Abstract
Background
The increased risk of gastrointestinal (GI) cancers after Hodgkin's lymphoma (HL) is well established. However, no large population-based study has described the actuarial survival after subsequent GI cancers in HL survivors (HL-GI).
Patients and methods
For 209 patients with HL-GI cancers (105 colon, 35 stomach, 30 pancreas, 21 rectum, and 18 esophagus) and 484 165 patients with first primary GI cancers (GI-1), actuarial survival was compared, accounting for age, gender, race, GI cancer stage, radiation for HL, and other variables.
Results
Though survival of HL patients who developed localized stage colon cancer was similar to that of the GI-1 group, overall survival (OS) of HL patients with regional or distant stage colon cancer was reduced [hazard ratio, (HR) = 1.46, P = 0.01]. The HL survivors with regional or distant stage colon cancer in the transverse segment had an especially high risk of mortality (HR: 2.7, P = 0.001 for OS). For localized stomach cancer, OS was inferior among HL survivors (HR = 3.46, P = 0.006).
Conclusions
The HL patients who develop GI cancer experience significantly reduced survival compared with patients with a first primary GI cancer. Further research is needed to explain the inferior survival of HL patients and to define selection criteria for cancer screening in HL survivors.
Keywords: cancer survivorship, gastrointestinal cancers, Hodgkin lymphoma, second cancers, SEER
introduction
Hodgkin's lymphoma (HL) has become a highly curable disease [1]. However, HL survivors face an increased risk of death from other cancers compared with the general population [2, 3]. Mortality from second cancers after HL exceeds that due to HL 15–30 years after treatment [4]. Of second cancers after HL, those in the gastrointestinal (GI) system account for the third largest absolute excess risk, following lung and breast cancer [5, 6]. Hodgson et al. [6] found that among HL patients treated before age 25, the risk of colorectal cancer by age 40 was higher than the risk in 50-year olds in the general population, for whom screening for colorectal cancer is recommended. Increasing awareness of the risk of second cancers among HL survivors has important implications for longitudinal clinical care for HL survivors, such as screening practices. However, information on the survival after second cancers among HL survivors is sparse [7–11]. Milano et al. [11] found that female HL survivors with subsequent breast cancer had worse survival compared with women with a primary breast cancer. Despite increasing awareness of the risk of second cancers in the GI system, no population-based study compares the characteristics and survival of subsequent GI cancers among HL survivors with patients in the general population with a primary GI cancer [12].
Among 18 671 HL survivors (221 021 person years of follow-up) reported to the Surveillance, Epidemiology, and End Results (SEER) program, we identified all subsequent GI cancers in HL survivors (HL-GI), including colon, stomach, pancreas, rectum, and esophagus. We hypothesized that overall survival (OS) and GI cancer cause-specific survival (CSS) would be worse compared with patients with a first primary GI cancer (GI-1) in the SEER program.
patients and methods
patient database
From the SEER 9 (1973–2007) database [13], we identified 18 671 HL patients who were diagnosed histopathologically, then actively followed using methods applied previously [11, 14]. A minimum latency was 2 months according to the standard latency adapted by the SEER program to exclude synchronous primary cancers [13]. Two hundred and thirty patients subsequently developed GI cancers (114 colon, 40 stomach, 35 pancreas, 23 rectum, and 18 esophagus). Twenty-one patients were excluded due to the lack of data on GI cancer stage. The remaining 209 patients (105 colon, 35 stomach, 30 pancreas, 21 rectum, and 18 esophagus) were included in our analysis. The same exclusion criteria were applied to the GI-1 group and 476 592 patients (240 459 colon, 53 131 stomach, 62 336 pancreas, 97 256 rectum, and 23 410 pancreas) were identified.
HL stage was determined as described previously [11, 14] using information from the SEER extent of disease fields that assign HL patients to three categories (stage I, II, and III-IV), according to the Ann Arbor classification. GI cancers were staged using the SEER historic stage A field, categorized as localized, regional, or distant (metastatic). As information on radiation dose and fields is not collected by the SEER program, whether radiotherapy was administered to the abdomen or pelvis could not be determined.
statistical analyses
Patient and GI-cancer characteristics were compared between HL-GI and GI-1 patients using the chi-square test or Fisher's exact test. Actuarial survival was calculated using the Kaplan–Meier method. Survival times were measured from the date of GI cancer diagnosis until the date of death or last follow-up. For the GI-1 group, actuarial survival was calculated for randomly selected subsets of patients, matched to the HL-GI group using strata defined by age at GI cancer diagnosis (age: <50, 50–60, 60–70, >70) and year at diagnosis (<1990, 1990–2000, >2000). The age-matched Kaplan–Meier curve of the GI-1 group was generated by repeating these matching 100 times and calculating weighted average 100 matched survival curves.
