental amalgam, body mass index, menarche, child
Introduction
Resin-based dental composites generally consist of a mixture of monomers, such as bisGMA (bisphenol-A-diglycidyl-dimethacrylate) or UDMA (urethane-dimethacrylate) in combination with co-monomers of lower viscosity, such as TEGDMA (triethylene-glycol-dimethacrylate). Composites allow for more conservative, esthetic, and desirable dental restoration techniques compared with amalgam. These advantages generally outweigh the risks of local toxicity and allergic reactions seen in laboratory experimental settings (Bakopoulou et al., 2009). However, the possibility of systemic adverse effects has not been thoroughly investigated.
Methacrylate monomers may have endocrine effects. Laboratory experiments in mice administered bisGMA and TEGDMA showed significant reductions in fertility and growth (Al-Hiyasat and Darmani, 2006; Darmani and Al-Hiyasat, 2006). BisGMA-based composites may release bisphenol A (BPA), possibly due to impurities from the synthesis process or resin degradation (Van Landuyt et al., 2011). BPA has both estrogenic and anti-androgenic effects (Mendiola et al., 2010; Melzer et al., 2011). Numerous rodent experiments have shown that BPA exposure during early development stimulates growth and alters weight gain, body composition, and pubertal development (Richter et al., 2007; Nah et al., 2011). Some studies could not demonstrate chemical or biological hydrolysis of bisGMA to BPA, and further research is needed to understand the possible mechanisms of BPA exposure resulting from bisGMA-based dental composites (Schmalz et al., 1999; Söderholm and Mariotti, 1999; Bakopoulou et al., 2009).
Compared with adults, children are likely to have greater exposure to monomers released from dental materials and increased vulnerability, due to their size and developmental stage (Scheuplein et al., 2002). Previously, we reported findings that children treated with bisGMA-based composites had more self-reported psychosocial problems after 5 yrs of follow-up, compared with children assigned amalgam (Bellinger et al., 2008; Maserejian et al., 2012), a finding consistent with those of prior studies on neuropsychological effects of BPA (Palanza et al., 2008; Braun et al., 2009; Yu et al., 2011). In this article, we present additional data from a randomized clinical trial of dental amalgam and composites to test the hypotheses that: (i) randomization to treatment with composites led to greater changes in body fat, body mass index (BMI), and height velocity, compared with amalgam; (ii) greater treatment levels of bisGMA-based composites or UDMA-based compomer, but not amalgam, were associated with these growth outcomes; and (iii) as exploratory analyses among girls, treatment assignment was associated with age at menarche.
Methods
Data were collected as part of the New England Children’s Amalgam Trial (NECAT), a randomized safety trial of amalgam with 5-year follow-up conducted from 1997 to 2006. Children (N = 534) aged 6 to 10 yrs at baseline were recruited in 1 rural (Farmington, ME) or 5 urban (Boston, MA) clinical sites. Eligibility criteria included: ≥ 2 posterior teeth with caries requiring restoration on occlusal surfaces, no prior amalgams, English language proficiency, and, by parent-report, no physician-diagnosed psychological, behavioral, neurological, immunosuppressive, or renal disease. Written informed consent/assent was obtained from parents/children. The study was approved by the Institutional Review Boards of all participating sites. Details of study procedures have been published (Children’s Amalgam Trial, 2003; Bellinger et al., 2006).
Children were randomized to a treatment plan of amalgam or resin-based composites for posterior tooth restorations (Fig.) at baseline and through follow-up. Randomization was stratified by number of teeth with caries (2-4 vs. ≥ 5) and rural/urban location. Children assigned to composites were treated with bisGMA-based composite (Z100, by 3M ESPE, St. Paul, MN, USA) for permanent teeth and UDMA-based compomer (Dyract AP, by Dentsply Caulk, De Trey, Konstanz, Germany) on primary teeth. Compomer is a polyacid-modified composite containing 72% (by weight) strontium-fluorosilicate-glass to allow for fluoride release. In the amalgam arm, posterior teeth were treated with Dispersalloy (by Dentsply Caulk, Milford, DE, USA); anterior teeth were treated with compomer/composite as in standard practice. Children in the amalgam group and those with greater amalgam treatment levels were found to have higher urinary mercury concentrations during follow-up (Bellinger et al., 2006; Maserejian et al., 2008). Prior studies have shown that Z100 composite released BPA, bisGMA, bisDMA, and BADGE (Pulgar et al., 2000; Al-Hiyasat et al., 2004; Ortengren et al., 2004; Yap et al., 2004; Martin et al., 2005; Sasaki et al., 2005). Dyract did not release detectable BPA or bisGMA in eluates from filled tooth samples (Hamid et al., 1998). Urine/blood specimens collected and analyzed for mercury content were discarded, per protocol, upon NECAT’s completion, and were therefore unavailable for measurement of BPA concentration.
