Abstract
Objective:
To compare the cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in patients with atrial fibrillation (AF).
Methods:
Using standard methods, we created a Markov decision model based on the estimated cost of apixaban and data from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial and other trials of warfarin therapy for AF. We quantified the cost and quality-adjusted life expectancy resulting from apixaban 5 mg twice daily compared with those from warfarin therapy targeted to an international normalized ratio of 2–3. Our base case population was a cohort of 70-year-old patients with no contraindication to anticoagulation and a history of stroke or TIA from nonvalvular AF.
Results:
Warfarin therapy resulted in a quality-adjusted life expectancy of 3.91 years at a cost of $378,500. In comparison, treatment with apixaban led to a quality-adjusted life expectancy of 4.19 years at a cost of $381,700. Therefore, apixaban provided a gain of 0.28 quality-adjusted life-years (QALYs) at an additional cost of $3,200, resulting in an incremental cost-effectiveness ratio of $11,400 per QALY. Our findings were robust in univariate sensitivity analyses varying model inputs across plausible ranges. In Monte Carlo analysis, apixaban was cost-effective in 62% of simulations using a threshold of $50,000 per QALY and 81% of simulations using a threshold of $100,000 per QALY.
Conclusions:
Apixaban appears to be cost-effective relative to warfarin for secondary stroke prevention in patients with AF, assuming that it is introduced at a price similar to that of dabigatran.
Anticoagulation reduces the risk of stroke from atrial fibrillation (AF) more effectively than antiplatelet therapy.1 For decades, vitamin K antagonists (VKAs) such as warfarin have been the standard oral anticoagulant drugs for this indication, but they require close monitoring to ensure effectiveness and to reduce the risk of associated hemorrhage. Several new oral anticoagulant drugs have more predictable metabolism and fewer drug and dietary interactions than VKAs, eliminating the need for laboratory monitoring. Dabigatran appears superior to warfarin and rivaroxaban is noninferior to warfarin for stroke prevention in patients with AF,2,3 and both drugs have been approved by the US Food and Drug Administration (FDA) for this indication. Apixaban appears to reduce rates of stroke, major hemorrhage, and death compared with warfarin in patients with AF4 and is awaiting FDA review.
By addressing some of the limitations of VKAs, these drugs may improve thromboprophylaxis for patients with AF, but questions remain regarding their widespread adoption. In addition to raising concerns about a lack of antidotes and reliable laboratory measures of their anticoagulant activity, the introduction of these agents also raises issues related to cost. Given the strong performance of apixaban for secondary stroke prevention in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial,5 it may become a compelling agent for preventing recurrent stroke in patients with AF. However, the cost-effectiveness of apixaban in this role is unknown. Using a decision model similar to that in our previously reported cost-effectiveness analysis of dabigatran vs warfarin,6 we compared the cost-effectiveness of apixaban and warfarin for stroke prevention in patients with AF and prior stroke or TIA.
METHODS
Decision model.
We used a Markov model to compare 2 strategies of anticoagulation after stroke or TIA in patients with AF: adjusted-dose warfarin with a goal international normalized ratio (INR) of 2–3 vs apixaban 5 mg twice daily. Our base case population was a hypothetical cohort of 70-year-old patients with no contraindication to anticoagulation and a history of stroke or TIA from nonvalvular AF.