Cox proportional hazards models were used to compare the survival after GI cancer between HL-GI and GI-1. Variables included in the Cox model included age and calendar year at GI cancer diagnosis, GI cancer stage, sex, race, socioeconomic status, and surgery or radiation for GI cancers. All analyses were conducted using a SAS software package [15].
dosimetry analysis
The radiation dose delivered to specific colon segments during the HL treatment was modeled using measurements from water and anthropomorphic phantom [16]. Average doses were calculated for both para-aortic and inverted-Y fields using anterior and posterior techniques to a total dose of 35 Gy. Segments of the colon were categorized as ascending (cecum, appendix, and ascending colon), transverse (hepatic flexure, transverse colon, and splenic flexure), and descending (descending and sigmoid colon).
results
patient and tumor characteristics
Patient and disease characteristics at the time of HL diagnosis for each subsequent GI cancer site are shown in Table 1. Median age at the time of HL diagnosis in the 209 patients was 46 years (51 for colon, 32 for stomach, 42 for pancreas, 52 for rectum, and 41 for esophagus). Overall, 39% of patients were diagnosed with HL before age 40, while 69% of patients with subsequent stomach cancer had HL before age 40. The majority of patients had classical HL, with nodular sclerosis being the most frequent type (49%). Fifty-four percent of all patients and 64% of patients younger than 40 years at the time of HL diagnosis received radiation for HL. Forty-seven percent of patients had stage III or IV HL.
Table 1.
Patient and tumor characteristics at time of HL diagnosis
| GI sites | All |
Colon |
Stomach |
Pancreas |
Rectum |
Esophagus |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Number | % | Number | % | Number | % | Number | % | Number | % | Number | % |
| Number of patients | 209 | 105 | 35 | 30 | 21 | 18 | ||||||
| Age at HL diagnosis (years) | ||||||||||||
| <15 | 4 | 2 | 1 | 1 | 1 | 3 | 1 | 3 | 0 | 0 | 1 | 6 |
| 15–39 | 79 | 37 | 30 | 29 | 23 | 66 | 12 | 40 | 5 | 24 | 7 | 39 |
| 40–59 | 73 | 35 | 39 | 37 | 6 | 17 | 11 | 37 | 9 | 43 | 8 | 44 |
| >60 | 55 | 26 | 35 | 33 | 5 | 14 | 6 | 20 | 7 | 33 | 2 | 11 |
| Year of diagnosis | ||||||||||||
| 1973–1979 | 77 | 37 | 35 | 33 | 20 | 57 | 12 | 40 | 4 | 24 | 6 | 33 |
| 1980–1989 | 79 | 38 | 44 | 42 | 11 | 31 | 10 | 33 | 8 | 38 | 6 | 33 |
| 1990–2007 | 53 | 25 | 26 | 25 | 4 | 11 | 8 | 27 | 9 | 43 | 6 | 33 |
| Subtype | ||||||||||||
| Nodular sclerosis | 102 | 49 | 47 | 45 | 18 | 51 | 16 | 53 | 10 | 48 | 11 | 61 |
| Mixed cellularity | 54 | 25 | 31 | 30 | 11 | 31 | 6 | 20 | 4 | 19 | 2 | 11 |
| Other | 38 | 18 | 12 | 11 | 6 | 17 | 8 | 27 | 7 | 33 | 5 | 28 |
| Unknown | 15 | 7 | 15 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stage of HLa | ||||||||||||
| I | 60 | 29 | 32 | 30 | 8 | 23 | 13 | 43 | 4 | 19 | 3 | 17 |
| II | 50 | 24 | 25 | 24 | 9 | 26 | 7 | 23 | 8 | 38 | 1 | 6 |
| III/IV | 99 | 47 | 48 | 46 | 18 | 51 | 10 | 33 | 9 | 43 | 14 | 78 |
| Radiotherapy | ||||||||||||
| Yes | 114 | 55 | 55 | 52 | 24 | 69 | 13 | 43 | 13 | 62 | 9 | 50 |
| No | 92 | 44 | 48 | 46 | 11 | 31 | 17 | 57 | 8 | 38 | 8 | 44 |
| Unknown | 3 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 6 |
aRefer to reference [11].
HL, Hodgkin's lymphoma; GI, gastrointestinal.
