Abstract
The objectives of this study were to determine the prevalence of Salmonella, trends, major serovars, and their clusters from fluff samples, in poultry hatcheries in Ontario between 1998 and 2008. Multi-level logistic regression modelling with random effects for hatchery and sampling visit (day on which samples were collected from a hatchery) was used to identify factors [poultry breeder type, year (trend), and season] associated with the prevalence of Salmonella and a cluster detection test was used to identify clusters of common serovars. The period prevalence of Salmonella in fluff samples was 8.7% in broiler-breeders, 3.1% in layer-breeders, 13.2% in turkey-breeders, and 11.9% in other-breeder birds, such as ducks, geese, quail, partridges, and pheasants. There was an overall increasing trend in Salmonella prevalence in broiler-breeders and other-breeder birds, and a decreasing trend in layer-breeders. The 4 most common serovars identified were Salmonella Kentucky, Heidelberg, Enteritidis, and Senftenberg in broiler-breeders; Salmonella Heidelberg, Senftenberg, Braenderup, and Typhimurium in layer-breeders; Salmonella Senftenberg, Heidelberg, Saintpaul, and Montevideo in turkey-breeders; and Salmonella Enteritidis, Thompson, Typhimurium, and Heidelberg in other-breeder birds. Temporal clusters were identified for 12 of 13 serovars examined in broiler-breeders, and 4 of 4 serovars in all other poultry-breeders. The seasonal effects varied by year with the highest probability of Salmonella most often occurring in the summer, followed by the fall season. Variance components suggested that control measures should be directed at the hatchery and the sampling visit levels. Further studies are needed to identify risk factors for Salmonella in broiler-breeder, turkey-breeder, and other-breeder bird hatcheries in order to implement necessary control measures.
Résumé
Les objectifs de cette étude étaient de déterminer la prévalence de Salmonella, les tendances, les sérovars principaux, et leurs regroupements à partir d’échantillons de duvet, dans des poulaillers en Ontario entre 1998 et 2008. Une modélisation par régression logistique à niveaux multiples avec des effets aléatoires pour le poulailler et la visite (journée à laquelle les échantillons ont été prélevés d’un poulailler) a été utilisée pour identifier les facteurs [type de reproducteur, année (tendance), et saison] associés avec la prévalence de Salmonella et un test de détection de regroupement a été utilisé pour identifier les regroupements de sérovars communs. La prévalence de Salmonella dans les échantillons de duvet durant la période était de 8,7 % chez les reproducteurs de poulets à griller, 3,1 % chez les reproducteurs de pondeuses, 13,2 % chez les reproducteurs de dindons, et de 11,9 % chez les reproducteurs d’autres types de volaille, tel que les canards, les oies, les cailles, les perdrix et les faisans. Une tendance générale à la hausse de la prévalence de Salmonella était notée chez les reproducteurs de poulets à griller ainsi que chez les reproducteurs des autres types de volaille, alors qu’une tendance à la baisse était notée chez les reproducteurs des pondeuses. Les quatre sérovars les plus fréquemment identifiés étaient Salmonella Kentucky, Heidelberg, Enteritidis et Senftenberg chez les reproducteurs de poulets à griller; Salmonella Heidelberg, Senftenberg, Braenderup et Typhimurium chez les reproducteurs de pondeuses; Salmonella Senftenberg, Heidelberg, Saintpaul et Montevideo chez les reproducteurs de dindons, et Salmonella Enteritidis, Thompson, Typhimurium et Heidelberg chez les reproducteurs des autres types de volaille. Des regroupements temporels ont été identifiés pour 12 des 13 sérovars examinés chez les reproducteurs de poulets à griller, et 4 des 4 sérovars retrouvés chez les reproducteurs des autres types de volaille. L’effet saisonnier variait selon l’année avec la probabilité la plus élevée d’isoler Salmonella en été, suivi de l’automne. Les composantes de la variance suggéraient que les mesures de maîtrise devraient être dirigées vers le poulailler et les visites d’échantillonnage. Des études supplémentaires sont nécessaires afin d’identifier les facteurs de risque pour Salmonella dans les couvoirs des reproducteurs de poulets à griller, des reproducteurs de dindons et des reproducteurs des autres types de volaille afin d’implémenter les mesures nécessaires pour maîtriser l’infection.
(Traduit par Docteur Serge Messier)
Introduction
Foodborne salmonellosis is a major public health issue in many countries, including Canada (1,2). In each year, 6000 to 12 000 Salmonella cases are reported in Canada (3), and Ontario had the 4th highest provincial incidence in 2006 with 21.3 cases per 100 000 persons; this figure was higher than the national average incidence rate of 18.0 cases per 100 000 person-years (4). There are many Salmonella enterica serovars that cause disease in humans, among which Salmonella Enteritidis, Typhimurium, and Heidelberg contributed the highest number of salmonellosis cases reported in Canada and the United States (US) (4–6).
Consumption of contaminated meat (including poultry), eggs, milk, cheese, salad croutons, and fresh produce, and contact with pet turtles, are associated with most of the infections and outbreaks in humans in Canada and the US (3,4,7). In Ontario, between 1997 and 2001, 47.8% of the total reported cases of salmonellosis were linked to consumption of chicken and eggs (8).
Both horizontal and vertical transmission is important in Salmonella contamination of poultry hatcheries. Horizontal transmission includes indirect transmission from the environment, transportation equipment, and vectors (e.g., rodents, flies, humans) (9–11). In vertical transmission, the bacterium is transferred directly from colonized breeder hens via the egg (10,12,13). Vertical transmission of some serovars (e.g., Salmonella Enteritidis, Typhimurium, and Heidelberg) is an important source of Salmonella contamination to hatcheries (14–16). The hatchery is a central point of distribution of chicks to commercial farms; therefore, control of Salmonella contamination in the hatchery is an important component in controlling Salmonella colonization in commercial poultry flocks.
Few studies have assessed the temporal distribution of Salmonella serovars in poultry breeder flocks (17,18) and none have assessed temporal patterns of Salmonella in hatcheries. Using fluff data from the Ontario Hatchery and Supply Flock Policy (OHSFP), which is a government monitoring program to detect Salmonella Pullorum and Salmonella Gallinarum in poultry hatcheries in Ontario (broiler-breeders, layer-breeders, turkey-breeders, and a miscellaneous category of other-breeder birds that comprises ducks, geese, quail, partridges, and pheasants) (19), the objectives of this study were to: i) determine the prevalence of Salmonella in hatcheries in Ontario between 1998 and 2008; ii) identify the most commonly isolated serovars for each poultry breeder type; iii) describe the overall and serovar-specific temporal trends in the prevalence of Salmonella, including major serovars of public health significance (e.g., Salmonella Enteritidis, Typhimurium, and Heidelberg); iv) describe the overall seasonal patterns in the prevalence of Salmonella; and v) identify temporal clusters of Salmonella serovars.
