Abstract
A one-pot cascade arylative epoxidation of enolizable ketones with bromopentafluorobenzene (PFPBr) and derivatives into perfluoroaryl oxiranes is reported. PFPBr is utilized as an equivalent of Br+ and PFP− in this highly efficient, easily scaled up and diastereoselective epoxidation reaction, which produces synthetically useful polyfluoroaryl oxiranes.
Keywords: bromopentafluorobenzene, polyfluoroarylepoxides, cascade transformation
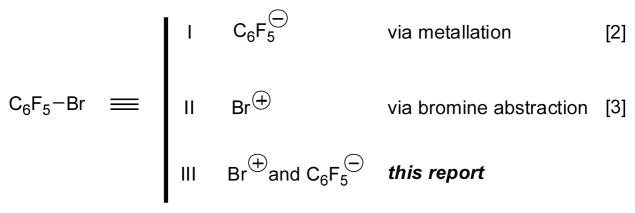
Bromopentafluorobenzene (PFPBr) is an important building block for the preparation of polyfluorophenyl containing compounds.[1] Under different conditions, the PFP–Br bond can be cleaved in a diverse manner (Scheme 1). Through metallation reactions with alkali metals, alkyl lithium reagents, or oxidative additions to Pd (0), PFPBr serves as an equivalent of the PFP anion[2] (Mode I), which participates in reactions with diverse electrophiles or in cross-coupling reactions. On the other hand, there have been reports on the Br+ abstraction from PFPBr by nucleophiles (Mode II).[3] Herein, we report an unprecedented mode III, in which PFPBr works as an equivalent of both Br+ and PFP− in the same transformation: a one-pot arylative epoxidation of ketones into pentafluorophenyloxiranes (Mode III).
Scheme 1.
Diverse Reactivity of PFPBr.
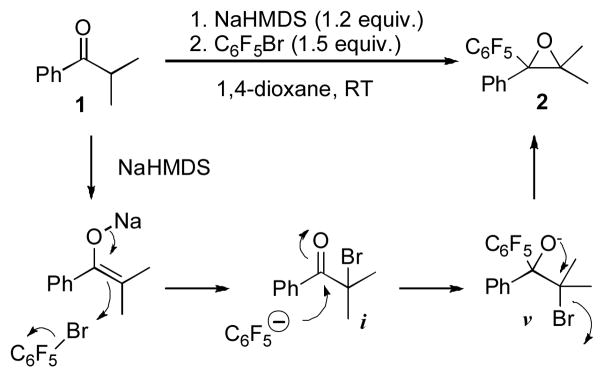
Our group has recently reported that alkynyl bromides and cyanogen bromide can act as equivalents of both Br+ and alkynyl- or cyanide anions in a highly efficient one-pot conversion of ketones into fully substituted alkynyl or cyanoepoxides.[4,5] Since the amphoteric reactivity of PFPBr as an equivalent of either Br+ or PFP− has been reported (Scheme 1), we hypothesized that it may also serve as a competent reagent for the one-pot cascade epoxidation reaction of ketones (Scheme 2). It was expected that an enolate would abstract Br+ from PFPBr to produce an α-bromoketone i and a PFP anion. Following nucleophilic addition of the latter at the carbonyl group would generate an alkoxide v, which, upon intramolecular SN2 reaction would produce the oxirane 2.
Scheme 2.
Proposed Reaction Path for Epoxidation of Ketones with PFPBr.
To test the above hypothesis, the reaction of isobutyrophenone (1a) and PFPBr (3a) has been examined (Table 1). Gratifyingly, it was found that employment of LiHMDS in THF led to 23% yield of the epoxide 2a (entry 3)! Further brief optimization of the reaction conditions revealed that isobutyrophenone in the presence of NaHMDS in 1,4-dioxane almost quantitatively was converted into tetrasubstituted epoxide 2a (entry 9). Under these reaction conditions, 1-bromoperfluorobutane and 1-iodoperfluorobutane did not undergo this cascade transformation.[6]
Table 1.
Screening of Reaction Conditions.

| |||
|---|---|---|---|
| Entry | Base | Solvent | Result[a] |
| 1 | LiHMDS | DMF | trace |
| 2 | LiHMDS | Et2O | N.R. |
| 3 | LiHMDS | THF | 23 |
| 4 | LiHMDS | 1,4-Dioxane | N.R. |
| 5 | LiOiPr | THF | N.R. |
| 6 | LiOiPr | 1,4-Dioxane | N.R. |
| 7 | NaHMDS | THF | 40% |
| 8 | NaHMDS | Et2O | 75% |
| 9 | NaHMDS | 1,4-Dioxane | 96% |
| 10 | KOtBu | THF | decomp |
| 11 | KOtBu | 1,4-Dioxane | decomp |
GC yields, using pentadecane as an internal standard.