Table 2 outlines patient and GI-cancer characteristics of HL-GI and GI-1 groups at the time of GI cancer diagnosis. There was no significant difference in gender between HL-GI and GI-1 for any site. Median age for HL-GI patients at the time of GI cancer diagnosis was 63 years for colon, 52 for stomach, 61 for pancreas, and 58 for rectum and esophagus. HL-GI patients were significantly younger at the time of GI cancer diagnosis for all sites compared with GI-1 patients (median age: 71 for colon, 69 for stomach, 69 for pancreas, 67 for rectum, and 66 for esophagus; P < 0.001 for all sites).
Table 2.
Patient and tumor characteristics at time of GI cancer diagnosis
| Characteristics | Colon |
Stomach |
Pancreas |
Rectum |
Esophagus |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HL-GI | GI-1 | HL-GI | GI-1 | HL-GI | GI-1 | HL-GI | GI-1 | HL-GI | GI-1 | |
| Number of patients | 105 | 240 459 | 35 | 53 131 | 30 | 62 336 | 21 | 97 256 | 18 | 23 410 |
| Age at GI cancer diagnosis (years) | ||||||||||
| Median | 63 | 71 | 52 | 69 | 61 | 69 | 58 | 67 | 58 | 66 |
| <49 | 20% | 7% | 46% | 10% | 30% | 7% | 24% | 10% | 28% | 9% |
| 50–69 | 44% | 39% | 40% | 42% | 43% | 44% | 52% | 47% | 61% | 55% |
| 70+ | 36% | 54% | 14% | 48% | 27% | 48% | 24% | 43% | 11% | 37% |
| P | <0.0001 | <0.0001* | 0.013* | 0.01* | 0.000587 | |||||
| Gender | ||||||||||
| Male | 54% | 48% | 71% | 63% | 63% | 51% | 76% | 56% | 78% | 76% |
| Female | 46% | 52% | 29% | 37% | 37% | 49% | 29% | 44% | 22% | 24% |
| P | 0.204 | 0.3819 | 1* | 0.1356 | 0.2016 | |||||
| Latency (years) | ||||||||||
| <10 | 30% | NA | 22% | NA | 34% | NA | 62% | NA | 28% | NA |
| 10–20 | 39% | NA | 46% | NA | 30% | NA | 24% | NA | 44% | NA |
| 20–30 | 21% | NA | 31% | NA | 37% | NA | 14% | NA | 28% | NA |
| Cancer stage | ||||||||||
| Localized | 33% | 37% | 23% | 23% | 3% | 12% | 48% | 46% | 22% | 32% |
| Regional | 39% | 40% | 40% | 36% | 43% | 32% | 43% | 37% | 61% | 33% |
| Metastatic | 28% | 23% | 37% | 41% | 53% | 69% | 10% | 18% | 17% | 35% |
| P | 0.4579 | 0.908 | 0.05482* | 0.6805* | 0.1479* | |||||
| Grade | ||||||||||
| I | 11% | 11% | 0% | 5% | 13% | 6% | 0% | 11% | 6% | 6% |
| II | 52% | 50% | 20% | 19% | 30% | 14% | 67% | 52% | 6% | 30% |
| III | 16% | 16% | 49% | 44% | 10% | 17% | 14% | 13% | 67% | 38% |
| IV | 3% | 1% | 11% | 4% | 0% | 2% | 0% | 1% | 6% | 3% |
| Unknown | 17% | 21% | 20% | 27% | 47% | 61% | 19% | 23% | 17% | 23% |
| P | 0.4596*a | 0.1682*a | 0.1018*a | 0.1018*a | 0.02706*a | |||||
| Surgery | ||||||||||
| Yes | 91% | 92% | 30% | 30% | 44% | 44% | 95% | 88% | 30% | 15% |
| No | 9% | 7% | 70% | 70% | 56% | 56% | 5% | 11% | 70% | 80% |
| Unknown | 0% | 1% | 0% | 0% | 0% | 0% | 0% | 1% | 0% | 5% |
| P | 0.5718a | 0.8598a | 0.04173a | 0.04173a | 0.4593a | |||||
| Radiotherapy | ||||||||||
| Yes | 3% | 3% | 29% | 29% | 17% | 17% | 59% | 19% | 29% | 10% |
| No | 96% | 97% | 90% | 90% | 56% | 56% | 81% | 70% | 90% | 18% |
| Unknown | 1% | 0% | 0% | 0% | 0% | 0% | 0% | 1% | 0% | 82% |
| P | 1*a | 0.8238a | 0.3447*a | 0.3447*a | 0.2266a | |||||
P values represent chi-squared test comparing the distribution between HL-GI and GI-1 patients for the GI sites and the indicated variables.
aRepresents chi-squared test omitting patients with unknown variables.
*Represents P values from fisher's exact test.