Materials and methods
Surveillance data, pertaining to Ontario hatcheries registered under the OHSFP and collected between 1998 and 2008, were obtained from the Animal Health Laboratory (AHL), Guelph, Ontario. Under this program, in general, 0.5 g of fluff from every hatcher in all Ontario hatcheries with a setting capacity of 1000 eggs or more (per 3 wk for chickens, per 4 wk for turkeys, and per 2 to 5 wk for other birds) were collected every 6 wk and submitted to the AHL (19). For chickens and turkeys, hatchers with a setting capacity of 1000 eggs or more accounted for almost 100% of hatching eggs in Ontario; for other birds, hatchers with a setting capacity of 1000 eggs or more accounted for more than 50% of hatching eggs in Ontario [(Al Dam, Ontario Ministry of Agriculture, Food and Rural Affairs), (Edward Malek, Canadian Food Inspection Agency), (Bob Guy, Ontario Broiler Hatching Egg & Chick Commission), (Janet Schlitt, Turkey Farmers of Ontario), (Pamela Kuipers, Egg Farmers of Ontario), 2012, personal communication]. A hatcher is a cabinet used for incubating eggs in the hatchery. Fluff is the hatching debris (e.g., down feathers) and egg shell remaining in the hatcher after hatching (19). Therefore, each sample in our study represented fluff material collected from an individual hatcher from a specific hatchery on a specific collection date. For each sampling visit (day on which samples were collected), generally, all hatchers within a hatchery were sampled.
All hatcheries were in compliance with the Canadian Hatchery Regulations that specify the minimum standards in which hatching eggs and chicks are stored and handled, and minimum standards for cleaning and disinfection. Modifications to the regulations were minimal over the study period.
The microbiological methods of culturing fluff samples in this study are identical to those described for culturing environmental samples from poultry breeder flocks (20), with the exception that in this study, 0.5 g of fluff was immersed into 100 mL of buffered peptone water. Salmonella-positive isolates were submitted to the Laboratory for Foodborne Zoonoses, Public Health Agency of Canada, for sero typing and phage typing (20).
The laboratory dataset contained information on submission identification (ID), sample ID, date of sample reception, name of the hatchery, Salmonella isolation results, poultry type, and reference number for each isolated Salmonella serovar.
Descriptive analysis
Descriptive analyses were carried out using computer software (Microsoft Office Excel, 2007; Microsoft Corporation, Redmond, Washington, USA and STATA, version 10.0; STATA Corporation, College Station, Texas, USA). The number of hatcheries, number of samples submitted, number of Salmonella-positive isolates, and poultry type-specific sample prevalence for the period between 1998 and 2008 were summarized. The frequencies of serovars identified from different poultry breeder types between 1998 and 2008 were tabulated.
Long-term trends in the prevalence of Salmonella in the different poultry types were graphically illustrated after estimating the annual prevalence for the period between 1998 and 2008. The annual sample-level prevalence was estimated by dividing the total number of Salmonella isolates for each year by the total number of samples during the same year.
Cluster detection
Temporal clusters of Salmonella serovars were identified using computer software (SaTScan, version 8.0; Martin Kulldorff and Information Management Services, Boston, Massachusetts, USA). Analyses were conducted separately for each poultry breeder type; serovars with a frequency of ≥ 10 isolates during the study period were included in the analysis (exception: we also included Salmonella Typhimurium in layer-breeders, which had only 9 isolates, because of its importance to public health). An odds ratio and log likelihood ratio for the temporal scan test were estimated using the Bernoulli model, because the data consisted of cases versus a non-case group. The case and non-case definitions, scanning window size (50% of the study period), level of statistical significance (P ≤ 0.05) used to indicate a significant cluster, and methods for detecting secondary clusters in this study are identical to those described for analyzing environmental samples from poultry breeder flocks (20).
Regression analysis
A logistic regression analysis was conducted using computer software (STATA, version 10.0), to test the association between the Salmonella status of fluff samples and poultry breeder type, year (trend), and season. All variables were categorical. Broiler-breeders, the year 1998, and winter were used as referent categories for poultry type, year, and season, respectively. Winter was defined as January to March, spring as April to June, summer as July to September, and fall as October to December. Because there was no Salmonella isolated in the year 2007 for layer-breeders, data for the years 2007 and 2008 were combined to facilitate model convergence.
Poultry type, year, and season were initially included in all models. The significance of each predictor variable was evaluated using a Wald test, with P ≤ 0.05 indicating statistical significance (21). All 2-way interactions were added individually to the main effects model and removed if they were not statistically significant based on a likelihood ratio test (21). Evaluation of confounding and collinearity between predictor variables were as previously described (20).
Using this approach, 4 types of models were built: i) fixed effects models; ii) mixed models with a random effect for hatchery; iii) mixed models with a random effect for sample visit (to account for potential clustering among hatchers on the day of a visit); and iv) mixed models with random effects for both hatchery and sample visit. The random effects models were built using adaptive Gaussian quadrature using the xtmelogit command in STATA (22). For the best fitting model within each model type (e.g., models with 2 random effects), residuals for all levels of the models were evaluated unless the complexity of the model limited the performance of post-estimation commands designed to estimate the best linear unbiased predictors (BLUPS). In these cases, the residuals based only on the fixed portion of the model were evaluated. The fit of the models was assessed based on Akaike’s Information Criterion (AIC) (22). The best fitting model was selected based on the lowest AIC score in conjunction with the significance of likelihood-ratio tests for the interaction terms (P ≤ 0.05).
From the final model, the predicted probabilities of Salmonella-positive status were visualized across season, year, and poultry type variables. The percentage variation explained at each level of the final multi-level model was determined using the latent variable technique (21).
Results
Overall, 28 269 fluff samples were submitted from poultry breeder hatcheries between 1998 and 2008; among the samples submitted, 63%, 20%, 11%, and 6% were from broiler-, layer-, turkey-, and other-breeder bird hatcheries, respectively. The sample-level prevalence of Salmonella for each breeder type is shown in Table I.
Table I.
Sample-level prevalence of Salmonella in poultry hatcheries, from fluff samples submitted through the Ontario Hatchery and Supply Flock Policy between 1998 and 2008
| Poultry type | Number of hatcheries | Number (and percentage) of samples submitted | Number of Salmonella isolates | Poultry type-specific sample-level prevalence (%) | 95% confidence interval |
|---|---|---|---|---|---|
| Broiler-breeders | 14 | 17 886 (63) | 1564 | 8.7 | 8.3–9.2 |
| Layer-breeders | 10 | 5750 (20) | 181 | 3.1 | 2.7–3.6 |
| Turkey-breeders | 4 | 3082 (11) | 406 | 13.2 | 12.0–14.4 |
| Other-breeder birdsa | 23 | 1551 (6) | 185 | 11.9 | 10.4–13.6 |
| Total | 51 | 28 269 | 2336 | 8.3 | 7.9–8.6 |
Other-breeder birds include ducks, geese, quail, partridges, and pheasants.
The frequency distribution of Salmonella serovars is summarized in Table II. A total of 43 different serovars were isolated, although most were infrequent. Overall, the 5 most common serovars were Salmonella Kentucky (29.1%), Heidelberg (24.7%), Senftenberg (13.6%), Enteritidis (9.8%), and Thompson (2.7%). Among the top 5 serovars, Salmonella Kentucky was almost exclusively isolated from broiler-breeders. More than 60% of Salmonella Heidelberg was isolated from broiler-breeders, whereas the majority of Salmonella Senftenberg (49.5%) was isolated from turkey-breeders. Salmonella Enteritidis was isolated only from broiler-breeders (76.0%) and other-breeder birds (24.0%); however, Enteritidis was not isolated from layer- or turkey-breeders. Among Salmonella Enteritidis isolates from broiler-breeders, the most common phage types (PT) were PT 13 (42.8%) and PT 8 (39.8%); among other-breeder birds, the most common was PT 9b (87.0%).
Table II.