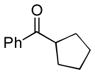
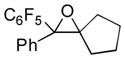
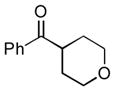
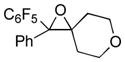
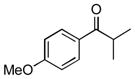
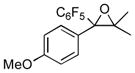
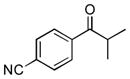
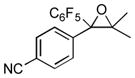
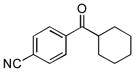
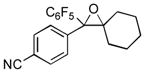
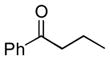
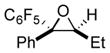
Next, the generality of the cascade transformation of different ketones with PFPBr was examined (Table 2). It was found that α,α-disubstituted methyl aryl ketones are suitable substrates for this transformation. Thus, isopropyl, cyclobutyl, cyclopentyl and cyclohexyl phenyl ketones (1a-d) smoothly reacted with PFPBr to produce epoxides 2a-d in good to excellent yields. Pyran-4-yl ketone 1e was also successfully converted to the corresponding product 2e. Diverse substituents at the phenyl ring, such as 4-methoxy (1f) and 4-cyano (1g, h), were tolerated in this reaction (entries 6–8). Moreover, different heteroaryl ketones, including pyridin-3-yl ketone (1j, j) and N-tosyl-indole-3-yl ketone (1k), were converted into the corresponding epoxides 2i-k in good yields (entries 9–11). Importantly, in contrast to the epoxidation reaction with alkynyl bromides[4] and cyanogen bromide,[5] PFPBr smoothly reacted with propiophenone 1l and butyrophenone 1m producing the corresponding trisubstituted oxiranes (2l, m) in good yield and very high diastereoselectivity[7] (entries 12, 13). It should be mentioned that this reaction is easily scalable, as 10 mmol scale reaction of propiophenone 1l with PFPBr resulted in outcome similar to that for 0.5 mmol reaction (entry 12).
Table 2.
Synthesis of Pentafluorophenyl Oxiranes.

| |||
|---|---|---|---|
| Entry | Ketone 1 | Product 2 | Yield, %[a] (dr) |
| 1 |
1a
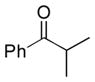
|
2a
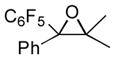
|
92 90[b] |
| 2 |
1b
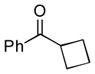
|
2b
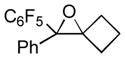
|
>99 |
| 3 |
1c
|
2c
|
74 |
| 4 |
1d
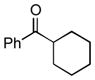
|
2d
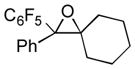
|
70 |
| 5 |
1e
|
2e
|
66 |
| 6 |
1f
|
2f
|
88 |
| 7 |
1g
|
2g
|
65 |
| 8 |
1h
|
2h
|
82 |
| 9 |
1i
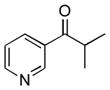
|
2i
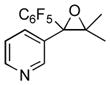
|
53 |
| 10 |
1j
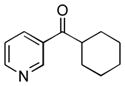
|
2j
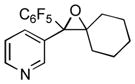
|
62 |
| 11 |
1k
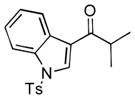
|
2k
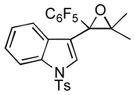
|
60 |
| 12 |
1l
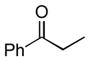
|
2l
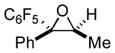
|
81 (95:5) 90 (95:5) [c] |
| 13 |
1m
|
2m
|
85 (93:7) |
Isolated yields of 0.5 mmol reactions.
Reaction was in toluene medium.
A 10 mmol reaction in toluene.
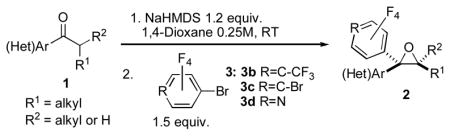
Next, the scope of bromopolyfluroarenes was tested. It was found that 1-bromo-4-trifluoromethytetrafluorolbenzene 3b, 1,4-dibromotetrafluorobenzne 3c, and 4-bromotetrafluoropyridine 3d were all competent reactants in this cascade transformation, producing the corresponding polyfluoroaryl (hetaryl) epoxides in good yields (2n-t). Although the reactions of 3b-d with propiophenone and butyrophenone produced good yields of the trisubstituted epoxides 2q-t, the diastereoselectivity was lower (entries 4–7) compared to that of the analogous reactions with PFPBr (Table 1, entries 12, 13).
