Abstract
This study aimed to establish the effect of a diet enriched with green-lipped mussel (GLM) on pain and functional outcomes in osteoarthritic dogs. Twenty-three client-owned dogs with osteoarthritis (OA) were fed a balanced control diet for 30 d and then a GLM-enriched balanced diet for the next 60 d. We assessed peak vertical force (PVF), which is considered to be the gold standard method, at Day (D)0 (start), D30 (end of control diet), and D90 (end of GLM-enriched diet). The owners completed a client-specific outcome measure (CSOM), which is a pain questionnaire, once a week. Motor activity (MA) was continuously recorded in 7 dogs for 12 wk. Concentrations of plasma omega-3 fatty acids were quantified as indicative of diet change. Statistical analyses were linear-mixed models and multinomial logistic regression for repeated measures. The GLM diet (from D30 to D90) resulted in an increase in concentrations of plasma omega-3 fatty acids (P < 0.016) and improvement of PVF (P = 0.003). From D0 to D30, PVF did not significantly change (P = 0.06), which suggests that the GLM diet had a beneficial effect on gait function. Moreover, PVF (P = 0.0004), CSOM (P = 0.006), and MA (P = 0.02) improved significantly from D0 to D90. In general, the balanced control diet could have contributed to reduced OA symptoms, an effect that was subsequently amplified by the GLM diet.
Résumé
L’objectif de cette étude était d’établir l’effet d’une diète équilibrée enrichie en moule verte (GLM) avec des évaluations fonctionnelles et de douleur sur des chiens arthrosiques. Vingt-trois chiens arthrosiques de propriétaires (région de Montréal, QC) ont été nourris d’abord avec une diète équilibrée contrôle pendant 30 j., puis avec la diète enrichie en GLM pour les 60 j. suivants. Les évaluations incluaient le pic de force verticale (PFV), considéré comme la méthode étalon, au Jour (J)0 (inclusion), J30 (fin de la diète contrôle) et J90 (fin de la diète GLM). Les propriétaires ont complété de manière hebdomadaire une échelle de mesure spécifique à chaque client (CSOM), qui est un questionnaire de quantification de la douleur. L’activité motrice (AM) a été enregistrée en continu sur 7 chiens pour toute la durée de l’étude (12 sem.). Les concentrations plasmatiques d’acides gras oméga-3 ont été quantifiées en tant que marqueurs de changement de diètes. Les analyses statistiques furent des modèles linéaires-mixtes et une régression logistique multinomiale pour mesures répétées. La diète GLM (de J30 à J90) augmenta les concentrations plasmatiques d’acides gras oméga-3 (P < 0,016) ainsi que le PFV (P = 0,003). De J0 à J30, les changements de PFV furent non-significatifs (P = 0,06), ce qui suggère que la diète GLM a eu un effet thérapeutique sur la fonction biomécanique. De plus, PFV (P = 0,0004), CSOM (P = 0,006) et AM (P = 0,02) s’améliorèrent significativement de J0 à J90. De manière globale, il est possible que la diète équilibrée contrôle ait contribué à améliorer les signes d’arthrose, un effet qui fut amplifié par la suite avec la diète GLM.
(Traduit par Docteur Eric Troncy)
Introduction
Osteoarthritis (OA) is a degenerative disease characterized by inflammatory bursts that lead to a whole-joint disorder, not just cartilage defects as initially thought. The disease can be treated by targeted therapeutics (1). Use of non-steroidal anti-inflammatory drugs (NSAIDs) as standard OA treatment shows the occurrence of adverse effects varying from 2.6% to 34% (2,3). As an alternative approach, nutritional supplements are used for OA multimodal therapy. Specifically, it has been shown that green-lipped mussel (GLM) products may have chondro-modulator and anti-inflammatory properties (2). Using the strength of evidence ranking promulgated by Aragon et al (4), GLM, like other structure-modifying agents such as glycosaminoglycan polysulfate and elk velvet antler, was classified as low to moderate for its effectiveness in reducing the clinical signs of OA in dogs (5,6). One study showed a positive therapeutic effect of a GLM-enriched diet only by using a subjective arthritis scoring system (7). Converging information suggests that a GLM-enriched diet may be of therapeutic benefit in the treatment of OA, but evidence-based medicine requires proof of evidence of such possible positive effects using objective outcome measures (5).
Spontaneous alterations in biomechanics, pain, and stress are present in osteoarthritic dogs and methods that reflect these changes are used to assess canine OA (8,9). Kinetic gait analysis, which uses force plate variables, allows neuromuscular and skeletal disorders to be evaluated objectively. This method, which is sensitive and repeatable under predefined standardized conditions, has been considered the gold standard for assessing OA in dogs (10,11). It represents an evaluation of biomechanical and neurophysiological (particularly nociceptive hypersensitization) alterations, with no possibility of distinguishing between them. Structural changes as assessed by radiographic OA lesions (12) or the more sensitive magnetic resonance imaging (13) do not correlate well with expressed pain or functional impairment. Some chronic pain composite scales (8,9,14–17) and client-specific outcome measures (CSOMs) (18) have been partially validated for use in dogs. Recently, motor activity (MA) was proposed as being a sensitive method for OA assessment (19). Conversely, the weak relationship between pain and OA lesions (1) suggests that the objective methods previously cited are not sufficient by themselves for evaluating subjective pain (9,20,21). Therefore, by using a combination of objective and subjective methods, this study aimed to quantify the effects of a GLM-enriched diet on pain and functioning in osteoarthritic dogs in a longitudinally controlled design in which the dogs were first fed a control diet for 30 d and then the GLM-enriched diet for the next 60 d.
Materials and methods
Animal selection
The Institutional Animal Care and Use Committee approved this study (Rech-1297) as in compliance with the Canadian Council on Animal Care guidelines. After obtaining informed consent from the owners, 30 adult dogs were selected. For inclusion, the dogs had to have exhibited sign(s) of stable lameness in 1 or more limbs for at least 6 mo, as described by our group in a previous study (22). Briefly, a physical examination was performed and a minimal database [complete blood count (CBC), chemistry panel, and urinalysis] was established and repeated at Day (D)30 and D90. According to these criteria, only dogs with no health concerns other than lameness were included. An orthopedic examination and peak vertical force (PVF) gait analysis established the severity of lameness in each joint and limb. A radiographic diagnosis confirmed OA with narrowing of joint space, sclerosis, and osteophyte formation. Six dogs had 1 OA lesion associated with lameness in a limb. This lame limb was evaluated throughout the study. Twenty-four dogs had multisite OA lesions, with the same joint (mostly hips) being affected bilaterally in 7 dogs. The remaining dogs presented from 2 to 6 radiographic OA joint alterations. The limb most affected by lameness, as shown by PVF gait analysis and orthopedic examination, and with OA lesions was followed throughout the study.