GI, gastrointestinal; HL-GI, cancer in the gastrointestinal tract after Hodgkin's lymphoma; GI-1, first or only primary cancer in the gastrointestinal tract; NA, not available.
The distribution of clinical stage at the time of HL-GI diagnosis was comparable to those observed for GI-1 for cancers of stomach, esophagus, rectum, and pancreas. While the distribution of clinical stages for all colon cancer considered together did not differ between HL-GI and GI-1 patients, cancers in the transverse colon were diagnosed at significantly more advanced stages in the former group (P = 0.024). Twenty-one HL patients developed cancer in the transverse segment, 19 of whom had regional or distant disease. Ten patients with second cancer in the transverse segment received radiation therapy (RT) for observed/expected (HL). While regional/distant (O/E) of these patients were elevated, it was not substantially significant [O/E 1.87; 95% confidence interval (CI) 0.9–3.44]. To investigate whether an association might exist between radiation dose to colon segment and stage of subsequent colon cancer, we calculated doses delivered to various segments of colon during typical abdominal radiotherapy for HL [5]. Dosimetry modeling showed that the transverse colon received 81% of the prescribed dose (versus 4–43% to other segments). No difference in cancer stage based upon sub-site within either stomach (cardia, fundus, body, and pylorus) or rectum (rectosigmoid junction and rectum) was noted. For colorectal, stomach, and pancreas cancers, tumor grade was comparable between HL-GI and GI-1 groups. However, a greater proportion of HL-esophagus patients had poorly differentiated cancer (P = 0.027). No difference in initial treatment for HL-GI and GI-1 cancers was observed in any site.
vital status at last follow-up and cause of death
Supplementary Table S3, available at Annals of Oncology online, outlines vital status at the time of last follow-up and cause of death for HL-GI and GI-1 groups. Overall median follow-up of the HL-GI group was 14 months after GI cancer diagnosis. HL survivors with subsequent colorectal cancer had a median follow-up of 23 months, while patients with stomach, pancreas, and esophagus cancers had a median follow-up of >10 months (9.0, 4.5, and 5.5 months respectively). Among patients alive at the last follow-up, the median follow-up was 49 months (54 months for colorectal and 23 months for other sites). Of 209 HL-GI patients, 147 (70%) were deceased; 59% of colorectal cancer patients and 83% of patients with other GI cancers had died. Among patients with localized HL-colon cancer, comparable percentages died from colon cancer (23%) and other cancers (23%), while 15% of deaths were due to HL. On the other hand, 63% of deaths among patients with regional or distant HL-colon cancer were attributed to colon cancer, similar to 63% of deaths in the GI-1 group. Sixty-eight percent of deaths among HL survivors with cancer of the stomach, esophagus, and pancreas were due to the respective GI cancers. Twenty-four HL-GI patients died of other cancers and 27 died of noncancer causes.
survival of HL-GI versus GI-1 groups
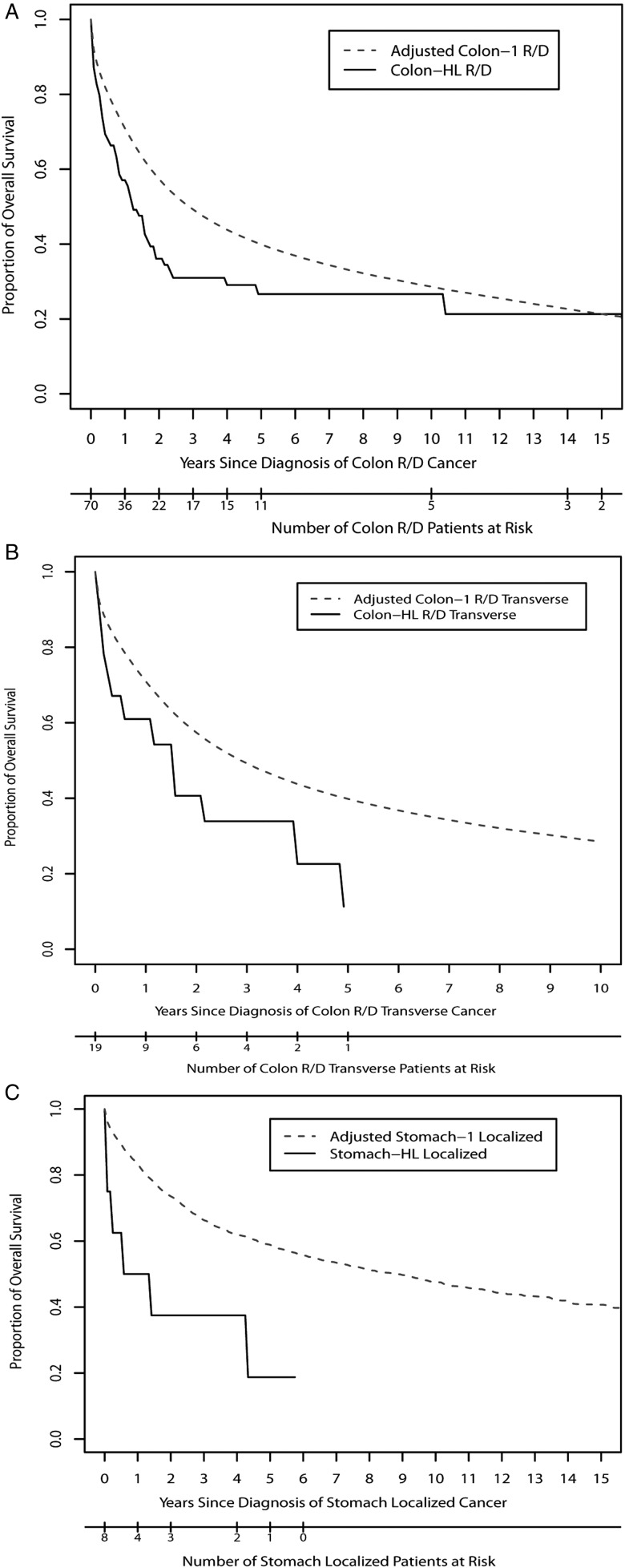