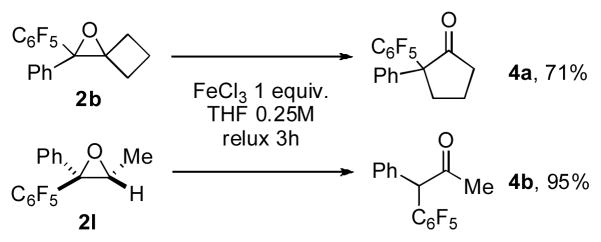
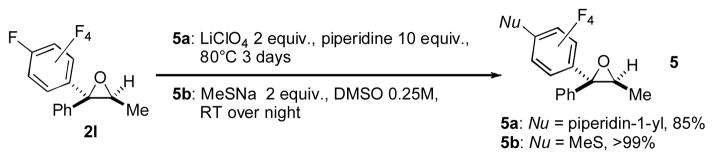
The synthetic usefulness of the obtained polyfluorophenyl oxiranes was showcased by their further transformations. First, it was demonstrated that oxiranes 2b and 2l in the presence of stoichiometric amount of FeCl3 undergo a facile semipinacol rearrangement[8] to produce the ring expansion product 4a and a H-migration product 4b, respectively, in good to excellent yields (Scheme 3). Secondly, it was shown that the obtained polyfluorophenyl oxiranes are excellent substrates for SNAr reactions[9] (Scheme 4). Thus, 2l underwent efficient substitution reaction with piperidine and sodium methylthiolate to produce the corresponding N- and S-containing products 5a and 5b in excellent yields with no oxirane ring opening[10] products detected.
Scheme 3.
Semipinacol Rearrangement of Oxiranes.
Scheme 4.
SNAr on Pentafluorophenyl Ring.
In conclusion, we have demonstrated that amphoteric bromopolyfluoroarenes(heteroarenes) could serve as equivalents of both Br+ and aryl(hetaryl) anions in the same cascade transformation. Thus, reaction of enolizable ketones with bromopolyfluoroarenes leads to a variety of valuable tri- and tetrasubstituted epoxides[11] in good to excellent yields and diastereoselectivity. A synthetic usefulness of the obtained polyfluorophenyl-containing oxiranes was further demonstrated in their transformations, including a semipinacol rearrangement and SNAr reactions.
Experimental Section
To an oven dried conical vial equipped with a magnetic stirring bar and a PTFE-topped screw cap, ketone (0.5 mmol) was added, and the vessel was evacuated and recharged by argon 3 times. Then anhydrous 1,4-dioxane (2.5 mL) was added dropwise and the reaction mixture was stirred until the ketone was completely dissolved. NaHMDS (0.6 mL, 1M in THF) was subsequently added to the solution of ketone aforementioned and the mixture was stirred for 5 minutes. Then PFPBr or other other arylbromide was added drop wise to the mixture and the reaction mixture was stirred for another 15 minutes and precipitation of NaBr was observed. Reaction mixture was filtered through a silica or zeolite pad, washed with 50 mL diethyl ether. The filtrate was concentrated under reduced pressure and the residue was purified by column chromatography of silica gel with hexane/ethyl acetate to obtain final product.
Table 3.
Synthesis of Polyfluoroaryl Oxiranes.
| ||||
|---|---|---|---|---|
| Entry | Ketone 1 | Bromide 3 | Product 2 | Yield, %[a] (dr) |
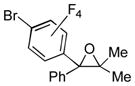
| 1 | 1a | 3b |
2n
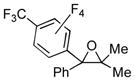
|
80 |
| 2 | 1a | 3c |
2o
|
84 |
| 3 | 1a | 3d |
2p
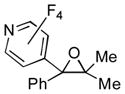
|
60 |
| 4 | 1l | 3b |
2q
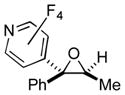
|
86 (85:15) |
| 5 | 1m | 3b |
2r
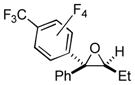
|
81 (77:23) |
| 6 | 1m | 3c |
2s

|
75 (90:10) |
| 7 | 1m | 3d |
2t
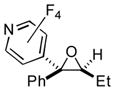
|
77 (90:10) |
Isolated yields of 0.5 mmol reactions.