The dogs were healthy, did not suffer from other orthopedic abnormalities, and had not undergone an orthopedic intervention within the past year. Partial cranial cruciate ligament rupture with slight instability at the time of presentation was accepted. The dogs could not receive oral nutraceuticals or sporadic non-steroidal anti-inflammatory drugs (NSAIDs) for a 4-wk withdrawal period before inclusion in the study. Dogs on OA prescription-type diets, fatty acid supplements, or oral or injectable anti-inflammatory drugs prescribed by a veterinarian (including both steroidal and NSAID drugs), or those receiving polysulphated glycosaminoglycans therapy, were not selected.
Experimental design
This was a prospective, double–blinded, longitudinally controlled study. The dogs were fed a commercial control diet from inclusion (D0) to D30 (Dog Chow; Nestlé Purina, St.-Louis, Missouri, USA) (Table I). Following this, the dogs were fed a GLM-enriched diet from D31 to D90 (Mobility Support JS; Medi-Cal/Royal Canin, Guelph, Ontario) (Table I). These foods are formulated to meet the nutritional standards of the dog food nutrient profiles established by the Association of American Feed Control Officials for adult (> 1 y old) maintenance (23). The first diet aimed to standardize the food regimen. A prolonged period was chosen to establish any positive effects of the GLM product, as previously indicated (24). A 14-d progressive transition was observed between the 2 diets for each regimen. The owners and investigators (technicians, veterinarians) were blinded to the treatment. Both foods looked similar and were packaged in identical bags. The daily food needs were calculated according to the manufacturer’s recommendations based on the body weight (BW) of the dogs as measured at D0 and D30. The owners were instructed to feed their dogs daily with the exact amount of food calculated. Adjustments to the food intake were not planned. The dogs stayed at home throughout the study. Data acquisition was performed at D0, D30, and D90 at the university teaching hospital of the Faculty of Veterinary Medicine. The owners were not given any recommendations for activity or exercise. A dog was examined at any time if the owner reported pain or abnormal behavior. If rescue analgesia was considered compulsory, the dog would be excluded from the trial.
Table I.
Composition of the main nutrients of diets for dogs
| Guaranteed analysis (except where indicated differently)a | Purina Dog Chow for adult dogs | Mobility Support JS24 large breedb |
|---|---|---|
| Protein (%Min) | 21.00 | 24.50 |
| Fat (%Min) | 10.00 | 10.50 |
| Fiber (%Max) | 4.50 | 6.30 |
| Moisture (%Max) | 12.00 | 10.00 |
| Eicosapentaenic acid (EPA) (%Min)c | Not present | 0.45 |
| Docosahexaenoic acid (DHA) (%Min)c | Not present | 0.20 |
| Glucosamine (mg/kg Min)c | Not present | 1900 |
| Chondroitin (mg/kg Min)c | Not present | 100 |
| Linoleic acid (%Min) | 1.50 | 2.22a |
| Calcium (%Min) | 1.00 | 0.77a |
| Phosphorus (%Min) | 0.80 | 0.52a |
| Zinc (mg/kg)c | 120 | 219.90a |
| Copper (mg/kg)c | d | 31.10a |
| Manganese (mg/kg)c | d | 72.1a |
| Vitamin A (IU/kg) | 10 000 | 28 000a |
| Vitamin E (IU/kg) | 100 | 600a |
Calculated from typical analysis of the diet.
A detailed list of ingredients is available at: http://products.royalcanin.us/products/veterinary/canine/mobility-support-js-large-breed.aspx
Natural source of nutrients provided by New Zealand green-lipped mussel (GLM).
No additional data were available from the nutritional details of this diet.
Plasma omega-3 fatty acid measurements
The fatty acids cis-5,8,11,14,17-eicosapentaenoic acid (EPA) and cis-4,7,10,13,16,19-docosahexaenoic acid (DHA) were quantified in dog plasma by high-performance liquid chromatography-electrospray tandem mass spectrometry (HPLC-ESI-MS/MS) using an analytical approach previously described by our group (25). The method consists of a simple protein precipitation extraction with ethanol followed by analysis using HPLC-ESI-MS/MS. The chromatographic separation was performed using a Waters Symmetry C18 100 × 2.1 mm combined with a 9-min linear gradient at a flow rate of 200 μL/min. The initial mobile phase composition ratio was 50:50 of methanol and 20 mM ammonium acetate in water and the final composition ratio was 90:10. The mass spectrometer was operated in the full scan mass spectrometry mode using one segment analysis (m/z 280–400). The quantification mass used was m/z 301 [M-H]− for EPA and m/z 327 [M-H]− for DHA. Calibration curves were constructed based on the peak-area of each analyte. Blood samples were collected at D0, D30, and D90 from fasted dogs.
Pain and functional assessments
Kinetic gait analysis
Ground reaction forces were recorded as the dogs trotted at a velocity of 1.9 to 2.2 m/s on a biomechanical force platform (Model OR6-6; Advanced Medical Technology, Watertown, Massachusetts, USA) coupled to software (Vetforce; Sharon Software, Dewitt, Michigan, USA) (11). The PVFBW was the average of 5 successful and reproducible loadings at a consistent velocity of the affected limb, normalized to the dog’s body weight (22).
Client-specific outcome measure
Each owner completed a client-specific outcome measure (CSOM) at D0 as described in previous studies (18,26). The owners continued to complete these questionnaires every week during the study. Briefly, the owners recorded up to 5 of their dog’s activities that were most impaired by the painful condition of OA. Since less than 5 activities were accepted, a median value was calculated for all of the selected activities (CSOM-med). The activities were subjectively clustered into 6 categories [Ct(i)] (Table II). All CSOM-med values and categories were ranked 0 (no problem) to 4 (greatest difficulty).
Table II.