Frequency of Salmonella serovars in poultry hatcheries, from fluff samples submitted through the Ontario Hatchery and Supply Flock Policy between 1998 and 2008
| Serovar | Group | Broiler-breeders | Layer-breeders | Turkey-breeders | Other-breeder birdsa | Total |
|---|---|---|---|---|---|---|
| Adabraka | E | 0 | 0 | 1 | 0 | 1 |
| Agona | B | 28 | 0 | 1 | 5 | 34 |
| Anatum | E1 | 6 | 0 | 7 | 2 | 15 |
| Bareilly | C2 | 2 | 0 | 0 | 0 | 2 |
| Berta | D | 1 | 0 | 0 | 0 | 1 |
| Blegdam | D | 4 | 0 | 0 | 0 | 4 |
| Braenderup | C1 | 3 | 10 | 0 | 7 | 20 |
| Brandenburg | B | 1 | 1 | 4 | 0 | 6 |
| Bredeney | B | 0 | 0 | 6 | 0 | 6 |
| Cerro | C1 | 0 | 2 | 0 | 1 | 3 |
| Cubana | G2 | 2 | 0 | 0 | 0 | 2 |
| Derby | B | 2 | 0 | 0 | 2 | 4 |
| Enteritidis | D | 173 | 0 | 0 | 56 | 229 |
| Give | E1 | 0 | 0 | 9 | 0 | 9 |
| Hadar | C | 29 | 1 | 9 | 11 | 50 |
| Havana | G2 | 2 | 0 | 0 | 12 | 14 |
| Heidelberg | B | 359 | 65 | 137 | 16 | 577 |
| Indiana | B | 2 | 0 | 0 | 0 | 2 |
| Infantis | C1 | 20 | 0 | 0 | 0 | 20 |
| Java | B | 0 | 0 | 2 | 0 | 2 |
| Johannesburg | R | 1 | 0 | 0 | 0 | 1 |
| Kentucky | C2 | 677 | 1 | 2 | 0 | 680 |
| Kiambu | B | 1 | 0 | 0 | 0 | 1 |
| Litchfield | C2 | 1 | 0 | 0 | 0 | 1 |
| Mbandaka | C1 | 11 | 0 | 0 | 1 | 12 |
| Montevideo | C1 | 13 | 8 | 12 | 0 | 33 |
| Muenchen | C2 | 1 | 0 | 0 | 0 | 1 |
| Muenster | E1 | 0 | 0 | 1 | 4 | 5 |
| Newport | C2 | 1 | 0 | 5 | 0 | 6 |
| Ohio | C1 | 1 | 3 | 1 | 0 | 5 |
| Oranienburg | C1 | 2 | 0 | 0 | 0 | 2 |
| Orion | E2 | 11 | 1 | 0 | 0 | 12 |
| Rissen | C1 | 1 | 0 | 0 | 0 | 1 |
| Saintpaul | B | 5 | 0 | 26 | 0 | 31 |
| Schwarzengrund | B | 1 | 1 | 1 | 4 | 7 |
| Senftenberg | E4 | 94 | 58 | 157 | 8 | 317 |
| Tennessee | C1 | 2 | 0 | 3 | 5 | 10 |
| Thompson | C1 | 33 | 5 | 0 | 24 | 62 |
| Tumodi | B | 1 | 0 | 0 | 0 | 1 |
| Typhimurium | B | 20 | 9 | 0 | 19 | 48 |
| Uganda | E1 | 0 | 0 | 0 | 4 | 4 |
| Westhampton | E1 | 2 | 5 | 0 | 0 | 7 |
| Worthington | G2 | 12 | 0 | 0 | 0 | 12 |
| Total | 1564 | 181 | 406 | 185 | 2336 |
Other-breeder birds include ducks, geese, quail, partridges and pheasants.
Among specific breeder types, the 4 most common serovars were Salmonella Kentucky (43.3%), Heidelberg (23.0%), Enteritidis (11.1%), and Senftenberg (6.0%) in broiler-breeder hatcheries; Salmonella Heidelberg (35.9%), Senftenberg (32.0%), Braenderup (5.5%), and Typhimurium (5.0%) in layer-breeder hatcheries; Salmonella Senftenberg (38.7%), Heidelberg (34.0%), Saintpaul (6.4%), and Montevideo (2.9%) in turkey-breeder hatcheries; and Salmonella Enteritidis (30.3%), Thompson (13.0%), Typhimurium (10.3%), and Heidelberg (8.6%) in other-breeder bird hatcheries.
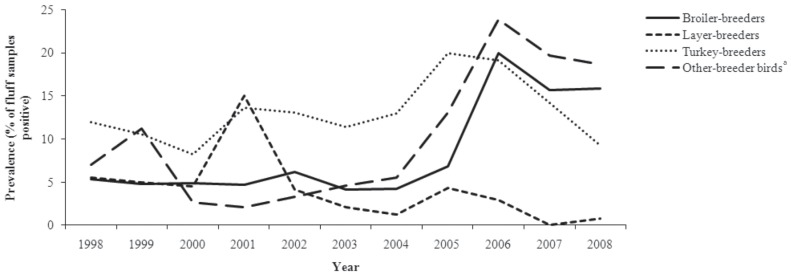
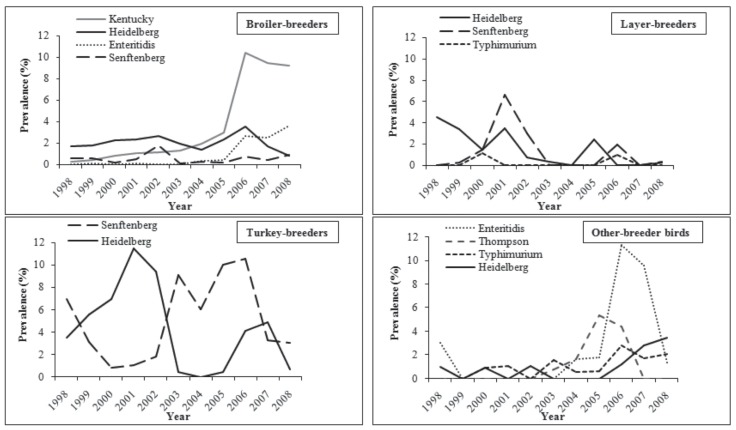
Trends in the prevalence of Salmonella in poultry hatcheries are illustrated in Figure 1. Trends in the prevalence of the most common Salmonella serovars for each poultry type are illustrated in Figure 2.
Figure 1.
Trends in the sample-level prevalence of Salmonella in poultry hatcheries, from fluff samples submitted through the Ontario Hatchery and Supply Flock Policy between 1998 and 2008.
a Other-breeder birds include ducks, geese, quail, partridges, and pheasants.
Figure 2.
Trends in the sample-level prevalence (percentage of fluff samples positive) of the most common Salmonella serovars in broiler-, layer-, turkey-, and other-breeder bird hatcheries, from fluff samples submitted through the Ontario Hatchery and Supply Flock Policy between 1998 and 2008. Other-breeder birds include ducks, geese, quail, partridges, and pheasants.
Significant temporal clusters were detected in 12 of 13 Salmonella serovars examined in broiler-breeder hatcheries, and 4 of 4 serovars in layer-breeder, turkey-breeder, and other-breeder bird hatcheries (Table III). A single cluster was detected for each examined serovar in other-breeder birds; all occurred in the latter half of the study period and were of long duration (≥ 6 mo). In all other poultry types, clusters were detected throughout the study period and varied in duration. Clusters of Salmonella Heidelberg were detected in all poultry types; 60% of these clusters were of long duration.
Table III.