Footnotes
The support of the National Science Foundation (CHE-1112055) and NIH (1P50 GM-086145) is gratefully acknowledged.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a) Frohn HJ, Franke H, Fritzen P, Bardin VV. J Organomet Chem. 2000;598:127. [Google Scholar]; b) Glover PB, Bassett AP, Nockemann P, Kariuki BM, Van Deun R, Pikramenou Z. Chem Eur J. 2007;13:6308. doi: 10.1002/chem.200700087. [DOI] [PubMed] [Google Scholar]; c) Brinkmann Y, Madhushaw RJ, Jazzar R, Bernardinelli G, Kündig EP. Tetrahedron. 2007;63:8413. [Google Scholar]; d) Kuprat M, Lehmann M, Schulz A, Villinger A. Organometallics. 2010;29:1421. [Google Scholar]; e) Romanato P, Duttwyler S, Linden A, Baldridge KK, Siegel JS. J Am Chem Soc. 2010;132:7828. doi: 10.1021/ja9109665. [DOI] [PubMed] [Google Scholar]; f) Trzeciak AM, Bartosz-Bechowski H, Ciunik Z, Niesyty K, Ziólkowski JJ. Can J Chem. 2011;79:752. [Google Scholar]; g) Lee JY. US Patent. Albemarle Corporation; (Richmond, VA) USA: Jan 2, 2001. (Ed.: U. S. Patent) [Google Scholar]
- 2.For examples on preparation of pentafluorophenyl lithium and magnesium reagents, see: De Pasquale RJ. J Organomet Chem. 1968;15:233.Abarbri M, Dehmel F, Knochel P. Tetrahedron Lett. 1999;40:7449.Tyrra W, Wickleder MS. J Organomet Chem. 2003;677:28.Franzke A, Pfaltz A. Chem Eur J. 2011;17:4131. doi: 10.1002/chem.201003314.For examples of metallation of PFPBr via oxidative addition with transition metals, see: Anderson BB, Behrens CL, Radonovich LJ, Klabunde KJ. J Am Chem Soc. 1976;98:5390.Albeniz AC, Espinet P, Martin-Ruiz B, Milstein D. J Am Chem Soc. 2001;123:11504. doi: 10.1021/ja016315b.Jin MY, Yoshikai N. J Org Chem. 2011;76:1972. doi: 10.1021/jo102417x.Patel NR, Kirchmeier RL. Inorg Chem. 1992;31:2537.Ivushkin VA, Sazonov PK, Artamkina GA, Beletskaya IP. J Organomet Chem. 2000;597:77.For examples of generation of PFP anion via electrophilic bromine abstraction by phosphines, see: Bardin VV, Pressman LS, Rogoza LN, Furin GG. J Fluorine Chem. 1991;53:213.
- 3.For examples of electrophilic bromine abstraction, see: Falvello LR, Fornies J, Navarro R, Rueda A, Urriolabeitia EP. Organometallics. 1996;15:309.Bolton R, Sandall JPB. J Fluorine Chem. 1976;7:540.c) See also ref. 2i.
- 4.Trofimov A, Chernyak N, Gevorgyan V. J Am Chem Soc. 2008;130:13538. doi: 10.1021/ja806178r. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Gevorgyan V. Angew Chem, Int Ed. 2011;50:2808. doi: 10.1002/anie.201006966. [DOI] [PubMed] [Google Scholar]
- 6.For example on electrophilic iodine abstraction from perfluoroalkyl iodides, see: Howell JL, Muzzi BJ, Rider NL, Aly EM, Abouelmagd MK. J Fluorine Chem. 1995;72:61.
- 7.a) Kang JY, Connell BT. J Org Chem. 2011;76:2379. doi: 10.1021/jo2001353. [DOI] [PubMed] [Google Scholar]; b) Alesi S, Emer E, Capdevila MG, Petruzziello D, Gualandi A, Cozzi PG. Molecules. 2011;16:5298. doi: 10.3390/molecules16065298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For examples of metal-catalyzed semipinacol rearrangement, see: Kulasegaram S, Kulawiec RJ. J Org Chem. 1997;62:6547.Ranu BC, Jana U. J Org Chem. 1998;63:8212.
- 9.For SNAr reaction at polyfluorophenyl ring, see: Tanaka K, Takeyama T, Yukawa R, Iwata S, Kurushima T. Supramol Chem. 2010;22:186.Ranjbar-Karimi R, Mashak-Shoshtari M, Darehkordi A. Ultrason Sonochem. 2011;18:258. doi: 10.1016/j.ultsonch.2010.05.017.Cano R, Ramón DJ, Yus M. J Org Chem. 2011;76:654. doi: 10.1021/jo1022052.Zhao J, Song T, Zhu S, Xu L. Tetrahedron. 2011;67:910.
- 10.For oxirane ring opening reactions with N- and S-nucleophiles, see: Rodriguez-Escrich S, Reddy KS, Jimeno C, Colet G, Rodriguez-Escrich C, Sola L, Vidal-Ferran A, Pericas MA. J Org Chem. 2008;73:5340. doi: 10.1021/jo800615d.Acetti D, Brenna E, Fuganti C, Gatti FG, Serra S. Tetrahedron: Asymmetry. 2009;20:2413.
- 11.For employment of oxiranes in synthesis, see: Hodgson DM, Bray CD. In: Aziridines and Epoxides in Organic Synthesis. Yudin AK, editor. Wiley-VCH: Weinheim; 2006. pp. 169–170.Hanson RM. Organic Reactions. John Wiley & Sons, Inc; 2004. p. 116.p. 118.Nagaki A, Takizawa E, Yoshida J. Chem Eur J. 2010;16:14149. doi: 10.1002/chem.201000815.