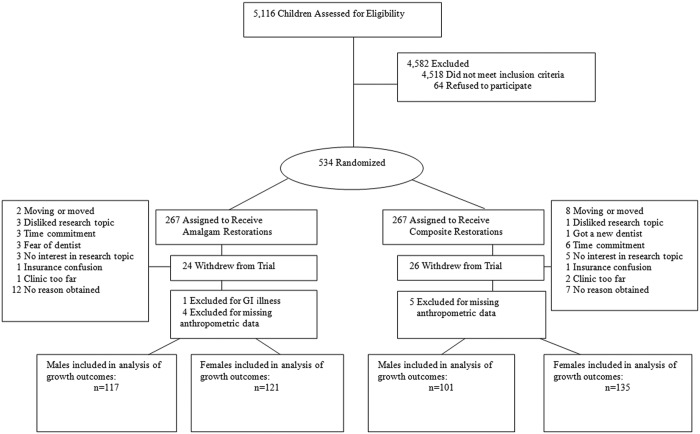
Figure.
Recruitment, randomization, and follow-up in the New England Children’s Amalgam Trial. The recruitment period was from September 1997 through September 1999, with follow-up ending March 2005. Some participants expressed more than one reason for withdrawal, all of which are recorded in the Fig.
NECAT data collectors measured height, weight, and body fat percentage annually. Height was measured with a tape measure and ruler and was recorded to the nearest tenth-inch. Weight and body fat percentage were measured on a bio-impedance scale (model TBF-551, Tanita Corp. of America, Inc.) (Rubiano et al., 1999) that was routinely calibrated. Menarche status was reported annually as no/yes, and if yes, month and year of menarche. Body mass index (kg/m2) and BMI-for-age z-score were calculated based on the Centers for Disease Control (CDC) data files, which define overweight or obese as BMI-for-age z-scores ≥ 85th percentile (Ogden et al., 2006; Krebs et al., 2007). To measure accelerated growth in height, height velocity was calculated as the change in height between consecutive annual measurements.
Statistical Analysis
The analyses included 474 children (218 males and 256 females), representing 89% of the 534 randomized participants, with complete data on growth outcomes and no self-reported gastrointestinal disorders (e.g., colitis) that may affect nutritional absorption. Exposure to dental materials was analyzed primarily as intent-to-treat randomized group analysis, and secondarily as observational treatment-level analyses. The primary outcomes for growth were changes in BMI-for-age z-score, body fat percentage, and height velocity. Associations were examined separately for boys and girls, given established sex differences in growth patterns and the possibility that endocrine-disrupting chemicals have sex-specific effects. The analysis had 80% power to detect differences between treatment groups of a mean change in BMI of 1.3 in boys and 1.2 in girls, change in BMI-for-age z-score of 0.3 in boys and 0.2 in girls, and change in body fat percentage of 3.7% in boys and 2.9% in girls.
For descriptive purposes, age-adjusted means for growth measures were obtained with generalized linear models. For the primary analyses, we used linear mixed-effects models with repeated measures of growth outcomes with subject-specific intercepts and age-specific slopes. We created each multivariable model by considering numerous factors related to growth (listed in Table 1) and used a backward elimination process to retain those with p < 0.10 that altered the association between dental material and the growth outcome by > 10%. Piece-wise linear splines were included in the mixed-effects models when significant.
Table 1.