Temporal clustersa of Salmonella serovars in poultry hatcheries from fluff samples submitted through the Ontario Hatchery and Supply Flock Policy between 1998 and 2008 (P < 0.05)
| Poultry type | Serovarb | First clusterc | Second clusterc,d | Third clusterc,e | Fourth clusterc,f |
|---|---|---|---|---|---|
| Broiler-breeders | Kentucky (677) | 2005/08–2008/12 (558) | 2000/07–2005/06 (108) | ||
| Heidelberg (359) | 2005/12–2006/08 (63) | 2005/04–2005/04 (19) | 1999/08–2003/10 (156) | ||
| Enteritidis (173) | 2005/07–2008/12 (166) | 2004/04–2004/06 (5) | |||
| Senftenberg (94) | 2002/04–2002/09 (25) | 2008/10–2008/11 (9) | |||
| Thompson (33) | 1998/03–1998/05 (5) | 2006/02–2006/02 (3) | |||
| Hadar (29) | 1998/03–1999/08 (12) | 2006/02–2006/10 (8) | |||
| Agona (28) | 1998/10–1999/01 (17) | 2006/12–2006/12 (9) | 1999/05–1999/08 (2) | ||
| Infantis (20) | 2006/10–2006/10 (6) | 1999/12–2004/11 (13) | |||
| Typhimurium (20) | 2005/09–2005/10 (4) | ||||
| Montevideo (13) | 1998/10–2001/11 (10) | ||||
| Worthington (12) | 2008/02–2008/02 (4) | 1998/08–1999/03 (5) | 2007/06–2007/06 (2) | ||
| Mbandaka (11) | No cluster detected | ||||
| Orion (11) | 2006/12–2007/01 (7) | 2005/02–2005/06 (4) | |||
| Layer-breeders | Heidelberg (65) | 1998/01–2002/01 (61) | 2008/04–2008/04 (3) | ||
| Senftenberg (58) | 2001/08–2002/03 (21) | 2005/01–2006/10 (26) | 2000/08–2000/11 (4) | 2008/02–2008/02 (3) | |
| Braenderup (10) | 2005/07–2005/11 (9) | ||||
| Typhimurium (9) | 2006/09–2006/10 (6) | 2000/03–2000/09 (3) | |||
| Turkey-breeders | Senftenberg (157) | 2004/10–2006/10 (69) | 2003/03–2003/07 (16) | 1998/06–1998/09 (18) | 2008/09–2008/11(9) |
| Heidelberg (137) | 2000/06–2002/12 (74) | 2006/10–2007/03 (22) | 1998/03–1999/12 (30) | 2007/09–2007/11 (5) | |
| Saintpaul (26) | 2004/09–2005/02 (19) | 2003/11–2006/06 (7) | |||
| Montevideo (12) | 2004/11–2006/02 (9) | ||||
| Other-breeder birds | Enteritidis (56) | 2006/01–2007/12 (45) | |||
| Thompson (24) | 2003/10–2006/09 (24) | ||||
| Typhimurium (19) | 2006/06–2008/02 (12) | ||||
| Heidelberg (16) | 2006/10–2008/10 (13) |
Iterative temporal scan performed using computer software (SaTScan, version 8.0; Martin Kulldorff and Information Management Services, Boston, Massachusetts, USA). Scanning window size is 50% of the study period. Dates are given as year/month.
Total number of isolates for each serovar is given in parentheses.
Number of isolates is given in parentheses.
Based on remaining data with first cluster removed.
Based on remaining data with first and second clusters removed.
Based on remaining data with first, second, and third clusters removed.
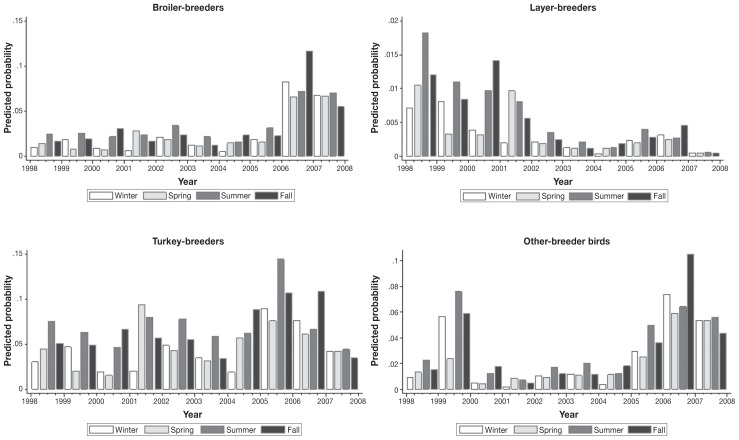
There was no evidence of high correlation among the predictor variables (Spearman’s rho < |0.8|). A comparison of the AIC statistic for selected regression models is shown in Table IV; the final random intercept logistic regression model is shown in Table V. There were significant interactions between poultry breeder type and year (LRT χ2 = 159.15; P < 0.0001), and between season and year (LRT χ2 = 50.40; P = 0.0041). The proportion of variation at the hatchery and sampling visit levels were 31% (σ2hatchery = 2.28, 95% CI: 1.19 to 4.38, P < 0.001) and 25% (σ2visit = 1.86, 95% CI: 1.57 to 2.20, P < 0.001), respectively. From the final model, predicted probabilities of Salmonella-positives by season and year for each poultry breeder type were estimated (Figure 3). The probabilities of Salmonella-positives in broiler-, layer-, turkey-, and other-breeder bird hatcheries varied by year. An increasing trend in the probabilities of Salmonella-positives in broiler-breeders was predicted during the latter part of the study period, while a decreasing trend in layer-breeder hatcheries was predicted. In other-breeder birds, the predicted probability was high early in the study period, and again in the latter years. In turkey-breeders, the probability of Salmonella-positives fluctuated. The predicted probabilities for the effect of season on Salmonella also varied by year; with the highest probability of testing Salmonella positive occurring most often in the summer season, and to a lesser extent, in the fall season.
Table IV.
Comparison of Akaike’s Information Criterion (AIC) statistics for logistic regression models with and without random intercepts for hatchery and/or sample visit date (n = 28 262 observations)
| Type of modela | AIC |
|---|---|
| Without random effect | |
| Poultry type, season, year | 15 232.30 |
| 1 random effect (hatchery) | |
| Poultry type, season, year | 14 455.11 |
| Poultry type*year, year*season | 14 171.41 |
| Poultry type*year, year*season, poultry type*season | 14 171.67 |
| 1 random effect (sample visit) | |
| Poultry type, season, year | 14 455.11 |
| Poultry type*year, year*season | 13 733.68 |
| Poultry type*year, year*season, poultry type*season | 13 737.12 |
| 2 random effects (hatchery, sample visit) | |
| Poultry type, season, year | 13 520.48 |
| Poultry type*year, year*season | 13 421.72 |
| Poultry type*year, year*season, poultry type*season | 13 425.81 |
Interactions between predictor variables are indicated by *. For example, poultry type*year indicates that, in addition to main effects for poultry type and year, an interaction term between poultry type and year was included in the model.
Table V.