Supplementary Table S4, available at Annals of Oncology online, and Figure 1 compare survival between HL-GI and GI-1 groups. The HL patients who developed localized colon cancer had similar survival to GI-1 patients, whereas those with regional or distant disease had a significantly increased risk of death [hazard ratio (HR) = 1.46, 95% CI 1.1–1.95]. In particular, HL survivors with regional or distant stage cancers in the transverse colon segment had especially high risks of mortality (HR = 2.7 and 2.6; 95% CI 1.5–4.6 and 1.4–4.7; P = 0.001 and 0.002 for OS and CSS, respectively). HL-GI and GI-1 outcomes after rectal cancer were similar to colon cancer. Mortality after regional and distant stage rectal cancer among HL survivors was increased more than twofold, while outcomes after localized disease were not significantly different between HL-GI and GI-1 groups. For localized stomach cancer, OS for HL-stomach was reduced compared with primary stomach cancer, with a HR of 3.46 (95% CI 1.44–8.32). However, this observation was based on eight deaths in the HL-GI group. Owing to the small number of HL-GI patients in each site, analyses based on patient or treatment factors that may affect the survival after GI cancers were not carried out.
Figure 1.
Comparison of overall survival between HL-GI and GI-1 for (A) regional or distant colon cancer, overall (B) regional or distant colon cancer in the transverse segment, (C) localized stomach cancer. For regional or distant colon, 25 340 colon-1 R/D patients were randomly selected from within strata matched to the 70 HL-colon R/D patients; for regional or distant transverse colon, 4294 colon-1 R/D transverse patients were randomly selected from within strata matched to the 19 HL-colon R/D patients; and for localized stomach, 1672 stomach-1 patients were randomly selected from within strata matched to the eight HL-stomach patients. The strata are defined by age and year at diagnosis (age: <50, 50–60, 60–70, ≥70 years and year at diagnosis: <1990, 1990–2000, ≥2000). These matching analyses were repeated 100 times, and the calculated Kaplan–Meier actuarial survivals of the group with first primary GI cancers represent a weighted average of 100 matched survival curves.
discussion
To our knowledge, this is the first population-based study on outcomes after a diagnosis of second primary GI cancers among HL survivors. The large cohort of HL patients (n = 18 671) enabled us to identify a substantial number of second GI cancers for comparison with the general US population, with all study subjects derived from the SEER program. After adjusting for age and calendar year at GI cancer diagnosis, socioeconomic status, and cancer treatment variables, we found that HL survivors with regional or distant stage colon cancer and localized stage stomach cancer had inferior OS than patients who developed de novo GI malignancies, with significant HRs of 1.46 and 3.46, respectively. HL survivors who developed localized esophageal cancer or regional or distant stage rectal cancer also experienced significantly increased risk of deaths, albeit based upon only 4 and 11 deaths, respectively.
risks of subsequent GI cancers
It is well established that HL survivors are at the increased risk of developing subsequent cancers in the GI system [5, 7–10, 17]. Factors associated with increased risk of second GI cancers include alkylating agents [17], radiotherapy [5, 7–10], and younger age at HL diagnosis [6, 7, 9], while other pertinent factors such as heritable cancer syndromes or geographic area have not been analyzed in these retrospective studies. Use of larger radiation field including abdomen and pelvis was shown to be associated with the higher risk of second cancers [5]. However, no prospective studies showed dose- and field-dependent association between abdominal radiation and second GI cancer risk. Hodgson et al. [6] pointed out the need for early screening for colorectal cancer in selected HL survivors as the risk of colorectal cancer by age 40 of patients who were treated before age 25 was higher than the risk in 50-year olds in the general population. A prospective clinical trial that looks into the benefit of colonoscopy screening on young patients who received abdominal radiation more than 10 years ago is in progress.