Categories of the client-specific outcome measure (CSOM)
| Categories (Ct(i)) | Activities cited by owners |
|---|---|
| Reduced mobility during activity [Ct(1)] | Walking (from 5 min to long walk > 25 min) |
| Playing | |
| Running | |
| Jumping | |
| Accompanying bike | |
| Climbing stairs | |
| Walking on slick floors | |
| Reduced mobility after exercise [Ct(2)] | Walking and climbing stairs after exercise, after a walk, after activities, or after playing |
| Reduced ability to change posture [Ct(3)] | Getting up |
| Lying down | |
| Sitting | |
| Reduced ability to change posture after rest or in the morning [Ct(4)] | Lying down after night, in the morning, or after rest |
| Getting up at the end of the day | |
| Resistance to manipulations [Ct(5)] | At the touch, stretching |
| Mood change [Ct(6)] | Going outside |
Motor activity
An accelerometer placed on the neck collar (Actical Mini Mitter; Bio-Lynx Scientific Equipment, Laval, Quebec) recorded continuous MA on 7 randomly selected dogs, as described in a previous study (27). The raw data output of this device is measured in activity counts (in arbitrary units from 0 to ∞) with an epoch length of 2 min.
A total of 60 480 counts (720 per d) were sampled for each dog. The data were expressed as 14-d average total intensities, delineating 6 consecutive periods (P(i)): P1, from D0 to D14, was the first transition between the home diet and the control diet; during P2, the dogs were fed only the control diet; P3, from D30 to D44, was the second transition; P4 to P6 were the successive 14-d periods of the GLM-enriched diet. In order to analyze variations in MA throughout each day, the activity counts were monitored during 3 daily time periods: at night from 20:00 to 07:00 h; in the morning (AM) from 07:02 to 13:00 h; and in the afternoon (PM) from 13:02 to 19:58 h.
Statistical analyses
We analyzed the changes in pain outcomes (PVFBW and MA) and concentrations of omega-3 fatty acids (EPA and DHA) using linear-mixed models with random intercepts for repeated measures. Both PVFBW and MA were log-transformed to reach normality and homogeneity of the variance. For CSOM-med and Ct(i), we used a multinomial logistic regression for repeated measures or Cochran- Mantel-Haenszel when the assumptions for use of the former were not satisfied. We analyzed the effects of the following 4 subject-dependent covariates on PVF, CSOM, and MA: age at inclusion (older or younger than 6 y); gender (male or female); the anatomical location of the most affected limb (cranial or caudal leg); and the severity of the OA lesion based on the number of joints diagnosed by radiography. The daily time periods (night, AM, and PM) were used to analyze variations in MA. The number of activities, as a fixed covariate, was analyzed by the effect on CSOM-med. Backwards selection of the covariates entered into the models was applied to examine associations between pain outcomes and the different covariates. Significant covariates were maintained in the final models. Missing outcomes were determined using the pairwise deletion method. The data are presented as mean [± standard deviation (SD)], except where stated otherwise. Statistical analyses were performed using SAS software (SAS 9.1; SAS Institute, Cary, North Carolina, USA). All analyses were conducted at α = 0.05 with a Bonferroni adjustment for pairwise multiple comparisons.
Results
The study included 30 dogs. Seven dogs were removed from the study: 3 because of a sudden deterioration in their condition (n = 1 before D30 and n = 2 during the GLM-enriched diet period), 1 for use of NSAIDs (during the GLM-enriched diet), and 3 because the owner did not comply with the guidelines. The demographic characteristics of the final sample (N = 23) are shown in Table III. Clinically relevant changes (general examination and blood analyses) related to the control and GLM-enriched diets were not observed during the course of the study.
Table III.
Demographic characteristics of the dogs’ sample
| Age groups | n [%] |
| 2.5 to 6 y | 12 [52.1] |
| 6 to 12 y | 11 [47.9] |
| Gender | n [%] |
| Male | 14 [60.9] |
| Female | 9 [39.1] |
| Breeds | n [%] |
| Labrador | 7 [30.4] |
| Golden retriever | 4 [17.4] |
| Mixed breeds | 3 [13.0] |
| Other pure breeds | 9 [39.1] |
| Origin of lameness in the most affected limb | n [%] |
| Hip (dysplasia) | 8 [34.8] |
| Stifle (chronic cranial cruciate ligament rupture) | 7 [30.4] |
| Shoulder (osteochondritis dissecans) | 3 [13.0] |
| Elbow (dysplasia) | 3 [13.0] |
| Tarsus/Carpus | 2 [8.9] |
| Number of affected joints | n [%] |
| 1 damaged joint | 4 [17.4] |
| Bilateral (same damaged joint on the right and left limb) | 6 [26.1] |
| Multisite (from 2 to 5 damaged joints) | 13 [56.5] |
Plasma omega-3 fatty acid measurements
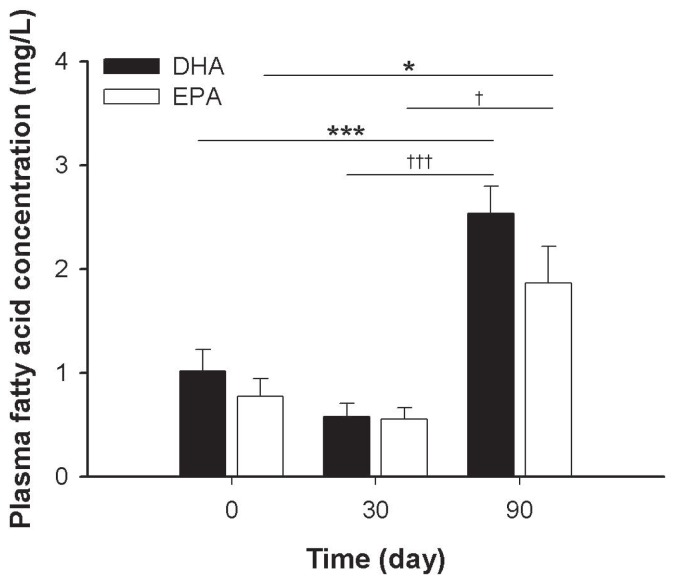
Plasma concentrations in DHA and EPA were significantly higher at D90 than at D0 (P < 0.001 and P = 0.016, respectively) and D30 (P < 0.001 and P = 0.026, respectively). There were no significant changes in DHA or EPA plasma concentrations in dogs fed the control diet (Figure 1).
Figure 1.
Histogram of the concentrations of plasma eicosapentaenic acid (EPA) and docosahexaenoic acid (DHA) by time period. Values are the mean ± standard error of the mean (SEM) of 23 dogs. Data were analyzed by linear-mixed model. Differences of least squares means were corrected by Bonferroni’s adjustment. Significance of difference from D0: *, P < 0.05; ***, P < 0.001. Significance of difference from D30: †, P < 0.05; †††, P < 0.001.