Logistic regression model with random intercepts for hatchery and sampling visita for Salmonella status in poultry hatcheries, from fluff samples submitted through the Ontario Hatchery and Supply Flock Policy between 1998 and 2008
| Variable | Level | Odds ratio | P-value | 95% confidence interval | |
|---|---|---|---|---|---|
| Poultry type | Broiler-breeders | Referent | |||
| Layer-breeders | 0.73 | 0.714 | 0.15 | 3.72 | |
| Turkey-breeders | 0.92 | 0.926 | 0.17 | 5.07 | |
| Other-breeder birds | 3.24 | 0.213 | 0.51 | 20.70 | |
| Year | 1998 | Referent | |||
| 1999 | 1.96 | 0.147 | 0.79 | 4.84 | |
| 2000 | 0.90 | 0.820 | 0.35 | 2.31 | |
| 2001 | 0.59 | 0.309 | 0.21 | 1.63 | |
| 2002 | 2.19 | 0.109 | 0.84 | 5.76 | |
| 2003 | 1.30 | 0.613 | 0.47 | 3.64 | |
| 2004 | 0.47 | 0.184 | 0.16 | 1.42 | |
| 2005 | 1.93 | 0.167 | 0.76 | 4.96 | |
| 2006 | 9.33 | < 0.001b | 3.96 | 21.98 | |
| 2007–2008 | 7.49 | < 0.001b | 3.48 | 16.15 | |
| Season | Winter | Referent | |||
| Spring | 1.49 | 0.301 | 0.70 | 3.18 | |
| Summer | 2.61 | 0.012b | 1.24 | 5.55 | |
| Fall | 1.72 | 0.155 | 0.81 | 3.64 | |
| Poultry type*year | Broiler-breeders*1998 | Referent | |||
| Layer-breeders*1999 | 0.58 | 0.278 | 0.22 | 1.55 | |
| Layer-breeders*2000 | 0.61 | 0.419 | 0.19 | 2.01 | |
| Layer-breeders*2001 | 0.46 | 0.207 | 0.14 | 1.53 | |
| Layer-breeders*2002 | 0.14 | 0.002b | 0.04 | 0.49 | |
| Layer-breeders*2003 | 0.13 | 0.006b | 0.03 | 0.56 | |
| Layer-breeders*2004 | 0.11 | 0.002b | 0.03 | 0.43 | |
| Layer-breeders*2005 | 0.17 | 0.001b | 0.06 | 0.50 | |
| Layer-breeders*2006 | 0.04 | < 0.001b | 0.02 | 0.13 | |
| Layer-breeders*2007–2008 | 0.01 | < 0.001b | 0.00 | 0.03 | |
| Turkey-breeders*1999 | 3.45 | 0.142 | 0.66 | 18.01 | |
| Turkey-breeders*2000 | 0.61 | 0.626 | 0.09 | 4.37 | |
| Turkey-breeders*2001 | 0.32 | 0.299 | 0.04 | 2.75 | |
| Turkey-breeders*2002 | 0.54 | 0.535 | 0.08 | 3.83 | |
| Turkey-breeders*2003 | 1.01 | 0.977 | 0.18 | 5.88 | |
| Turkey-breeders*2004 | 0.85 | 0.841 | 0.17 | 4.32 | |
| Turkey-breeders*2005 | 1.78 | 0.461 | 0.38 | 8.24 | |
| Turkey-breeders*2006 | 0.96 | 0.959 | 0.22 | 4.15 | |
| Turkey-breeders*2007–2008 | 0.85 | 0.827 | 0.21 | 3.50 | |
| Other-breeder birds*1999 | 0.80 | 0.665 | 0.30 | 2.15 | |
| Other-breeder birds*2000 | 0.69 | 0.489 | 0.24 | 1.98 | |
| Other-breeder birds*2001 | 1.12 | 0.817 | 0.42 | 2.99 | |
| Other-breeder birds*2002 | 0.75 | 0.562 | 0.28 | 2.01 | |
| Other-breeder birds*2003 | 0.88 | 0.818 | 0.30 | 2.60 | |
| Other-breeder birds*2004 | 1.27 | 0.655 | 0.44 | 3.67 | |
| Other-breeder birds*2005 | 1.63 | 0.339 | 0.60 | 4.42 | |
| Other-breeder birds*2006 | 0.28 | 0.008b | 0.11 | 0.72 | |
| Other-breeder birds*2007–2008 | 0.19 | < 0.001b | 0.08 | 0.44 | |
| Year*Season | 1998*Winter | Referent | |||
| 1999*Spring | 0.27 | 0.024b | 0.09 | 0.84 | |
| 1999*Summer | 0.52 | 0.234 | 0.18 | 1.52 | |
| 1999*Fall | 0.61 | 0.376 | 0.21 | 1.81 | |
| 2000*Spring | 0.54 | 0.333 | 0.16 | 1.88 | |
| 2000*Summer | 0.95 | 0.939 | 0.31 | 2.95 | |
| 2000*Fall | 2.15 | 0.190 | 0.68 | 6.76 | |
| 2001*Spring | 3.37 | 0.045b | 1.03 | 11.10 | |
| 2001*Summer | 1.60 | 0.436 | 0.49 | 5.27 | |
| 2001*Fall | 1.69 | 0.386 | 0.51 | 5.64 | |
| 2002*Spring | 0.80 | 0.372 | 0.18 | 1.89 | |
| 2002*Summer | 0.63 | 0.427 | 0.20 | 1.96 | |
| 2002*Fall | 0.66 | 0.483 | 0.21 | 2.09 | |
| 2003*Spring | 0.61 | 0.447 | 0.17 | 2.16 | |
| 2003*Summer | 0.66 | 0.514 | 0.20 | 2.23 | |
| 2003*Fall | 0.56 | 0.370 | 0.17 | 1.95 | |
| 2004*Spring | 2.11 | 0.239 | 0.61 | 7.37 | |
| 2004*Summer | 1.33 | 0.659 | 0.37 | 4.83 | |
| 2004*Fall | 2.95 | 0.087 | 0.86 | 10.23 | |
| 2005*Spring | 0.56 | 0.310 | 0.18 | 1.71 | |
| 2005*Summer | 0.65 | 0.440 | 0.23 | 1.91 | |
| 2005*Fall | 0.70 | 0.535 | 0.24 | 2.11 | |
| 2006*Spring | 0.52 | 0.217 | 0.19 | 1.46 | |
| 2006*Summer | 0.33 | 0.029b | 0.12 | 0.89 | |
| 2006*Fall | 0.85 | 0.762 | 0.32 | 2.30 | |
| 2007–2008*Spring | 0.66 | 0.373 | 0.27 | 1.63 | |
| 2007–2008*Summer | 0.39 | 0.043b | 0.16 | 0.97 | |
| 2007–2008*Fall | 0.46 | 0.097 | 0.19 | 1.15 | |
| σ2(Hatchery) | 2.283 | 1.19 | 4.38 | ||
| σ2(Visit) | 1.856 | 1.57 | 2.20 | ||
51 hatcheries; 4545 sampling visits; 28 262 samples.
Data were statistically significant (P < 0.05).
Figure 3.
Predicted probability of Salmonella in poultry hatcheries, from fluff samples submitted through the Ontario Hatchery and Supply Flock Policy between 1998 and 2008. Other-breeder birds include ducks, geese, quail, partridges, and pheasants. Winter was defined as January to March, spring as April to June, summer as July to September, and fall as October to December.
Discussion
This study analyzed the OHSFP data from all hatcheries of all poultry types in Ontario to determine the temporal patterns of Salmonella prevalence in fluff by year and season, and identify temporal clusters of specific Salmonella serovars. Descriptive results showed that there was variation in the trends in the prevalence of Salmonella in different poultry types during the period of study. Increasing trends were observed in broiler-breeder and other-breeder bird hatcheries, while a decreasing trend was observed in layer-breeder hatcheries. A fluctuating trend was observed in turkey-breeder hatcheries. The regression analysis revealed that there was significant variation in Salmonella prevalence among hatcheries, and among sampling visits. The season of the year had a significant effect on the prevalence of Salmonella independent of poultry type. However, the seasonal effect, as well as the effect of poultry type, varied by year.