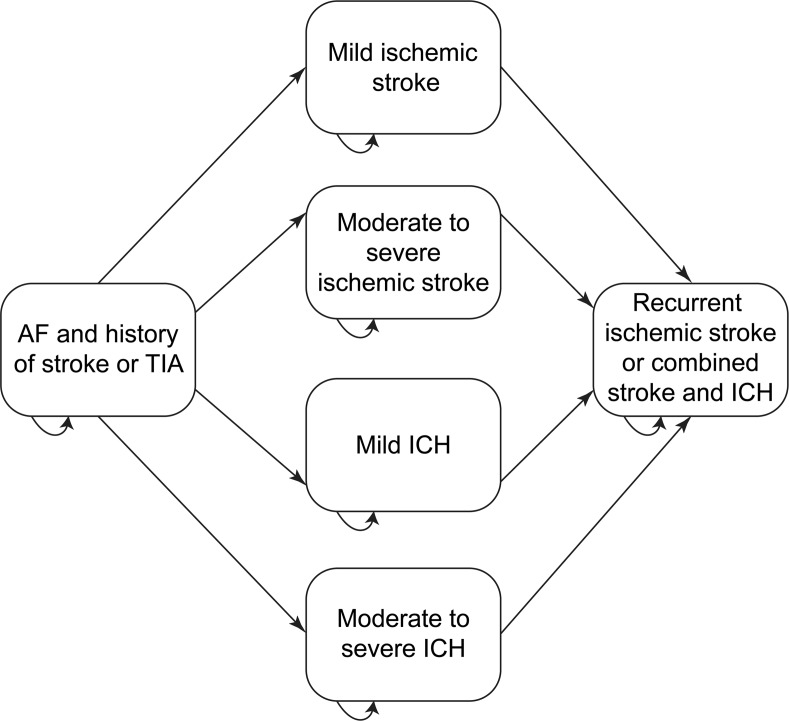
The base case included the following health states: no disability (history of TIA or ischemic stroke with full recovery), TIA, ischemic stroke (fatal or resulting in moderate to severe, mild, or no disability), intracerebral hemorrhage (ICH) (fatal or resulting in moderate to severe or mild disability), recurrent stroke or combined stroke and ICH, extracranial hemorrhage, myocardial infarction (MI), and death (figure 1).
Figure 1. Schematic depiction of a Markov decision model comparing warfarin with apixaban for secondary stroke prevention in patients with atrial fibrillation (AF).
All patients start with AF and prior stroke or TIA and then move between health states in 30-day cycles for 20 years or until death. From any of the health states shown, patients may die or transiently develop TIA, myocardial infarction, or extracranial hemorrhage. Potential health states are the same for both apixaban and warfarin, but the probability of transitions differ. ICH = intracerebral hemorrhage.
Our model adopted a societal perspective. We excluded costs unrelated to our treatment strategies and disease states because our focus was on the incremental cost-effectiveness of apixaban compared with that of warfarin. Estimates of quality-adjusted life expectancy were obtained by multiplying the probability of adverse events in our model by their expected utilities, which quantify the quality of life associated with an event or health state, ranging from 0 for death to 1 for perfect health.7
We applied costs and utilities in 1-month cycles for each outcome over its expected duration. Quality-adjusted life expectancy and net costs were quantified over 20 years or until death, whichever occurred first. We discounted life-years and costs at 3% per year7 and report them as quality-adjusted life-years (QALYs) and 2010 US dollars (rounded to the nearest $100).8 We built our model and performed all analyses using TreeAge Pro Suite 2011 (TreeAge Software, Williamstown, MA). The methods and reporting of our study follow guidelines for the conduct of cost-effectiveness analyses.7
Standard protocol approvals, registrations, and patient consents.
This study did not involve human subjects and therefore did not require approval by our ethics committees.
Probability of outcomes in the decision model.
Rates of outcomes in our model were primarily based on a substudy of the ARISTOTLE trial involving patients with prior stroke or TIA.5 Outcomes not included in our model were assumed to occur with equal frequency in both treatment groups. We adjusted mortality rates for age, the presence of AF and prior stroke or TIA, and type of antithrombotic therapy (table e-1 on the Neurology® Web site at www.neurology.org). In our base case, the time in a therapeutic INR range (TTR) for patients receiving warfarin therapy was 66%, the average TTR in the ARISTOTLE trial.4 In sensitivity analyses, the risks of adverse events with apixaban relative to warfarin were adjusted for quartiles of TTR.9
The rate of ischemic stroke in our base case was 2.23% per year among those receiving warfarin and 1.92% per year among those receiving apixaban.5 All ischemic stroke rates were increased by a factor of 1.4 per decade of life, compounded monthly.10 TIAs represented 28% of ischemic events.