Increased risk of second cancers among HL survivors translates into increased mortality. Studies by Aleman et al. [2] and Ng et al. [18] showed that mortality from second cancers after HL exceeds that due to HL 15–30 years after treatment.
Cancer mortality, however, is not only influenced by incidence, but also by disease characteristics such as clinical stage and tumor biology. Recent reports [11, 19] analyzed the characteristics and outcome of second breast cancer among HL survivors compared with those of first primary cancers in matched cohorts, and found that second cancers occur earlier and survival is worse. While the increased risk of second GI cancer among HL survivors is well established, our study is the first to report inferior outcome of second GI cancers compared with primary GI cancers.
survival after subsequent colon cancer
HL survivors developed colon cancer at a significantly younger age than patients with a first primary colon cancer. Though survival of HL patients who developed localized colon cancer was similar to that of the GI-1 group, age-matched OS of HL patients with regional or distant stage colon cancer was significantly reduced by almost 50%. Among colon cancer patients in the general population, younger age has been reported to be a poor prognostic factor, perhaps attributable to more aggressive disease in the young [20–23]. However, O'Connell et al. [24] reported that the inferior survival among young patients was attributed to a higher frequency of stage III-IV disease; the stage-specific survival of younger patients was similar or better compared with older patients. While the age-matched OS of HL patients with regional or distant stage colon cancer was worse, we could not appreciate inferior CSS or increased deaths due to other medical causes.
In our study, no significant difference in pathologic stage of colon cancer existed between HL-GI and GI-1 groups, when examined by age. In subgroup analyses, however, the frequency of more advanced stage disease in the transverse colon was greater in HL-GI patients, who also experienced a significantly reduced OS and CSS. While we were able to show that transverse segment of colon receives significantly higher dose of radiation in modeling of abdominal RT for HL, there was no significantly increased risk of second cancer in transverse segment after RT. Whether higher radiation doses result in the development of more aggressive colon cancers has not been studied to our knowledge and could not be answered from our study.
survival after subsequent stomach cancer
For early noninvasive stomach cancer in the general population, younger age is associated with improved survival [25, 26]. In contrast, we found that HL survivors developed localized stomach cancers at a younger age compared with the patients with primary localized stomach cancer and had a poorer outcome (HR = 3.46). However, it is important to note that a small number (n = 8) of patients warrants cautious interpretation of this result. Also, we could not analyze the independent effect of age on survival. It is possible that radiation-induced stomach cancer has more aggressive tumor biology. While further research is needed to explain the inferior survival after localized stomach cancer among HL survivors, early diagnostic evaluation upon presentation of dyspectic symptoms in patients who have additional risk factors for stomach cancer, such as a history of infection with Helicobacter pylori [27, 28], and tobacco use [29], may improve outcomes. In Japan, where the general population has an eightfold greater incidence of gastric cancer than in Western populations [30, 31], endoscopic screening results in diagnosis of gastric cancer at an earlier age, earlier nodal stage at diagnosis, and improved CSS [32].
noncancer mortality
Overall, 51 of 147 deaths were due to other cancers or noncancer causes (data not shown). Because of the small number of deaths from these causes, we were not able to compare mortality from other cancers or noncancer causes between HL-GI and GI-1 subgroups, as we had done in a similar analysis of breast cancer patients [11].
summary
Through a large population-based analysis that minimized selection bias and improved generalizability, we report significantly inferior OS among HL survivors who developed subsequent advanced colorectal or localized stomach malignancies compared with patients presenting with similar de novo cancers. This finding along with the increased risk of developing second GI malignancies among HL survivors, which persists more than years, and the importance of lifelong vigilance in this vulnerable population. Strengths of the current study include the sizable number of patients (476 592 patients with de novo GI cancer and 209 patients with GI cancer after HL) identified in a large population-based setting. Substantial patient numbers allowed for analyses of outcomes according to GI site and stage. Known limitations of SEER data include a lack of detailed information with regard to radiotherapy and an absence of chemotherapy data. Further, lifestyle and social factors associated with the development of cancers in the GI tract are not recorded (i.e. diet and alcohol use). Future research should continue to address the relationship between the dose of chemotherapy or radiation to specific organs and the subsequent risk of GI cancers, as well as the interaction with other established risk factors. Improved understanding of treatment-related risk regarding second GI cancer will help in the consideration of screening guidelines for patients at highest risk.