Cluster detection analysis results showed that temporal clusters were detected from all poultry types. Similar temporal clusters of some serovars (e.g., Salmonella Heidelberg) were detected among hatchery fluff samples and breeder flock environmental samples (20), which indicate that a possible source of contamination to hatcheries is the breeder flocks via vertical or horizontal transmission. In other-breeder birds, clusters of Salmonella Enteritidis, Typhimurium, Heidelberg, and Thompson were detected during the latter half of the study period and were of long duration, indicating that several serovars of public health importance have recently become endemic in the breeder sector in this commodity. In all other poultry breeder types, clusters were detected throughout the study period and were of short (< 6 mo) and long duration. Long-duration clusters likely indicate farm-to-farm transmission, exposure to a continuous common source, or secondary infections, whereas short-duration clusters likely indicate a point source of contamination (17,23).
One of the key findings of this study was that Salmonella Enteritidis was not isolated from layer- and turkey-breeder hatchery fluff samples. Further, it was infrequent in environmental samples from layer and turkey breeder flocks during the same period (20). This might be due to active control measures taken by industry to control this particular serovar, especially in layer-breeders. Salmonella Enteritidis might not be especially competent at colonizing or disseminating within populations of turkeys, as our findings are comparable with a previous study, which showed that turkeys were not a significant source of Salmonella Enteritidis for humans in Canada (24).
However, Salmonella Enteritidis was the 3rd most common serovar in fluff from broiler hatcheries; PT 13 and 8 were the most common phage types. An increasing trend in the prevalence of Salmonella Enteritidis was observed in broiler hatcheries between 2005 and 2008. These findings are consistent with increased numbers of human isolates of Salmonella Enteritidis in Ontario between 2005 and 2008 identified through the Canadian Integrated Program for Antimicrobial Resistance Surveillance (25) and the National Enteric Surveillance Program in Canada (4,26). Between 2005 and 2008, PT 13, 8, 1, and 4 were the most common phage types of Salmonella Enteritidis in humans (4,26,27) in Ontario; although, in 2005, the majority of the PT 13 cases occurred during an outbreak attributed to the consumption of mung bean sprouts (4,27). The similarities in Salmonella Enteritidis trends and phage types between broiler-breeder hatcheries and human cases suggest that control measures implemented at the broiler hatchery level could have a positive effect on public health.
A large cluster of 166 isolates of Salmonella Enteritidis from broiler fluff was detected over a 3.5-year period (July 2005 to December 2008), during a period in which the prevalence of this serovar in environmental samples from broiler breeder flocks in Ontario was low (20). Together, these findings suggest that domestic-origin broiler breeder flocks were unlikely a major source of this serovar to the hatcheries, and that an external, persistent common source was more likely. Hatching broiler eggs from the US contaminated with Salmonella Enteritidis might have been one possible source of this serovar and might explain the increasing prevalence of Salmonella Enteritidis in Ontario broiler hatcheries [(Agnes Agunos, Public Health Agency of Canada), (Keith Harron, Animal Health Laboratory, University of Guelph), 2010, personal communication], as imports from the US increased between 2004 and 2007 (Bob Guy, 2010, personal communication). Thus, the prevalence of this important public health serovar might be reduced by implementing: i) surveillance activities aimed at detecting Salmonella Enteritidis in imported hatching broiler eggs; and ii) control measures aimed at reducing Salmonella Enteritidis in US broiler breeder flocks supplying hatching eggs to Canada.
In contrast, in the spring of 2004, Salmonella Enteritidis was identified in fluff samples by the broiler breeder industry. The hatchery traced the source to a single parent flock, which was depopulated after consecutive positive test results (Rachel Ouckama, Maple Lodge Hatcheries, 2011, personal communication). This event was detected in our study without prior knowledge of its occurrence at the time of data analysis (a short-duration cluster of 5 isolates of Salmonella Enteritidis from broiler fluff was detected from April to June 2004). Because short duration clusters indicate a point source of the bacteria (for example, contamination of hatchers via humans, equipment or egg trays, or in this case, a single colonized parent flock), this event highlights that prompt investigation and implementation of control measures at the hatcheries after early identification of a cluster can prevent further transmission.
In other-breeder bird hatcheries, Salmonella Enteritidis was the most common serovar; PT 9b was the predominant phage type. An increasing trend in prevalence was observed between 2003 and 2006. The long duration (2-year) cluster of Salmonella Enteritidis suggests a continual source of the bacteria, possibly from contaminated eggs from parent flocks or from an endemic environmental source. Surveillance data on Salmonella in breeder flocks of other birds is limited because submission of environmental samples is optional for these producers (20), thereby limiting the inferences that can be made about how the source(s) and transmission of Salmonella Enteritidis in hatcheries for other-breeder birds might or might not differ from that in broiler-breeder hatcheries.
Salmonella Heidelberg was another prominent serovar among all poultry types in this study. Temporal clusters detected during the early half of the study period were long duration, whereas those detected during the latter half were predominantly short. In breeder flocks, clusters of Salmonella Heidelberg were detected mainly during the early half of the study period and were predominantly long duration (20). As discussed by Sivaramalingam et al (20), the temporal similarities (trends and clusters) between Salmonella Heidelberg at the breeder flock and hatchery levels suggests that control measures at the breeder level for all poultry types is likely to result in a continual decline in Salmonella Heidelberg prevalence at the hatchery and lower levels of the production chain. Further, because temporal clusters of Salmonella Heidelberg isolates in humans have been temporally linked to clusters of Salmonella Heidelberg isolates in chickens (most of which were broiler breeders) (17,28), interventions at both the breeder flock and hatchery levels might reduce the spread of Salmonella Heidelberg to humans via the food chain.
Compared with other serovars, Salmonella Typhimurium was not common in broiler-breeder fluff samples and it was not detected from turkey-breeder fluff samples. However, it was the 3rd and 4th most common serovar in fluff samples from other-breeder birds and layer-breeders, respectively. In humans, Salmonella Typhimurium was the most common serovar in Canada between 2002 and 2004 (4). Previous studies have shown that dairy cattle and swine were the major sources of Salmonella Typhimurium for humans (4). However, several outbreaks have been either associated with, or linked temporally with, poultry and poultry products (4,28,29). Clusters of Salmonella Typhimurium isolates were detected in fluff samples from layer-breeders and other-breeder birds in different periods. In layer-breeders, cluster duration varied from short to long, whereas in other-breeder birds, cluster duration was long. These indicate that the sources and transmission of Salmonella Typhimurium might differ among poultry types. Although it was infrequently isolated from the fluff samples in our study, Salmonella Typhimurium is particularly pathogenic to humans; therefore, early recognition of clusters would be useful in preventing outbreaks among poultry and humans.
Salmonella Kentucky was almost exclusively isolated from broiler-breeder fluff samples. It was the most common serovar in broiler fluff and showed an increasing trend, especially between 2005 and 2008. Similarly, an increasing trend was observed in chickens from passive surveillance data in Canada between 2002 and 2006 (4) and in the US between 2001 and 2005 (7). In these reports, Salmonella Kentucky was ranked as the 2nd most common serovar in chicken. Salmonella Kentucky was also isolated from chicken meat, pork chops, and dairy cattle in 2006 (30).