Kinetic gait analysis
The anatomical location of the most affected limb was found to be a significant factor of PVFBW, but no significant effect was found for the other covariates, i.e., age (P = 0.78), gender (P = 0.33), and severity of OA (P = 0.43). While PVFBW differed over time (P = 0.0004) and with the anatomical location of the most affected limb (P < 0.0001), there was no interaction effect. At D90, PVFBW was significantly higher than at D0 (P = 0.0004) and D30 (P = 0.003) (Table IV). As descriptive analysis, the level of improvement in PVF, i.e., the group mean of the difference between D30 and D90, was of 2.5 (4.2) %BW for the GLM-enriched diet (Table V). There was no evidence of a statistically significant difference between D0 and D30 (P = 0.06) (Table IV). The non-significant level of improvement was of 1.6 (5.3) %BW for this 30-d control period.
Table IV.
Body weight, peak vertical force (PVF), and clientspecific outcome measure (CSOM) by time period (D0, D30, and D90) in N = 23 dogs
| Method | D0 | D30 | D90 |
|---|---|---|---|
| BW (kg) Mean (SD)a |
40.4 (8.9) | 40.3 (8.5) | 41.4 (8.38) |
| PVFBW (%BW) Mean (SD)a |
65.4 (17.2) | 67.3 (19.8) | 69.9 (21.3)c,d |
| CSOM-med (score) Median (P5, P95)b |
2.0 (0.8, 3.0) | 1.5 (0.0, 2.0)c | 1.0 (0.0, 2.0)c |
Data are presented as (a) mean [standard deviation (SD)] of body weight (BW), peak vertical ground reaction force adjusted to body weight change (PVFBW) and (b) median of CSOM-med [5th (P5) and 95th (P95) percentiles]. Data were analyzed by linear-mixed model or multinomial logistic regression. Contrast analysis of the difference of least squares means was corrected by Bonferroni’s adjustment.
Significant difference from D0, P < 0.05.
Significant difference from D30, P < 0.05.
Table V.
Systematic review of clinical trials using peak vertical force (PVF) for establishing treatment efficacy compared to placebo in canine osteoarthritis
| Class of agent | Tested agent | Study design | Reference | Changes in PVF | Time elapsed |
|---|---|---|---|---|---|
| NSAID | Etodolac | Randomized, blinded, placebo-controlled trial | (34) | 2.3 (0.4) %BWa Low dosage (n = 34)b,c | 8 d |
| 0.4 (0.4) %BWaPlacebo (n = 33)NS | 8 d | ||||
| NSAID | Carprofen | Randomized, placebo- controlled trial | (10) | N = 29 (=80.6%) of Pos. Resp. (n = 36)c | 14 d |
| N = 19 (=55.9%) of Pos. Resp. Placebo (n = 34) | 14 d | ||||
| NSAID | Carprofen | Randomized, blinded, placebo-controlled trial | (11) | 2.43 %BW [−3.39 to 17.02]d (n = 16)b | 60 d |
| −1.4 %BW [−10.5 to 3.6] Placebo (n = 17)NS | 30 d | ||||
| NSAID | Meloxicam | Randomized, blinded, placebo-controlled trial | (11) | 4.7 %BW [−4.89 to 92.20]d (n = 16)b | 60 d |
| −1.4 %BW [−10.5 to 3.6] Placebo (n = 17)NS | 30 d | ||||
| Nutraceutical supplement | Quality elk velvet antler | Randomized, blinded, placebo-controlled trial | (29) | 4.1 ± 1.0 %BWe (n = 13)b,c | 60 d |
| 2.4 ± 0.7 %BWe (n = 25)b | 60 d | ||||
| 0.05 ± 1.5 %BWePlacebo (n = 13)NS | 30 d | ||||
| NSAID | Licofelone | Randomized, double-blinded, placebo-controlled trial | (35) | 1.7 ± 0.8 %BWe (n = 14)b,c | 14 d |
| −0.3 ± 0.6 %BWePlacebo (n = 16)NS | 14 d | ||||
| 2.9 ± 1.7 %BWe (n = 13)c | 28 d | ||||
| −1.9 ± 0.7 %BWePlacebo (n = 17)b | 28 d | ||||
| Nutraceutical supplement | Green-lipped mussel capsules | Randomized, double-blinded, doubled control trial | (24) | 0.2 [−5.6 to 12.0] %f GLM (n = 14)NS | 56 d |
| 3.2 [−8.2 to 11.8] %f Carprofen (n = 15)b | 56 d | ||||
| −0.9 [−33.6 to 10.0] %fPlacebo (n = 13)NS | 56 d | ||||
| Homeopathic combination | Zeel | Randomized, double-blinded, doubled control trial | (30) | 2.3 [−3.4 to 10.2] %f Zeel (n = 14)b,c | 56 d |
| 3.2 [−8.2 to 11.8] %f Carprofen (n = 15)b | 56 d | ||||
| −0.9 [−33.6 to 10.0] %fPlacebo (n = 13)NS | 56 d | ||||
| Therapeutic diet | Prescription Diet Canine j/d, Hill’s Science Diet | Randomized, blinded, placebo-controlled trial | (31) | 3.9 ± 1.3 %BWe (n = 22)b | 90 d |
| N = 18 (=82%) of increase in PVF | 90 d | ||||
| 0.4 ± 1.6 %BWePlacebo (n = 16)NS | 90 d | ||||
| N = 6 (38%) of increase in PVF Placebo | 90 d | ||||
| Therapeutic diet | Mobility Support, Medi-Cal/Royal Canin | Double-blinded, placebo-controlled trial | Present study | 2.5 (4.2) %BWa GLM diet (n = 23)b,c | 60 d |
| 1.6 (5.3) %BWaPlacebo (n = 23)NS | 30 d | ||||
| 4.2 (7.8) %BWaPlacebo + GLM diet (n = 23)b | 90 d | ||||
| Therapeutic diet | JM Joint Mobility, Nestlé Purina PetCare | Randomized, double-blinded, placebo-double-controlled trial | (32) | 3.5 (6.8) %BWa (n = 14)b | 90 d |
| 0.5 (6.1) %BWaPlacebo 1 (n = 14)NS | 90 d | ||||
| 1.6 (4.1) %BWaPlacebo 2 (n = 14)b | 90 d | ||||
| Herbal therapy | Brachystemma calycinum D don | Randomized, double-blinded, placebo-controlled trial | (33) | 3.5 (5.5) %BWa (n = 16)b,c | 42 d |
| 0.5 (5.3) %BWaPlacebo (n = 17)NS | 42 d |
Mean [standard deviation (SD)] value.