In our base case, 1.49% of patients receiving warfarin developed ICH per year, compared with 0.55% of patients receiving apixaban.5 Rates of ICH were increased by a factor of 1.97 per decade of life, compounded monthly.11 We assumed that patients who developed ICH stopped anticoagulation and began lifelong aspirin therapy, which was associated with a 0.3% annual rate of ICH and a 6.3% annual rate of ischemic stroke.12,13 Conversely, because our cohort represented a high-risk group with prior thromboembolism, patients who had a major extracranial hemorrhage resumed anticoagulation therapy after 1 month. Rates of major extracranial hemorrhage were 2.39% with warfarin and 2.29% with apixaban.5
The rate of MI was 0.91% per year among patients receiving warfarin and 0.57% per year among patients receiving apixaban.5 The rate of MI among patients receiving aspirin was 0.5%.14 MI risk was increased by a factor of 1.3 per decade of life, compounded monthly.15 The short-term mortality of MI was 7.8%.16
Quality-of-life estimates.
Utilities for ischemic stroke and ICH were based on their severity (table e-1). Ischemic stroke was classified as fatal or resulting in moderate to severe, mild, or no disability. ICH was classified as fatal or resulting in moderate to severe or mild disability. We assigned a temporary (1 month) decrement in utility to patients who developed TIA, nondisabling stroke, MI, or major extracranial hemorrhage.
Based on published data from a survey of patients receiving warfarin, we assigned warfarin therapy a utility of 0.987, given its associated bleeding risk and need for laboratory testing.17 No direct data regarding the utility of apixaban are available. Ximelagatran, an older direct thrombin inhibitor, was estimated to have a utility of 0.994 by physicians with expertise in anticoagulation,17 and recent cost-effectiveness analyses have assigned dabigatran the same utility as ximelegatran.6,18,19 To maintain consistency across studies, we used the same estimate of utility of apixaban in our base case and varied it widely in sensitivity analyses.
Costs.
Because apixaban is not yet approved for use in the United States, we estimated its cost from its European price ($210 per month20) and added the cost of office visits for routine clinical monitoring (Current Procedural Terminology [CPT] code 99211). The cost of warfarin therapy was its wholesale cost21 plus Medicare reimbursement (CPT code 99363)22 and 14 laboratory tests to check the INR23 for each 90-day period of anticoagulation management. In sensitivity analyses, we examined the costs of patients initiating warfarin therapy, which required up to 8 additional laboratory tests and was reimbursed at a higher rate (CPT code 99364).22
The one-time costs of hospitalization for TIA, ischemic stroke, ICH, MI, and major extracranial hemorrhage were estimated from costs published by the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project under the relevant diagnosis-related group (DRG).24 Chronic costs of care for these adverse events were calculated from the median value of published studies and Medicare reimbursement rates for the appropriate DRG.24–29
Sensitivity analyses.
In one-way sensitivity analyses, we varied all model inputs over plausible ranges based on confidence intervals from the ARISTOTLE substudy5 or the variation in published values. In addition, we examined the effects of baseline stroke and ICH risk by varying them in both treatment groups over the same range. For ischemic stroke, this range of baseline relative risk varied from 0.25 to 2, corresponding approximately to CHADS2 scores of 1 to 5.30 To address concerns about the real-world bleeding risk of apixaban and other new oral anticoagulant drugs,31 we varied the baseline risks of ICH and extracranial hemorrhage to 4 times the base case risks and also varied the relative risks of bleeding with apixaban vs warfarin to 25% higher than the 95% confidence intervals reported in the ARISTOTLE substudy.5 Given emerging data about an increased short-term risk of stroke upon discontinuation of rivaroxaban,32 we explored the possibility of a similar effect with apixaban by ranging the relative short-term risk of stroke after apixaban discontinuation to 4 times the risk after warfarin discontinuation. Last, a probabilistic sensitivity analysis was performed using a first-order Monte Carlo simulation over 10,000 iterations. For each iteration, the values for all input variables were randomly sampled from their respective distributions. A normal distribution was used for age, a log-normal distribution for costs and relative risks, a beta distribution for utilities, and a Dirichlet distribution for mutually exclusive categorizations of stroke and ICH severity.