Baseline Characteristics, by Assigned Treatment Plan
| Assigned Treatment |
||
|---|---|---|
| Amalgam | Composites | |
| N | 266 | 267 |
| Age, mean (SD) (yrs) | 7.5 (1.3) | 7.4 (1.4) |
| Sex, n (%) | ||
| Female | 131 (49%) | 156 (58%) |
| Male | 135 (51%) | 111 (42%) |
| Number of carious teeth, mean (SD) | 5.4 (3.0) | 5.3 (2.8) |
| Number of carious surfaces, mean (SD) | 9.8 (6.9) | 9.2 (6.2) |
| Race/ethnicity, n (%)* | ||
| Non-Hispanic White | 165 (62%) | 161 (60%) |
| Non-Hispanic Black | 54 (20%) | 54 (20%) |
| Hispanic | 15 (6%) | 24 (9%) |
| Other | 32 (12%) | 28 (11%) |
| Household Income ($US) | ||
| ≤ $20,000 | 78 (29%) | 89 (33%) |
| $20,000-40,000 | 113 (43%) | 110 (41%) |
| >$40,000 | 67 (25%) | 65 (24%) |
| Missing | 8 (3%) | 3 (1%) |
| Maternal Education | ||
| Less than high school | 34 (13%) | 39 (15%) |
| High school | 198 (74%) | 197 (74%) |
| College | 18 (7%) | 17 (6%) |
| Post-graduate | 9 (3%) | 12 (5%) |
| Missing | 7 (3%) | 2 (1%) |
| Socio-economic status, n (%)† | ||
| Low | 83 (31%) | 86 (32%) |
| Medium | 99 (37%) | 85 (32%) |
| High | 84 (32%) | 96 (36%) |
| Geographic location, n (%) | ||
| Urban (Boston, MA) | 144 (54%) | 147 (55%) |
| Rural (Farmington, ME) | 122 (46%) | 120 (45%) |
| Drinking water source, n (%) | ||
| Bottled | 67 (32%) | 65 (32%) |
| Tap | 81 (38%) | 77 (38%) |
| Mixed | 59 (28%) | 58 (28%) |
| Do not know | 4 (2%) | 4 (2%) |
| Fruit and vegetable servings/day, mean (SD) | 1.3 (0.6) | 1.3 (0.6) |
| Birth weight* | ||
| Missing | 37 (14%) | 32 (12%) |
| < 2500 g | 12 (5%) | 17 (6%) |
| 2500-3500 g | (48%) | (48%) |
| >= 3500 | 89 (34%) | 89 (33%) |
| Body fat %, mean (SD) | 22.3 (10.3) | 23.4 (10.7) |
| Body mass index, mean kg/m2 (SD) | 17.9 (3.7) | 18.1 (4.2) |
| Body mass index-by-age Z-score (SD) | 0.58 (1.09) | 0.61 (1.11) |
| Height, cm (SD) | 128.2 (10.4) | 127.4 (10.3) |
Self-reported by the mother of the participant during baseline interview.
Socio-economic status was calculated based on both household income and maternal education levels and standardized to the general U.S. population of New England area households.
To test whether the assigned treatment arm was associated with growth outcomes, we adjusted the models for randomization stratum, age, and the relevant baseline anthropometric measure. We examined ‘received treatment’ levels by creating separate models for UDMA-based compomer and bisGMA-based composite on posterior-occlusal tooth surfaces. A surface-years exposure metric (each treated surface weighted by yrs present in the mouth during follow-up) was calculated for each material. To evaluate the potential for confounding by factors related to severity of dental disease, we conducted the same analyses for amalgam treatment levels separating primary and permanent tooth fillings, thereby allowing for parallel comparisons with the randomly assigned amalgam data.
Exploratory analysis of age at menarche was restricted to girls from the rural Maine site, because menarche data collection was incomplete in the Boston site. Proportional hazards models were used to assess (1) randomized treatment arm and (2) levels of each treatment material received at baseline on posterior-occlusal surfaces. The proportional hazards assumption was tested with an interaction term between exposure and time, which indicated no violation of proportional hazards. Multivariable models considered adjustment for age, body fat percentage, birth weight, maternal education, household income, race/ethnicity, and randomization stratum. The final models retained age, birth weight, and income. With a total of 113 girls in a log rank test of proportions, there was 80% power to detect a hazard ratio of 0.50 when the treatment groups were compared. Statistical analyses were conducted at significance level alpha = 0.05 with SAS v.9.2 (Cary, NC, USA).