funding
This work was supported by the National Center for Research Resources [TL1 RR 024135 to PY]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR or the NIH.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We thank Laura Finger for editorial assistance. This paper was selected for presentation at the 2011 ASCO meeting.
references
- 1.Donaldson SS, Hancock SL, Hoppe RT. The Janeway lecture. Hodgkin's disease–finding the balance between cure and late effects. Cancer J Sci Am. 1999;5:325–333. [PubMed] [Google Scholar]
- 2.Aleman BM, van den Belt-Dusebout AW, Klokman WJ, et al. Long-term cause-specific mortality of patients treated for Hodgkin's disease. J Clin Oncol. 2003;21:3431–3439. doi: 10.1200/JCO.2003.07.131. doi:10.1200/JCO.2003.07.131. [DOI] [PubMed] [Google Scholar]
- 3.Hoppe RT. Hodgkin's disease: complications of therapy and excess mortality. Ann Oncol. 1997;8:115–118. doi:10.1023/A:1008238908676. [PubMed] [Google Scholar]
- 4.Mauch PM, Kalish LA, Marcus KC, et al. Long-term survival in Hodgkin's disease relative impact of mortality, second tumors, infection, and cardiovascular disease. Cancer J Sci Am. 1995;1:33–42. [PubMed] [Google Scholar]
- 5.Ng AK, Bernardo MV, Weller E, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long-term risks and risk factors. Blood. 2002;100:1989–1996. doi: 10.1182/blood-2002-02-0634. doi:10.1182/blood-2002-02-0634. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol. 2007;25:1489–1497. doi: 10.1200/JCO.2006.09.0936. doi:10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- 7.Dores GM, Metayer C, Curtis RE, et al. Second malignant neoplasms among long-term survivors of Hodgkin's disease: a population-based evaluation over 25 years. J Clin Oncol. 2002;20:3484–3494. doi: 10.1200/JCO.2002.09.038. doi:10.1200/JCO.2002.09.038. [DOI] [PubMed] [Google Scholar]
- 8.van Leeuwen FE, Klokman WJ, Veer MB, et al. Long-term risk of second malignancy in survivors of Hodgkin's disease treated during adolescence or young adulthood. J Clin Oncol. 2000;18:487–497. doi: 10.1200/JCO.2000.18.3.487. [DOI] [PubMed] [Google Scholar]
- 9.Swerdlow AJ, Barber JA, Hudson GV, et al. Risk of second malignancy after Hodgkin's disease in a collaborative British cohort: the relation to age at treatment. J Clin Oncol. 2000;18:498–509. doi: 10.1200/JCO.2000.18.3.498. [DOI] [PubMed] [Google Scholar]
- 10.Behringer K, Josting A, Schiller P, et al. Solid tumors in patients for Hodgkin's disease: a report from the German Hodgkin Lymphoma Study Group. Ann Oncol. 2004;15:1079–1085. doi: 10.1093/annonc/mdh273. doi:10.1093/annonc/mdh273. [DOI] [PubMed] [Google Scholar]
- 11.Milano MT, Li H, Gail MH, et al. Long-term survival among patients with Hodgkin's lymphoma who developed breast cancer: a population-based study. J Clin Oncol. 2010;28:5088–5096. doi: 10.1200/JCO.2010.29.5683. doi:10.1200/JCO.2010.29.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birdwell SH, Hancock SL, Varghese A, et al. Gastrointestinal cancer after treatment of Hodgkin's disease. Int J Radiat Oncol Biol Phys. 1997;17:67–73. doi: 10.1016/s0360-3016(96)00489-0. doi:10.1016/S0360-3016(96)00489-0. [DOI] [PubMed] [Google Scholar]
- 13.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 9 Regs Limited-Use, November 2009 Sub (1973–2007) (Katrina/Rita Population Adjustment). Linked To County Attributes: Total US, 1969–2007 Counties. Bethesda, MD: National Cancer Institute, Division of Cancer Control and Population Sciences; Surveillance Research Program, Cancer Statistics Branch, (April 2010, released), based on the November 2009 submission www.seer.cancer.gov . [Google Scholar]
- 14.Milano MT, Li H, Constine LS, Travis LB. Survival after second primary lung cancer: a population-based study of 187 Hodgkin lymphoma patients. Cancer. 2011;117:5538–5547. doi: 10.1002/cncr.26257. doi:10.1002/cncr.26257. [DOI] [PubMed] [Google Scholar]