Significantly different from baseline.
Significantly different from placebo.
Median [minimum to maximum] value.
Mean ± standard error of the mean (SEM) value.
Median [minimum to maximum] of percentage of change.
NSAID — Non-steroidal anti-inflammatory drug; %BW — Percentage of body weight;
— nonsignificant change from baseline; Pos. Resp. — Positive response defined as a 5% or greater increase in peak vertical force (PVF) compared to baseline value.
Client-specific outcome measure
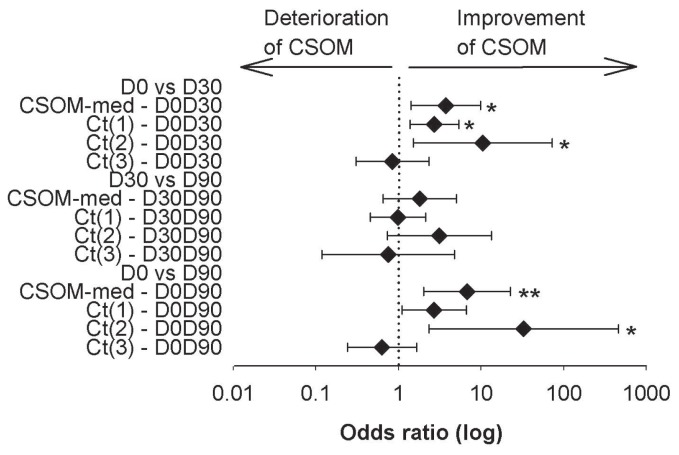
There was no covariate effect on CSOM-med. The CSOM-med showed a higher score, indicating more negative OA symptoms, at D0 than at D30 (odds ratio, OR = 3.8, P = 0.03) and D90 (OR = 6.7, P = 0.006) (Table IV). The CSOM-med remained unchanged from D30 to D90 (P = 0.75) (Figure 2). The score for “reduced mobility during activity” [Ct(1)] was higher at D0 only when compared to D30 (OR = 2.7, P = 0.02). The score for “reduced mobility after exercise” [Ct(2)] was higher at D0 that at both D30 (OR = 10.5, P = 0.05) and D90 (OR = 32.7, P = 0.03) (Figure 2). The score for “reduced ability to change posture after rest or in the morning” [Ct(4)] was higher at D0 than at D90 [Row Mean Score Difference (RMSD) = 13.0, P = 0.05]. The other time comparisons of Ct(4) and all time comparisons for Ct(3), Ct(5), and Ct(6) showed no differences (P > 0.05) (data not shown for Ct(4) to Ct(6)).
Figure 2.
Variations of client-specific outcome measures (CSOMs) within time. Interval plot of the 95% confidence interval of the odds ratio for improvement of the activities summarized by time comparison of the CSOM-med and categories 1 [Ct(1)] to 3 [Ct(3)]. Odds ratio could not be calculated for categories 4 [Ct(4)] to 6 [Ct(6)]. Time comparisons were performed between D0 and D30, D30 and D90, and D0 and D90. For each time comparison, the diamonds represent the estimated mean odds ratio (log); the left and right vertical lines are the upper and lower bounds of the 95% confidence interval; the dotted vertical line is the odds ratio = 1. Significant odds ratio was reached when 95% of the confidence interval did not cross the odds ratio of 1: *, P < 0.05; **, P < 0.01.
Motor activity
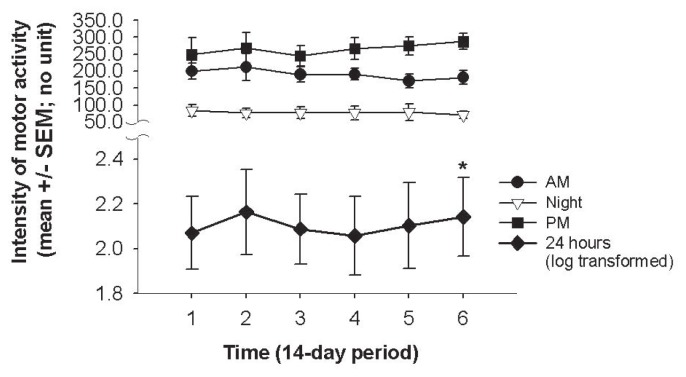
In the 7 dogs studied for MA, both age and the daily monitoring periods had significant effects on MA (P < 0.001), but no significant effect was found for gender (P = 1.0), the anatomical location of the most affected limb (P = 0.98), or the severity of OA (P = 0.51). Specifically, the MA intensity was lower for dogs older than 6 y (P < 0.001). For the daily monitoring periods, MA was lower during the night (P < 0.002), but there was no evidence of a significant difference between both AM and PM periods (P = 0.14). There was no interaction between the daily monitoring periods and the 14-d periods. The 14-d average total intensities of MA changed over time (P = 0.04). The post-hoc analysis showed a significant difference between the first (P1) and last (P6) periods (P = 0.02), as well as between P1 and the pooled P4, P5, and P6 periods (P = 0.012). There was no evidence of a difference among the other 14-d periods (Figure 3).
Figure 3.
Motor activity (MA) by time period. Data are presented as mean ± standard error of the mean (SEM) of log-transformed total activity counts (24 h; dark diamonds); activity counts in the morning from 07:02 to 13:00 h (AM, dark circles); afternoon from 13:02 to 19:58 h (PM; dark squares); and night from 20:00 to 07:00 h (open triangles). Time scale was expressed as every 14-d averaged total intensity from period 1 (P1 = week 1 to 2) to 6 (P6 = week 11 to 12). Significant difference of least squares means compared with P1: *, P < 0.05.
Discussion
The results of this study showed that values of PVF improved significantly in parallel with increased concentrations of plasma DHA and EPA when dogs with OA were fed the GLM-enriched diet. Our findings highlight the potent OA-modifying activity of a GLM-enriched diet. Analysis has shown that GLM powder contains glycosaminoglycans (chondroitin sulfate), amino acid (glutamine), omega-3 fatty acids including DHA and EPA (as demonstrated in our study as proof of absorption) and eicosatetraenoic acid (28), minerals (zinc, copper, manganese), and vitamins E and C (Table I). It was suspected that these components and others included in the diet, such as glucosamine, provided chondroprotective, antioxidant, and anti-inflammatory beneficial effects. This study provides strong clinical evidence to support feeding osteoarthritic dogs a GLM-enriched diet. However, our study failed to establish a positive effect of a GLM diet on CSOM and MA values. Surprisingly, PVF, CSOM, and MA improved throughout the study, from D0 to D90, which suggests a cumulative positive effect of both the standardized control and the therapeutic diets on the assessments of pain and function.