Results
Children assigned to the composites treatment plan were similar to those in the amalgam group in age, race/ethnicity, socio- economic status, and baseline anthropometric measures (Table 1). The numbers of boys and girls were similar in the amalgam group, but there were more girls (58%) than boys in the composites group. Overall, the mean (SD) number of fillings placed at baseline was 8.8 (SD 5.5) surfaces, with more fillings placed on primary vs. permanent teeth (Appendix Table). Over the 5-year follow-up, children had, on average, about 1 new decayed surface per yr, accumulating a mean (SD) 36.6 surface-years exposure to dental restorations, with no significant differences by assigned treatment. At baseline, 35% of boys and 32% of girls in NECAT were overweight or obese. The prevalence of overweight/obesity increased by the last study visit (when participants were ages 11-15 yrs) to 43% of boys and 47% of girls.
There were no associations between treatment group and changes in body fat percentage, BMI-for-age z-score, or height velocity throughout follow-up in either boys (Table 2a) or girls (Table 2b). Adjustment for additional covariates or excluding children with prior composite fillings did not appreciably change the results (data not shown). In secondary analysis of level of treatment received by material type, there were no associations with increasing levels of treatment with bisGMA-based composite or amalgam on permanent teeth (data not shown). Among both girls and boys, greater treatment levels on primary teeth were associated with greater changes in body fat percentage (boys, β = 1.9, 95% CI 0.4-3.3, p = 0.01; girls, β = 1.4, 95% CI 0.4-2.4, p = 0.005). However, results were comparable with those for amalgam (boys, β = 1.6, 95% CI 0.4-2.9, p = 0.01; girls, β = 1.7, 95% CI 0.6-2.8, p = 0.003), suggesting that the increased body fat percentage was independent of the dental material used for treatment.
Table 2.
Growth Outcomes: Baseline, Follow-up, and 5-year Change, by Randomized Treatment Assignment, and Estimates of Treatment Arm Effects from Repeated-measurement Models
| Table 2a. Males | ||||||||
|---|---|---|---|---|---|---|---|---|
| Composites Arm |
Amalgam Arm |
Composites vs. Amalgam† |
||||||
| Growth Outcome | Age-adjusted Baseline | Means (SE) Year 5 | 5-year Change (SE) | Age-adjusted Baseline | Means (SE) Year 5 | 5-year Change (SE) | β (SE) | p Value |
| Body fat percentage | 19.3 (1.0) | 24.3 (1.1) | 4.9 (0.9) | 18.7 (1.0) | 24.4 (1.1) | 5.7 (0.9) | 0.57 (0.82) | 0.49 |
| BMI-for-age z-score | 0.66 (0.12) | 0.81 (0.11) | 0.13 (0.08) | 0.52 (0.11) | 0.77 (0.11) | 0.25 (0.07) | -0.21 (0.23) | 0.36 |
| Height (cm) | 129.4 (0.7) | 163.6 (0.9) | 34.4 (0.6) | 128.6 (0.7) | 161.8 (0.9) | 33.5 (0.6) | 0.48 (0.83)‡ | 0.56 |
| Table 2b. Females | ||||||||
| Composites Arm |
Amalgam Arm |
Composites vs. Amalgam† |
||||||
| Growth Outcome | Age-adjusted Baseline | Means (SE) Year 5 | 5-year Change (SE) | Age-adjusted Baseline | Means (SE) Year 5 | 5-year Change (SE) | β (SE) | p Value |
| Body fat percentage | 26.3 (0.8) | 35.2 (0.9) | 8.8 (0.7) | 25.1 (0.9) | 32.7 (1.0) | 7.7 (0.8) | 0.05 (0.83) | 0.95 |
| BMI-for-age z-score | 0.53 (0.10) | 0.90 (0.09) | 0.36 (0.06) | 0.54 (0.10) | 0.74 (0.10) | 0.21 (0.07) | 0.08 (0.12) | 0.49 |
| Height | 126.8 (0.6) | 157.6 (0.7) | 30.7 (0.5) | 126.9 (0.6) | 158.1 (0.7) | 31.2 (0.5) | 0.77 (1.18)‡ | 0.51 |
From linear mixed-effects models, adjusted for randomization stratum, age, and baseline value of the growth outcome of interest. Models for body fat percentage also included height as a time-varying co-variable, and, among males only, a knot at age 13 yrs (p < 0.001). A positive β (> 0) indicates that the composites arm had increased growth compared with the amalgam arm.