In our study, Salmonella Senftenberg was the most common serovar isolated from turkey-breeders and the 2nd most common serovar from layer-breeder fluff samples. Among the total isolated Salmonella Senftenberg, approximately half of the isolates were from turkey-breeder fluff samples. Salmonella Senftenberg has been identified in turkey-breeder birds in Ontario and the US from passive surveillance data (5,18). However, in other studies, Salmonella Senftenberg was not a common serovar in turkey-breeders (17,24). A decreasing trend of Salmonella Senftenberg with multiple peaks was observed among the turkey-breeder fluff samples, especially during the latter half of the study period. Among the layer-breeder fluff samples, there were 2 peaks in Salmonella Senftenberg prevalence; the 1st in 2001 and the 2nd in 2006.
The low prevalence of Salmonella isolated from layer-breeder fluff samples might be due to the small number of breeding farms for laying hens existing in Ontario. For example, one hatchery, which supplies over half of the day-old layer chicks in Ontario, is supplied by 2 farms under the supervision of a single manager. Therefore, the risk of acquiring a disease might be relatively low (Mike Petrik, McKinley Hatchery, 2010, personal communication).
The trend in the prevalence of Salmonella isolated from hatchery fluff samples varied among the different poultry types. The increasing trend in broiler-breeders was mainly driven by increasing trends in the prevalence of Salmonella Kentucky and Enteritidis, while the increasing trend in other-breeder birds was mainly due to increasing trends in Salmonella Enteritidis, Typhimurium, and Heidelberg. These serovars can be transmitted to progeny chicks vertically or horizontally (16,31). The hatcheries might initially become contaminated by eggs from carrier flocks and once established in the hatchery, it is possible that these bacteria persist in the hatchery environment and contaminate subsequent batches of eggs or chicks. Some serovars, such as Salmonella Enteritidis and Senftenberg, can survive for long periods after cleaning and disinfection (32). The increasing trends might also be due to factors such as poor biosecurity measures and sanitation at the breeder flock and/or hatchery levels (33–36), and the import of contaminated hatching eggs from the US.
The influence of 1 or a few serovars on overall trends indicates that the population dynamics of Salmonella in poultry can be quite serovar-specific. Specific serovars can become prevalent in 1 or more reservoir populations and then decline within variable periods of time (7,37). The reasons behind the rise and fall in prevalence are usually unknown, but might include microbial adaptation, such as changes in the survivability or transmissibility of the serovar; changes in ecological niches; competition among serovars; immunity of the poultry population through infection or vaccination; serovar-specific control strategies, such as testing and depopulation; management changes; or other factors (7,38). Future studies should seek to better understand the underlying mechanisms for these types of trends in Salmonella serovar prevalence.
Finally, a multi-level analysis allowed for the estimation of variance components at different hierarchical levels (21). The moderate percentage of variation at each of the hatchery-, and visit- (day on which samples were collected from a hatchery) levels suggests that interventions or further investigation at each level is warranted. The variation in our study might be influenced by the number of hatchers in a hatchery (although it was difficult to investigate this from our data), location of the hatchery (35) and associated environmental sources of Salmonella, the hatchery’s US supplier of hatching eggs and chicks, or management practices and biosecurity measures adopted by the hatchery itself or the owners of the breeder flocks supplying the hatchery.
Our data were collected at the population level and interpretations of the results are representative of broiler-breeder, layer-breeder, and turkey-breeder hatcheries in Ontario, and are likely representative of other-breeder bird hatcheries. Additional research is required to better understand the serovar-specific dynamics of Salmonella contamination in poultry hatcheries, particularly with respect to changes in serovar dominance over time. There is also a need to better determine the underlying reasons for temporal clustering, and to develop techniques to use cluster information to identify the sources of Salmonella in hatcheries and to develop appropriate control measures. Future studies are also needed to identify factors that explain the increased prevalence of Salmonella in hatcheries during the summer and fall. Additional research is needed to identify the potential local environmental sources of Salmonella, as well as management practices, biosecurity protocols, and sanitation programs at the hatcheries that are associated with Salmonella prevalence. Finally, from a public health perspective, further work is required to identify whether there is a link between clusters of Salmonella identified in poultry (at all levels of the production chain) and human illness.
Acknowledgments
The authors thank the Poultry Industry Council for funding to carry out this project. The authors also thank the Ontario Hatchery and Supply Flock Policy for providing the data. We are grateful to the Animal Health Laboratory for their technical support for the diagnosis of Salmonella. We also thank the Laboratory for Foodborne Zoonoses, Public Health Agency of Canada, Guelph, Ontario, for carrying out serotyping of Salmonella. The technical information and cooperative discussions of Dr. Agnes Agunos, Dr. Durda Slavic, Mr. Keith Harron, Dr. Mike Petrik, Dr. Rachel Ouckama, Mr. Edward Malek, Mr. Al Dam, Mr. Bob Guy, Ms. Janet Schlitt, and Ms. Pamela Kuipers is greatly acknowledged.
References
- 1.Denny J, Boelaert F, Borck B, Heuer OE, Ammon A, Makela P. Zoonotic infections in Europe: Trends and figures — A summary of the EFSA-ECDC annual report. Euro Surveill. 2007. [Last accessed October 13, 2012]. p. 12. Available from http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3336. [DOI] [PubMed]
- 2.Ravel A, Greig J, Tinga C, et al. Exploring historical Canadian foodborne outbreak data sets for human illness attribution. J Food Prot. 2009;72:1963–1976. doi: 10.4315/0362-028x-72.9.1963. [DOI] [PubMed] [Google Scholar]
- 3.Health Canada. It’s Your Health. Salmonella prevention. 2011. [Last accessed August 23, 2011]. Available from http://www.hc-sc.gc.ca/hl-vs/alt_formats/pacrb-dgapcr/pdf/iyh-vsv/food-aliment/salmonella-eng.pdf.