Measuring ground reaction forces using a force plate has been considered to be the most objective measure of outcome in canine OA. A previous study concluded that GLM alleviated pain in dogs with OA because pain scored subjectively decreased but PVF did not improve clearly (24). The apparent discrepancy between our studies could be explained by technical differences in PVF data acquisition and management, differences in the nature of GLM supplementation (tablets versus therapeutic diet) and dosing, as well as variability in the different cohorts. The asymmetry of loading between the front and hind limbs in our sample population was taken into account in order to demonstrate a significant change over the course of the study.
Interestingly, the level of improvement in PVF, i.e., the group mean of the difference between the start and the end of the experimental study, should be taken into consideration. A descriptive analysis of all clinical trials using PVF to establish treatment efficacy compared to the placebo in dogs with OA produced interesting information (see Table V). First, most studies expressed the change in PVF as % BW (Table V). Therefore, a significant difference varying from 1.6% to 4.7% BW does not look so high, but the level of improvement in maximal loading represents an increment of 0.5 to 2.5 kg being applied to the afflicted and painful limb; this is a major change. Second, a plateau or ceiling effect appeared in the level of improvement of PVF. It can be assumed that the level of improvement in PVF cannot reach a supra-maximal value because tissue injuries limit joint biomechanics during gait or non-irreversible pain remodelling. In the present study, this GLM diet resulted in a level of improvement close to those observed in similar studies that tested nutraceuticals (29), homeopathic combinations (30), other therapeutic diets (31,32), herbal therapy (33), and NSAIDs, which are the gold standard for relieving pain in canine OA (10,11,24,34,35). Third, the degree of precision of the level of PVF improvement was hugely variable (see the range of precision in Table V). This highlights the presence of both responders and non-responders in the placebo and treatment groups. In half of the studies reviewed (7/15), comparisons of the tested product to the placebo were not significant. In most cases (6/7) of these studies, we suspect that the deficiency in the power of analysis related to the insufficient sample size would counteract the inter-individual variability in responses in both the placebo and treatment groups. We could also hypothesize that in 2 of the 8 successful demonstrations of a treatment effect compared to placebo, despite the limited power of analysis, the between-group comparison was significant because of the observed decrease in PVF in the placebo group (30,35).
Finally, introducing a standardized control diet led to a significant placebo effect on PVF in 1 study (32) and the difference in PVF between D0 and D30 was close to significance in the present study. As used in previous studies (36), the standardized control diet aimed to homogenize food intake and food habits. Changes in the PVF were not significant throughout the placebo period, but a level of improvement of 1.6% BW might have been confusing to interpret in terms of biomechanical change in dogs with OA. Therefore, we hypothesized that the change in diet was a confounding factor for PVF improvement. A single standardized control diet may have a positive impact on OA symptoms (37), for example, by decreasing BW (38). Significant weight reduction did not occur here, but the result suggests a potent placebo-control diet effect as previously observed using high-quality food (32,39). In summary, our findings indicate that PVF improved greatly from D0 to D90, which suggests a cumulative beneficial effect of the 2 diets on gait parameters.
The main goals of owners are to keep their dogs as comfortable, mobile, and pain-free as possible throughout their lifetime. Both CSOM (18) and MA (19,40) have therefore been used to supply a dynamic assessment of how pain interferes with life activities. In the present study, CSOM and MA changed significantly from D0 to D90, but we did not establish a significant effect of the GLM-enriched diet from D30 to D90. First, as observed for the PVF, a change in environmental habits (related to the classic placebo effect) after enrolment in a clinical trial, but mostly a change in nutrition and the high-quality standard of the (control) diet, appears to show a real therapeutic effect (improvement in CSOM from D0 to D30). It is acknowledged that the inclusion of an osteoarthritic dog in a clinical study could lead to a closer follow-up of the dog by the owner and a change in the level of attention and daily life activities dedicated to the dog that could sustain such a placebo effect. Moreover, the statistical regression to the mean and evolutionary compliance of the owners might have contributed to the improvement in CSOM from D0 to D30. Secondly, a low CSOM-median value of 1.5 at D30 suggested that the level of improvement in CSOM was within a small range of 1.5 to 0 from D30 to D90. Since the highest CSOM score was 4.0, the low CSOM (such as at D30), meaning a low level of discomfort, suggested a ceiling effect of the data. Third, the CSOM is distinct from standardized pain scales (9,14,15) because each CSOM is unique for each dog. In this cohort, the dogs that felt enhanced pain with activity, pain/symptoms in relation to inactivity, and stiffness at night, after activity, or in the morning were more sensitive to a dietary effect. Interestingly, some categories on the CSOM were found to be more sensitive to a dietary effect, such as “reduced mobility after exercise” [Ct(2)] and “reduced ability to change posture after rest or in the morning” [Ct(4)]. To the best of our knowledge, this is the first time that precise limitations in activity affecting dogs with OA have been classified using CSOM. Different symptoms may imply different mechanisms of processing and managing the disease.
Finally, the GLM-enriched diet affected MA during the last 14-d period (P6) only when compared to the first 14-d period, P1. Considering our low statistical power in this exploratory analysis of n = 7 dogs, we can only propose to extend our assessment of the therapeutic efficacy of this promising tool in future studies. Continuous recording of motor activity with an accelerometer provided interesting information in the present study. When compared to P1, the difference observed for the pooled periods of P4, P5, and P6 is suggestive P4 to be the time point at which the GLM-enriched diet started to induce a significant increase in MA. Moreover, the descriptive data of MA in the daily monitoring periods (night, AM, and PM) showed that MA improved continuously during the PM period throughout the GLM-enriched diet phase. Taken together, this suggests a delayed and positive cumulative effect of the GLM diet on this active PM period in this cohort.