Height velocity during follow-up was the outcome of interest in the repeated-measures model for treatment arm effect estimates.
Exploratory analyses of menarche, limited to the 113 girls from the rural Maine site (Table 3), found that girls assigned to composites (n = 62) were less likely to have reached menarche (48% vs. 67%) and had a lower risk of menarche (multivariable-adjusted hazard ratio = 0.57, 95% CI 0.35-0.95, p = 0.03) during the 5-year follow-up, compared with girls assigned to amalgam. However, among the 64 girls who reached menarche, the mean age at menarche was not significantly different by treatment assignment (composites, 12.5 ± 1.1 yrs; amalgam, 12.3 ± 1.0 yrs; multivariable-adjusted p = 0.48). There were no associations among greater bisGMA-based, UDMA-based, or total composite (p = 0.29) treatment levels received at baseline and onset of menarche during the follow-up period (data not shown). Results were similar in sensitivity analyses limited to girls (n = 109) with no prior composite restorations.
Table 3.
Descriptive Statistics in Exploratory Analysis of Menarche during the 5-year Follow-up among Girls from the Rural Maine Study Site
| Assigned Treatment |
|||
|---|---|---|---|
| Total | Amalgam | Composites | |
| N | 113 | 51 | 62 |
| Baseline age, mean (SD) yrs | 7.3 (1.4) | 7.4 (1.4) | 7.2 (1.3) |
| Menarche during follow-up, n (%) | 64 (56.6%) | 34 (66.7%) | 30 (48.4%) |
| By age at baseline, n (%)* | |||
| 6 yrs | 11 (24.4%) | 8 (42.1%) | 3 (11.5%) |
| 7 yrs | 15 (62.5%) | 8 (61.5%) | 7 (63.6%) |
| 8 yrs | 16 (76.2%) | 8 (100.0%) | 8 (61.5%) |
| 9-10 yrs | 22 (95.7%) | 10 (90.9%) | 12 (100.0%) |
| Age at menarche, mean (SD) yrs† | 12.4 (1.0) | 12.3 (1.0) | 12.5 (1.1) |
| Race/ethnicity, n (%)‡ | |||
| Non-Hispanic White | 110 (97.4%) | 51 (100.0%) | 59 (95.2%) |
| Non-Hispanic Black | 1 (0.9%) | 0 | 1 (1.6%) |
| Hispanic (non-mixed) | 0 | 0 | 0 |
| Other | 2 (1.8%) | 0 | 2 (3.2%) |
| Birth weight‡ | |||
| Missing | 4 (3.5%) | 3 (5.9%) | 1 (1.6%) |
| < 2500 g | 6 (5.3%) | 2 (3.9%) | 4 (6.5%) |
| 2500-3500 g | 58 (51.3%) | 28 (54.9%) | 30 (48.4%) |
| >= 3500 g | 45 (39.8%) | 18 (35.3%) | 27 (43.6%) |
| Baseline anthropometric measures, mean (SD) | |||
| Body fat % | 23.9 (7.7) | 23.0 (6.8) | 24.6 (8.3) |
| Body mass index, kg/m2 | 17.2 (3.1) | 16.8 (2.6) | 17.5 (3.4) |
| BMI-for-age z-score | 0.34 (1.08) | 0.20 (1.02) | 0.45 (1.13) |
| Height, cm (SD) | 126.0 (11.2) | 127.1 (11.8) | 125.1 (10.7) |
| Baseline number of carious surfaces, mean (SD) | 9.1 (5.8) | 8.9 (6.0) | 9.2 (5.7) |
Percentage of girls who reached menarche during follow-up, among girls in that age category at baseline.