- 4.Public Health Agency of Canada. Laboratory Surveillance Data for Enteric Pathogens in Canada Annual Summary 2006. Vol. 2007. Canada: [Last accessed October 13, 2012]. pp. 1–108. Available from http://www.nml-lnm.gc.ca/NESP-PNSME/assets/pdf/2006AnnualReport.pdf. [Google Scholar]
- 5.CDC. Salmonella Surveillance: Annual Summary, 2006. Atlanta, Georgia: US Department of Health and Human Services, CDC; 2008. [Last accessed August 23, 2011]. Available from http://www.cdc.gov/ncidod/dbmd/phlisdata/salmtab/2006/SalmonellaAnnualSummary2006.pdf. [Google Scholar]
- 6.Ravel A, Smolina E, Sargeant JM, et al. Seasonality in human salmonellosis: Assessment of human activities and chicken contamination as driving factors. Foodborne Pathog Dis. 2010;7:785–794. doi: 10.1089/fpd.2009.0460. [DOI] [PubMed] [Google Scholar]
- 7.Foley SL, Lynne AM, Nayak R. Salmonella challenges: Prevalence in swine and poultry and potential pathogenicity of such isolates. J Anim Sci. 2008;86:E149–E162. doi: 10.2527/jas.2007-0464. [DOI] [PubMed] [Google Scholar]
- 8.Lee MB, Middleton D. Enteric illness in Ontario, Canada, from 1997 to 2001. J Food Prot. 2003;66:953–961. doi: 10.4315/0362-028x-66.6.953. [DOI] [PubMed] [Google Scholar]
- 9.Hacking WC, Mitchell WR, Carlson HC. Sources of Salmonellae in broiler chickens in Ontario. Can J Comp Med. 1978;42:392–399. [PMC free article] [PubMed] [Google Scholar]
- 10.Cox NA, Berrang ME, Cason JA. Salmonella penetration of egg shells and proliferation in broiler hatching Eggs — A review. Poult Sci. 2000;79:1571–1574. doi: 10.1093/ps/79.11.1571. [DOI] [PubMed] [Google Scholar]
- 11.Bailey JS, Stern NJ, Fedorka-Cray P, et al. Sources and movement of Salmonella through integrated poultry operations: A multistate epidemiological investigation. J Food Prot. 2001;64:1690–1697. doi: 10.4315/0362-028x-64.11.1690. [DOI] [PubMed] [Google Scholar]
- 12.Poppe C, Duncan CL, Mazzocco A. Salmonella contamination of hatching and table eggs: A comparison. Can J Vet Res. 1998;62:191–198. [PMC free article] [PubMed] [Google Scholar]
- 13.Lister SA, Barrow P. Enterobacteriaceae. In: Pattison M, McMullin PF, Bradbury JM, Alexander DJ, editors. Poultry Diseases. 6th ed. Philadelphia, Pennsylvania: Saunders; 2008. pp. 110–145. [Google Scholar]
- 14.McGarr C, Mitchell WR, Carlson HC, Fish NA. An epidemiological study of Salmonellae in broiler chicken production. Can J Public Health. 1980;71:47–57. [PubMed] [Google Scholar]
- 15.Chao MR, Hsien CH, Yeh CM, et al. Assessing the prevalence of Salmonella enterica in poultry hatcheries by using hatched eggshell membranes. Poult Sci. 2007;86:1651–1655. doi: 10.1093/ps/86.8.1651. [DOI] [PubMed] [Google Scholar]
- 16.Kim A, Lee YJ, Kang MS, Kwag SI, Cho JK. Dissemination and tracking of Salmonella spp. in integrated broiler operation. J Vet Sci. 2007;8:155–161. doi: 10.4142/jvs.2007.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerin MT, Martin SW, Darlington GA, Rajic A. A temporal study of Salmonella serovars in animals in Alberta between 1990 and 2001. Can J Vet Res. 2005;69:88–99. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, McEwen B, Mann E, Martin W. Detection of clusters of Salmonella in animals in Ontario from 1991 to 2001. Can Vet J. 2005;46:517–523. [PMC free article] [PubMed] [Google Scholar]
- 19.Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) Ontario Hatchery and Supply Flock Policy (OHSFP) Review. 2001. pp. 1–10. [Google Scholar]
- 20.Sivaramalingam T, McEwen SA, Pearl DL, Ojkic D, Guerin MT. A temporal study of Salmonella serovars from environmental samples from poultry breeder flocks in Ontario between 1998 and 2008. Can J Vet Res. 2013;77:1–11. [PMC free article] [PubMed] [Google Scholar]
- 21.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. 2nd ed. Charlottetown, Prince Edward Island, Canada: AVC Inc; 2003. [Google Scholar]
- 22.Rabe-Hesketh S, Skrondal A, editors. Multilevel and Longitudinal Modeling using Stata. 2nd ed. College Station, Texas: Stata press; 2008. [Google Scholar]
- 23.Sato K, Carpenter TE, Case JT, Walker RL. Spatial and temporal clustering of Salmonella serotypes isolated from adult diarrheic dairy cattle in California. J Vet Diagn Invest. 2001;13:206–212. doi: 10.1177/104063870101300304. [DOI] [PubMed] [Google Scholar]
- 24.Irwin RJ, Poppe C, Messier S, Finley GG, Oggel J. A national survey to estimate the prevalence of Salmonella species among Canadian registered commercial turkey flocks. Can J Vet Res. 1994;58:263–267. [PMC free article] [PubMed] [Google Scholar]
- 25.Government of Canada. Annual Report 2008. Guelph (Ontario): Public Health Agency of Canada; 2011. [Last accessed August 23, 2011]. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) Available from http://www.phac-aspc.gc.ca/cipars-picra/2008/pdf/cipars-picra-2008-eng.pdf. [Google Scholar]
- 26.Public Health Agency of Canada. National Enteric Surveillance Program (NESP) Annual Summary 2009. Canada 2011. [Last accessed August 23, 2011]. Available from http://www.nml-lnm.gc.ca/NESP-PNSME/assets/pdf/NESP_2009_Annual_Report_ENG.pdf.
- 27.Public Health Agency of Canada. Laboratory surveillance data for enteric pathogens in Canada: Annual summary 2005. Canada. 2007. [Last accessed October 13, 2012]. pp. 1–101. Available from http://www.nml-lnm.gc.ca/NESPPNSME/assets/pdf/2005%20Annual%20Report%20Final.pdf.
- 28.Guerin MT, Martin SW, Darlington GA. Temporal clusters of Salmonella serovars in humans in Alberta, 1990–2001. Can J Public Health. 2005;96:390–395. doi: 10.1007/BF03404039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedberg CW, David MJ, White KE, MacDonald KL, Osterholm MT. Role of egg consumption in sporadic Salmonella Enteritidis and Salmonella Typhimurium infections in Minnesota. J Infect Dis. 1993;167:107–111. doi: 10.1093/infdis/167.1.107. [DOI] [PubMed] [Google Scholar]
- 30.Government of Canada. Canadian National Enteric Pathogen Surveillance System (C-EnterNet) 2006. Guelph: Public Health Agency of Canada; 2007. [Last accessed October 13, 2012]. Available from http://www.phac-aspc.gc.ca/publicat/2007/c-enternet06/pdf/06-areport_e.pdf. [Google Scholar]
- 31.Byrd JA, DeLoach JR, Corrier DE, Nisbet DJ, Stanker LH. Evaluation of Salmonella serotype distributions from commercial broiler hatcheries and grower houses. Avian Dis. 1999;43:39–47. [PubMed] [Google Scholar]
- 32.Pedersen TB, Olsen JE, Bisgaard M. Persistence of Salmonella Senftenberg in poultry production environments and investigation of its resistance to desiccation. Avian Pathol. 2008;37:421–427. doi: 10.1080/03079450802216561. [DOI] [PubMed] [Google Scholar]
- 33.Cox NA, Bailey JS, Berrang ME. Diminishing incidence and level of Salmonellae in commercial broiler hatcheries. J Appl Poultry Res. 1997;6:90–93. [Google Scholar]
- 34.Heyndrickx M, Vanderkerchove D, Herman L, Rollier I, Grijspeerdt K, De Zutter L. Routes for Salmonella contamination of poultry meat: Epidemiological study from hatchery to slaughter house. Epidemiol Infect. 2002;129:253–265. doi: 10.1017/s0950268802007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arsenault J, Letellier A, Quessy S, Normand V, Boulianne M. Prevalence and risk factors for Salmonella spp. and Campylobacter spp. caecal colonization in broiler chicken and turkey flocks slaughtered in Quebec, Canada. Prev Vet Med. 2007;81:250–264. doi: 10.1016/j.prevetmed.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Davies RH, Wray C. An approach to reduction of Salmonella infection in broiler chicken flocks through intensive sampling and identification of cross-contamination hazards in commercial hatcheries. Int J Food Microbiol. 1994;24:147–160. doi: 10.1016/0168-1605(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 37.Wegener HC, Hald T, Wong LF, et al. Salmonella control programs in Denmark. Emerg Infect Dis. 2003;9:774–780. doi: 10.3201/eid0907.030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cogan TA, Humphrey TJ. The rise and fall of Salmonella Enteritidis in the UK. J Appl Microbiol. 2003;94:114S–119S. doi: 10.1046/j.1365-2672.94.s1.13.x. [DOI] [PubMed] [Google Scholar]