The present study had some limitations. Natural fluctuations in the symptoms, rather than measurement errors, may have caused intra-individual variation in outcomes and potential bias. Evaluation during a significant baseline period would improve the outcome interpretation. Indeed, confidence levels could be calculated from these data and would have implications in monitoring the effects of OA intervention. Dropouts were the main concern in this study because 13.3% of the dogs did not complete the study and another 10% were excluded because their owners did not comply with instructions. Treatment-related dropouts should be included as clinical outcomes in themselves. Finally, GLM is a complex natural product comprised of several potent bioactive compounds. Our goal in monitoring plasma concentrations of EPA and DHA was to document the intestinal absorption of some potential therapeutic agents included in the diet. Nevertheless, biochemical details of GLM activity are still far from being understood and further investigation is required of the bioavailability and synergetic effects of all these diet components on the structure and function of joints.
In conclusion, the GLM-enriched diet modified gait in dogs with OA in that the PVF significantly increased over the 60-d period when GLM was introduced into a standardized control diet. This shows the efficacy of a GLM-enriched diet, which should definitely be proposed as an adjunctive treatment to conventional medication. In clinical trials, however, when treatment is incorporated into food, a change in the diet might be a confounding factor on OA symptoms.
Acknowledgments
The authors thank Anne-Andrée Mignault and Katherine Bernier for technical assistance, Dr. Mary Klinck for text editing, and Dr. Guy Beauchamp for statistical analyses. An unrestricted operating grant from Medi-Cal/Royal Canin Canada was used for study design, interpretation of data, and critical revision of the manuscript. An ongoing New Opportunities Fund grant from the Canada Foundation for Innovation (#9483) provided the equipment necessary for the study and a Discovery grant from the Natural Sciences and Engineering Research Council of Canada (#327158-2008) supported bioanalyses and salaries.
References
- 1.Schaible H-G, Richter F, Ebersberger A, et al. Joint pain. Exp Brain Res. 2009;196:153–162. doi: 10.1007/s00221-009-1782-9. [DOI] [PubMed] [Google Scholar]
- 2.Pollard B, Guilford WG, Ankenbauer-Perkins KL, Hedderley D. Clinical efficacy and tolerance of an extract of green-lipped mussel (Perna canaliculus) in dogs presumptively diagnosed with degenerative joint disease. N Z Vet J. 2006;54:114–118. doi: 10.1080/00480169.2006.36622. [DOI] [PubMed] [Google Scholar]
- 3.Lascelles BD, McFarland JM, Swann H. Guidelines for safe and effective use of NSAIDs in dogs. Vet Ther. 2005;6:237–251. [PubMed] [Google Scholar]
- 4.Aragon CL, Hofmeister EH, Budsberg SC. Systematic review of clinical trials of treatments for osteoarthritis in dogs. J Am Vet Med Assoc. 2007;230:514–521. doi: 10.2460/javma.230.4.514. [DOI] [PubMed] [Google Scholar]
- 5.Sanderson RO, Beata C, Flipo RM, et al. Systematic review of the management of canine osteoarthritis. Vet Rec. 2009;164:418–424. doi: 10.1136/vr.164.14.418. [DOI] [PubMed] [Google Scholar]
- 6.Johnston SA, McLaughlin RM, Budsberg SC. Nonsurgical management of osteoarthritis in dogs. Vet Clin North Am Small Anim Pract. 2008;38:1449–1470. doi: 10.1016/j.cvsm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Bui LM, Bierer TL. Influence of green lipped mussels (Perna canaliculus) in alleviating signs of arthritis in dogs. Vet Ther. 2003;4:397–407. [PubMed] [Google Scholar]
- 8.Hielm-Björkman AK, Rita H, Tulamo R-M. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic signs of pain caused by osteoarthritis. Am J Vet Res. 2009;70:727–734. doi: 10.2460/ajvr.70.6.727. [DOI] [PubMed] [Google Scholar]
- 9.Hielm-Björkman AK, Kuusela E, Liman A, et al. Evaluation of methods for assessment of pain associated with chronic osteoarthritis in dogs. J Am Vet Med Assoc. 2003;222:1552–1558. doi: 10.2460/javma.2003.222.1552. [DOI] [PubMed] [Google Scholar]
- 10.Vasseur PB, Johnson AL, Budsberg SC, et al. Randomized, controlled trial of the efficacy of carprofen, a nonsteroidal anti-inflammatory drug, in the treatment of osteoarthritis in dogs. J Am Vet Med Assoc. 1995;206:807–811. [PubMed] [Google Scholar]
- 11.Moreau M, Dupuis J, Bonneau NH, Desnoyers M. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet Rec. 2003;152:323–329. doi: 10.1136/vr.152.11.323. [DOI] [PubMed] [Google Scholar]
- 12.Gordon WJ, Conzemius MG, Riedesel E, et al. The relationship between limb function and radiographic osteoarthrosis in dogs with stifle osteoarthrosis. Vet Surg. 2003;32:451–454. doi: 10.1053/jvet.2003.50051. [DOI] [PubMed] [Google Scholar]
- 13.Libicher M, Ivancic M, Hoffmann M, Wenz W. Early changes in experimental osteoarthritis using the Pond-Nuki dog model: Technical procedure and initial results of in vivo MR imaging. Eur Radiol. 2005;15:390–394. doi: 10.1007/s00330-004-2486-y. [DOI] [PubMed] [Google Scholar]
- 14.Wiseman-Orr ML, Nolan AM, Reid J, Scott EM. Development of a questionnaire to measure the effects of chronic pain on health-related quality of life in dogs. Am J Vet Res. 2004;65:1077–1084. doi: 10.2460/ajvr.2004.65.1077. [DOI] [PubMed] [Google Scholar]
- 15.Brown DC, Boston RC, Coyne JC, Farrar JT. Development and psychometric testing of an instrument designed to measure chronic pain in dogs with osteoarthritis. Am J Vet Res. 2007;68:631–637. doi: 10.2460/ajvr.68.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown DC, Boston RC, Coyne JC, Farrar JT. Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc. 2008;233:1278–1283. doi: 10.2460/javma.233.8.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiseman-Orr ML, Scott EM, Reid J, Nolan AM. Validation of a structured questionnaire as an instrument to measure chronic pain in dogs on the basis of effects on health-related quality of life. Am J Vet Res. 2006;67:1826–1836. doi: 10.2460/ajvr.67.11.1826. [DOI] [PubMed] [Google Scholar]