Among those who had menarche during the study period.
Self-reported by mother during the baseline study interview.
Discussion
This secondary analysis of a randomized trial of amalgam and composites found no evidence of associations between dental restoration material and changes in BMI, body fat percentage, or height velocity among children through 5 yrs of follow-up. Girls assigned to composites tended to have slightly greater increases in BMI and body fat percentage compared with girls assigned to amalgam, but the differences were not statistically significant. The finding that greater treatment level on primary teeth was associated with greater increases in body fat percentage over follow-up was observed for both treatment groups. Thus, overall, children who received composite or amalgam restoration materials experienced similar growth during the study period.
A limitation of this study is the lack of biomarker data on the children’s exposure to monomers used in dental composites. Numerous experimental studies have shown that the bisGMA-based composite resulted in bisGMA, TEGDMA, and BPA release (Pulgar et al., 2000; Al-Hiyasat et al., 2004; Ortengren et al., 2004; Yap et al., 2004; Sasaki et al., 2005), and one small study found that children’s urinary BPA concentrations remained elevated 14 days post-treatment (Martin et al., 2005). Chronic low-dose exposure to these resins over the life of the restoration is plausible, because chemical and mechanical interactions in the oral environment cause degradation over time, allowing unpolymerized monomers to leach out (Van Landuyt et al., 2011). However, the quantity and duration of resins released in or absorbed by the human body after the placement of dental materials and during the entire life of the restoration in the mouth have not been adequately studied.
A strength of the current study is that repeated measures of growth and development, with detailed longitudinal data on dental treatments, were collected prospectively through 5 yrs of follow-up. Moreover, the randomized trial design ensured no self-selection in dental treatment materials, minimizing the possibility of confounding for intent-to-treat analyses. In analyses of received treatment levels, separating bisGMA- and UDMA-based composites, we had the unique advantage of conducting parallel analyses using the amalgam group primary/permanent teeth data. This comparison indicated that the observed associations between UDMA-based compomer and greater increases in body fat were attributable to confounding by factors related to severity of dental disease on primary teeth, rather than to dental treatment itself. Plausible factors underlying these associations include diet, such as consumption of sugar-sweetened beverages (Blum et al., 2005; Ebbeling et al., 2006). NECAT did not collect detailed dietary data. Nevertheless, in the primary analysis of randomized treatment group, the groups were balanced in dietary intakes of general food groups, such as fruits/vegetables and fish.
Our analysis of age of menarche was exploratory and had several limitations. The menarche analysis was restricted to a small number (n = 113) of girls from one geographic stratum (rural Maine). NECAT did not have a sufficiently long follow-up period to determine age of menarche for all female participants. Although the median age at menarche of 12.5 yrs in NECAT was similar to that reported in NHANES 2001 (Parent et al., 2003), our calculation of age at menarche excluded 43% of the girls, because they were pre-menarcheal at the end of the study (their median age was 12.1 yrs at study’s end). Given the higher obesity prevalence in NECAT, it is interesting that menarche did not occur earlier in NECAT compared with the general population (Ogden et al., 2006). In the survival analysis, girls assigned to composites had a lower risk of menarche during the follow-up period. However, there was no association between received treatment level of composites and menarche.
Mercury has also been implicated as an endocrine disruptor (Tan et al., 2009). The only known published study of mercury exposure and menarche concurrently measured fasting blood mercury concentrations and self-reported menarche (yes/no) among 138 girls aged 10 to 16.9 yrs old in the Akwesasne Mohawk Nation (U.S. and Canadian border). Higher mercury concentrations were associated with higher odds of a girl’s having reached menarche, but the association was not significant after adjustment for age and socio-economic status (Denham et al., 2005). Interactions among various environmental exposures, diet, and genetic susceptibility may together determine onset of puberty and age of menarche (Parent et al., 2003). Thus, analysis of these exploratory NECAT data helps inform hypotheses for future testing in larger samples, rather than providing tenable conclusions. Additional studies of environmental contributors to menarche are necessary.