- 15.SAS Institute Inc. SAS 9.1.3 Help and Documentation. Cary, NC: SAS Institute INC.; 2000–2004. [Google Scholar]
- 16.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. doi:10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 17.van den Belt-dusebout AW, Aleman BM, Besseling G, et al. Roles of radiation dose and chemotherapy in the etiology of stomach cancer as a second malignancy. Int J Radiat Oncol Biol Phys. 2009;75:1420–1429. doi: 10.1016/j.ijrobp.2009.01.073. doi:10.1016/j.ijrobp.2009.01.073. [DOI] [PubMed] [Google Scholar]
- 18.Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin's disease treated at age 50 or younger. J Clin Oncol. 2002;20:2101–2108. doi: 10.1200/JCO.2002.08.021. doi:10.1200/JCO.2002.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Elkin EB, Klem ML, Gonzales AM, et al. Characteristics and outcomes of breast cancer in women with and without a history of radiation for Hodgkin's lymphoma: a multi-institutional, matched cohort study. J Clin Oncol. 2011;29:2466–2473. doi: 10.1200/JCO.2010.32.4079. doi:10.1200/JCO.2010.32.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee PY, Fletcher WS, Sullivan ES, et al. Colorectal cancer in young patients: characteristics and outcome. Am Surg. 1994;60:607–612. [PubMed] [Google Scholar]
- 21.Leff DR, Chen A, Roberts D, et al. Colorectal cancer in the young patient. Am Surg. 2007;73:42–47. [PubMed] [Google Scholar]
- 22.Griffin PM, Liff JM, Greenberg RS, et al. Adenocarcinomas of the colon and rectum in persons under 40 years old. A population-based study. Gastroenterology. 1991;100:1033–1041. doi: 10.1016/0016-5085(91)90279-t. [DOI] [PubMed] [Google Scholar]
- 23.Liang JT, Huang KC, Cheng AL, et al. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br J Surg. 2003;90:205–214. doi: 10.1002/bjs.4015. doi:10.1002/bjs.4015. [DOI] [PubMed] [Google Scholar]
- 24.O'Connell JB, Maggard MA, Liu JH, et al. Do young colon cancer patients have worse outcomes? World J Surg. 2004;28:558–562. doi: 10.1007/s00268-004-7306-7. doi:10.1007/s00268-004-7306-7. [DOI] [PubMed] [Google Scholar]
- 25.Haugstvedt TK, Viste A, Eide GE, et al. Norwegian multicentre study of survival and prognostic factors in patients undergoing curative resection for gastric carcinoma. The Norwegian Stomach Cancer Trial. Br J Surg. 1993;80:475–488. doi: 10.1002/bjs.1800800423. doi:10.1002/bjs.1800800423. [DOI] [PubMed] [Google Scholar]
- 26.Winslet MC, Mohsen YM, Powell J, et al. The influence of age on the surgical management of carcinoma of the stomach. Eur J Surg Oncol. 1996;22:220–224. doi: 10.1016/s0748-7983(96)80006-0. [DOI] [PubMed] [Google Scholar]
- 27.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. doi:10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 28.Leung WK, Wu M, Kakugawa Y and the Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. doi:10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 29.Sasazuki S, Sasaki S, Tsugane S and Japan Public Health Center Study Group. Cigarette smoking, alcohol consumption, and subsequent gastric cancer risk by subsite and histologic type. Int J Cancer. 2002;101:560–566. doi: 10.1002/ijc.10649. doi:10.1002/ijc.10649. [DOI] [PubMed] [Google Scholar]
- 30.Hisamichi S. Screening for gastric cancer. World J Surg. 1989;13:31–37. doi: 10.1007/BF01671151. doi:10.1007/BF01671151. [DOI] [PubMed] [Google Scholar]
- 31.Fukao A, Tsubono Y, Tsuji I, et al. The evaluation of screening for gastric cancer in Miyagi prefecture, Japan. A population-based case-control study. Int J Cancer. 1995;60:45–48. doi: 10.1002/ijc.2910600106. doi:10.1002/ijc.2910600106. [DOI] [PubMed] [Google Scholar]
- 32.Kunisaki C, Ishino J, Nakajima S, et al. Outcomes of mass screening for gastric carcinoma. Ann Surg Oncol. 2006;13:221–228. doi: 10.1245/ASO.2006.04.028. doi:10.1245/ASO.2006.04.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.