- 18.Gingerich DA, Strobel JD. Use of client-specific outcome measures to assess treatment effects in geriatric, arthritic dogs: Controlled clinical evaluation of a nutraceutical. Vet Ther. 2003;4:376–386. [PubMed] [Google Scholar]
- 19.Dow C, Michel KE, Love M, Brown DC. Evaluation of optimal sampling interval for activity monitoring in companion dogs. Am J Vet Res. 2009;70:444–448. doi: 10.2460/ajvr.70.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn MM, Keuler NS, Maria YL, Faria LE, Muir P, Markel MD. Evaluation of agreement between numerical rating scales, visual analogue scoring scales, and force plate gait analysis in dogs. Vet Surg. 2007;36:360–367. doi: 10.1111/j.1532-950X.2007.00276.x. [DOI] [PubMed] [Google Scholar]
- 21.Waxman AS, Robinson DA, Evans RB, Hulse DA, Innes JF, Conzemius MG. Relationship between objective and subjective assessment of limb function in normal dogs with an experimentally induced lameness. Vet Surg. 2008;37:241–246. doi: 10.1111/j.1532-950X.2008.00372.x. [DOI] [PubMed] [Google Scholar]
- 22.Moreau M, Troncy E, Bichot S, Lussier B. Influence of changes in body weight on peak vertical force in osteoarthritic dogs: A possible bias in study outcome. Vet Surg. 2010;39:43–47. doi: 10.1111/j.1532-950X.2009.00621.x. [DOI] [PubMed] [Google Scholar]
- 23.Association of American Feed Control Officials (AAFCO) Official Publication. Oxford: Association of American Feed Control Officials; 2003. [Google Scholar]
- 24.Hielm-Björkman AK, Tulamo R-M, Salonen H, Raekallio M. Evaluating complementary therapies for canine osteoarthritis Part I: Green-lipped mussel (Perna canaliculus) Evid Based Complement Alternat Med. 2009;6:365–373. doi: 10.1093/ecam/nem136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douat J, Vachon P, Beaudry F. Characterization of in vitro metabolism of capsazepine, a vanilloid transient receptor potential channel antagonist, by liquid chromatography quadrupole ion trap mass spectrometry. Biomed Chromatogr. 2011;25:479–492. doi: 10.1002/bmc.1471. [DOI] [PubMed] [Google Scholar]
- 26.Lascelles BDX, Gaynor JS, Smith ES, et al. Amantadine in a multimodal analgesic regimen for alleviation of refractory osteoarthritis pain in dogs. J Vet Intern Med. 2008;22:53–59. doi: 10.1111/j.1939-1676.2007.0014.x. [DOI] [PubMed] [Google Scholar]
- 27.Moreau M, Rialland P, Pelletier J-P, et al. Tiludronate treatment improves structural changes and symptoms of osteoarthritis in the canine anterior cruciate ligament model. Arthritis Res Ther. 2011;13:R98. doi: 10.1186/ar3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy KJ, Mann NJ, Sinclair AJ. Fatty acid and sterol composition of frozen and freeze-dried New Zealand Green Lipped Mussel (Perna canaliculus) from three sites in New Zealand. Asia Pac J Clin Nutr. 2003;12:50–60. [PubMed] [Google Scholar]
- 29.Moreau M, Dupuis J, Bonneau NH, Lecuyer M. Clinical evaluation of a powder of quality elk velvet antler for the treatment of osteoarthrosis in dogs. Can Vet J. 2004;45:133–139. [PMC free article] [PubMed] [Google Scholar]
- 30.Hielm-Björkman AK, Tulamo R-M, Salonen H, Raekallio M. Evaluating complementary therapies for canine osteoarthritis Part II: A homeopathic combination preparation (Zeel(R)) Evid Based Complement Alternat Med. 2009;6:465–471. doi: 10.1093/ecam/nem143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roush JK, Cross AR, Renberg WC, et al. Evaluation of the effects of dietary supplementation with fish oil omega-3 fatty acids on weight bearing in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236:67–73. doi: 10.2460/javma.236.1.67. [DOI] [PubMed] [Google Scholar]
- 32.Moreau M, Troncy E, del Castillo JRE, Bédard C, Gauvin D, Lussier B. Effects of feeding a high omega-3 fatty acids diet in dogs with naturally occurring osteoarthritis. J Anim Physiol Anim Nutr (Berl) 2012. http://dx.doi.org/10.1111/j.1439-0396.2012.01325.x. [DOI] [PubMed]
- 33.Moreau M, Lussier B, Pelletier J-P, Martel-Pelletier J, Bédard C, Gauvin D, Troncy E. Brachystemma calycinum D. don effectively reduces the locomotor disability in dogs with naturally occuring osteoarthritis: A randomized placebo-controlled trial. Evid Based Complement Alternat Med. 2012. http://dx.doi.org/10.1155/2012/646191. [DOI] [PMC free article] [PubMed]
- 34.Budsberg SC, Johnston SA, Schwarz PD, DeCamp CE, Claxton R. Efficacy of etodolac for the treatment of osteoarthritis of the hip joints in dogs. J Am Vet Med Assoc. 1999;214:206–210. [PubMed] [Google Scholar]
- 35.Moreau M, Lussier B, Doucet M, Vincent G, Martel-Pelletier J, Pelletier JP. Efficacy of licofelone in dogs with clinical osteoarthritis. Vet Rec. 2007;160:584–588. doi: 10.1136/vr.160.17.584. [DOI] [PubMed] [Google Scholar]
- 36.Bierer TL, Bui LM. Improvement of arthritic signs in dogs fed green-lipped mussel (Perna canaliculus) J Nutr. 2002;132:1634S–1636S. doi: 10.1093/jn/132.6.1634S. [DOI] [PubMed] [Google Scholar]
- 37.Budsberg SC, Bartges JW. Nutrition and osteoarthritis in dogs: Does it help? Vet Clin North Am Small Anim Pract. 2006;36:1307–1323. doi: 10.1016/j.cvsm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Impellizeri JA, Tetrick MA, Muir P. Effect of weight reduction on clinical signs of lameness in dogs with hip osteoarthritis. J Am Vet Med Assoc. 2000;216:1089–1091. doi: 10.2460/javma.2000.216.1089. [DOI] [PubMed] [Google Scholar]
- 39.Fritsch DA, Allen TA, Dodd CE, et al. A multicenter study of the effect of dietary supplementation with fish oil omega-3 fatty acids on carprofen dosage in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;236:535–539. doi: 10.2460/javma.236.5.535. [DOI] [PubMed] [Google Scholar]
- 40.Brown DC, Boston RC, Farrar JT. Use of an activity monitor to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc. 2010;237:66–70. doi: 10.2460/javma.237.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]