In summary, there were no associations between composite dental materials and physical development, including changes in BMI, body fat percentage, and height velocity, over 5 yrs of follow-up. Numerous genetic, dietary, environmental, and behavioral factors determine physical development and weight changes. In the context of these other predictors of physical development, the effects of resin-based dental materials, if any, may be difficult to discern. Nonetheless, there is compelling evidence that chemical monomers shown to have adverse effects in experimental studies are released from resin-based dental materials, and additional studies on the safety of these materials are warranted. As new resin materials are developed, a combination of toxicological, endocrinological, and epidemiological studies should monitor their safety and their potential to be exposure sources of endocrine-disrupting chemicals.
Supplementary Material
Footnotes
The analyses presented in this paper were funded by Award Number R01ES019155 from the National Institute of Environmental Health Sciences (NIEHS). The NIEHS had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The data collection was supported by a cooperative agreement (U01 DE11886) between the New England Research Institutes and the National Institute of Dental and Craniofacial Research (NIDCR), both of which participated in the design and conduct of the New England Children’s Amalgam Trial, including collection and management of data. The NIDCR had no role in the analyses or interpretation of data for this manuscript, or in the preparation, review, or approval of the manuscript. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Institute of Dental and Craniofacial Research, or the National Institutes of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Al-Hiyasat AS, Darmani H. (2006). In vivo effects of BISGMA—a component of dental composite—on male mouse reproduction and fertility. J Biomed Mater Res A 78:66-72. [DOI] [PubMed] [Google Scholar]
- Al-Hiyasat AS, Darmani H, Elbetieha AM. (2004). Leached components from dental composites and their effects on fertility of female mice. Eur J Oral Sci 112:267-272. [DOI] [PubMed] [Google Scholar]
- Bakopoulou A, Papadopoulos T, Garefis P. (2009). Molecular toxicology of substances released from resin-based dental restorative materials. Int J Mol Sci 10:3861-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC, Trachtenberg F, Barregard L, Tavares M, Cernichiari E, Daniel D, et al. (2006). Neuropsychological and renal effects of dental amalgam in children: a randomized clinical trial. J Am Med Assoc 295:1775-1783. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Trachtenberg F, Zhang A, Tavares M, Daniel D, McKinlay S. (2008). Dental amalgam and psychosocial status: the New England Children’s Amalgam Trial. J Dent Res 87:470-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum JW, Jacobsen DJ, Donnelly JE. (2005). Beverage consumption patterns in elementary school aged children across a two-year period. J Am Coll Nutr 24:93-98. [DOI] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. (2009). Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect 117:1945-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Children’s Amalgam Trial Study Group (2003). Children’s Amalgam Trial: design and methods. Control Clin Trials 24:795-814. [DOI] [PubMed] [Google Scholar]
- Darmani H, Al-Hiyasat AS. (2006). The effects of BIS-GMA and TEG-DMA on female mouse fertility. Dent Mater 22:353-358. [DOI] [PubMed] [Google Scholar]
- Denham M, Schell LM, Deane G, Gallo MV, Ravenscroft J, DeCaprio AP. (2005). Relationship of lead, mercury, mirex, dichlorodiphenyldichloroethylene, hexachlorobenzene, and polychlorinated biphenyls to timing of menarche among Akwesasne Mohawk girls. Pediatrics 115:e127-e134. [DOI] [PubMed] [Google Scholar]
- Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. (2006). Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics 117:673-680. [DOI] [PubMed] [Google Scholar]
- Hamid A, Okamoto A, Iwaku M, Hume WR. (1998). Component release from light-activated glass ionomer and compomer cements. J Oral Rehabil 25:94-99. [DOI] [PubMed] [Google Scholar]
- Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. (2007). Assessment of child and adolescent overweight and obesity. Pediatrics 120(Suppl 4):193-228. [DOI] [PubMed] [Google Scholar]
- Martin MD, Bajet D, Woods JS, Dills RL, Poulten EJ. (2005). Detection of dental composite and sealant resin components in urine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 99:429. [Google Scholar]
- Maserejian NN, Trachtenberg FL, Assmann SF, Barregard L. (2008). Dental amalgam exposure and urinary mercury levels in children: the New England Children’s Amalgam Trial. Environ Health Perspect 116:256-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maserejian NN, Trachtenberg FL, Hauser R, McKinlay S, Shrader P, Tavares M, et al. (2012). Dental composite restorations and psychosocial function in children. Pediatrics [Epub ahead of print July 16/2012] (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Harries L, Cipelli R, Henley W, Money C, McCormack P, et al. (2011). Bisphenol A exposure is associated with in vivo estrogenic gene expression in adults. Environ Health Perspect 119:1788-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Jorgensen N, Andersson AM, Calafat AM, Ye X, Redmon JB, et al. (2010). Are environmental levels of bisphenol A associated with reproductive function in fertile men? Environ Health Perspect 118:1286-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nah WH, Park MJ, Gye MC. (2011). Effects of early prepubertal exposure to bisphenol A on the onset of puberty, ovarian weights, and estrous cycle in female mice. Clin Exp Reprod Med 38:75-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. (2006). Prevalence of overweight and obesity in the United States, 1999-2004. J Am Med Assoc 295:1549-1555. [DOI] [PubMed] [Google Scholar]
- Ortengren U, Langer S, Goransson A, Lundgren T. (2004). Influence of pH and time on organic substance release from a model dental composite: a fluorescence spectrophotometry and gas chromatography/mass spectrometry analysis. Eur J Oral Sci 112:530-537. [DOI] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, vom Saal FS, Parmigiani S. (2008). Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environ Res 108:150-157. [DOI] [PubMed] [Google Scholar]
- Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. (2003). The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev 24:668-693. [DOI] [PubMed] [Google Scholar]
- Pulgar R, Olea-Serrano MF, Novillo-Fertrell A, Rivas A, Pazos P, Pedraza V, et al. (2000). Determination of bisphenol A and related aromatic compounds released from bis-GMA-based composites and sealants by high performance liquid chromatography. Environ Health Perspect 108:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. (2007). In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 24:199-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubiano F, Nuñez C, Heymsfield SB. (1999). Validity of consumer model bioimpedance analysis system with established dual energy x-ray absorptiometry (DXA). Medicine & Science in Sports & Exercise 31:S202. [Google Scholar]
- Sasaki N, Okuda K, Kato T, Kakishima H, Okuma H, Abe K, et al. (2005). Salivary bisphenol-A levels detected by ELISA after restoration with composite resin. J Mater Sci Mater Med 16:297-300. [DOI] [PubMed] [Google Scholar]
- Scheuplein R, Charnley G, Dourson M. (2002). Differential sensitivity of children and adults to chemical toxicity. I. Biological basis. Regul Toxicol Pharmacol 35:429-447. [DOI] [PubMed] [Google Scholar]
- Schmalz G, Preiss A, Arenholt-Bindslev D. (1999). Bisphenol-A content of resin monomers and related degradation products. Clin Oral Investig 3:114-119. [DOI] [PubMed] [Google Scholar]
- Söderholm KJ, Mariotti A. (1999). BIS-GMA-based resins in dentistry: are they safe? J Am Dent Assoc 130:201-209. [DOI] [PubMed] [Google Scholar]
- Tan SW, Meiller JC, Mahaffey KR. (2009). The endocrine effects of mercury in humans and wildlife. Crit Rev Toxicol 39:228-269. [DOI] [PubMed] [Google Scholar]
- Van Landuyt KL, Nawrot T, Geebelen B, De Munck J, Snauwaert J, Yoshihara K, et al. (2011). How much do resin-based dental materials release? A meta-analytical approach. Dent Mater 27:723-747. [DOI] [PubMed] [Google Scholar]
- Yap AU, Han VT, Soh MS, Siow KS. (2004). Elution of leachable components from composites after LED and halogen light irradiation. Oper Dent 29:448-453. [PubMed] [Google Scholar]
- Yu C, Tai F, Song Z, Wu R, Zhang X, He F. (2011). Pubertal exposure to bisphenol A disrupts behavior in adult C57BL/6J mice. Environ Toxicol Pharmacol 31:88-99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.