RESULTS
Base case.
Warfarin therapy resulted in a quality-adjusted life expectancy of 3.91 years at a cost of $378,500. In comparison, treatment with apixaban led to a quality-adjusted life expectancy of 4.19 years at a cost of $381,700. Therefore, apixaban provided a gain of 0.28 QALYs at an additional cost of $3,200, resulting in an incremental cost-effectiveness ratio (ICER) of $11,400.
Sensitivity analyses.
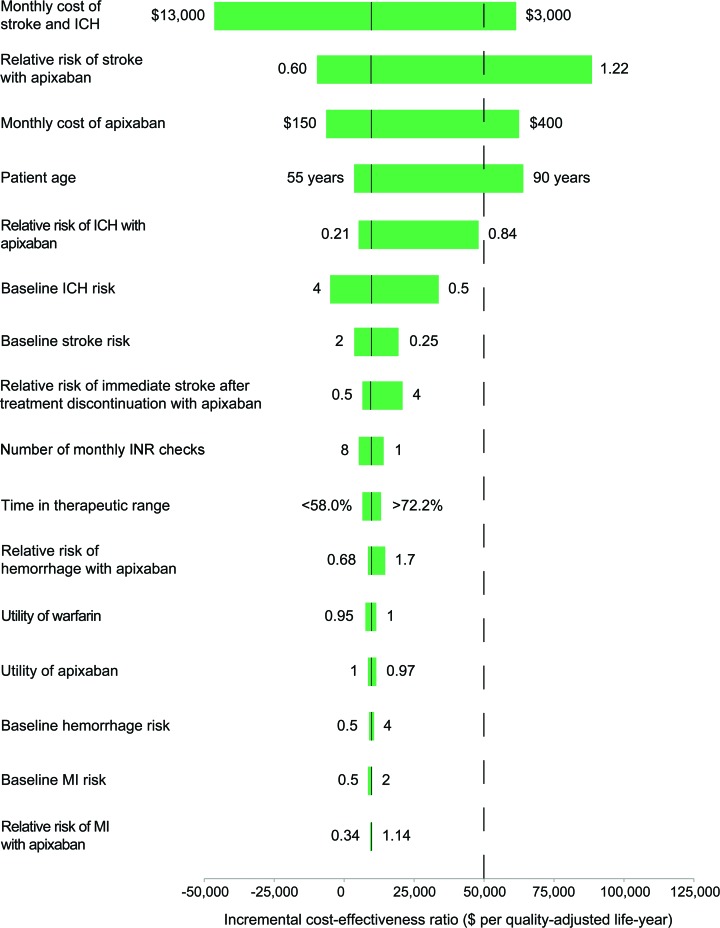
Variables with the greatest influence on our results were the monthly cost of recurrent stroke or combined stroke and ICH, the starting age of the cohort, the relative risk of ischemic stroke with apixaban vs warfarin, and the cost of apixaban (figure 2). First, the cost-effectiveness of apixaban improved as the monthly cost of care for patients with recurrent stroke or combined stroke and ICH increased. Above a monthly cost of $4,100, the ICER for apixaban dropped below $50,000 per QALY and above $8,200, apixaban was dominant. Second, our model was moderately sensitive to the cost of apixaban, but apixaban remained cost-effective at monthly costs up to $350, which would be roughly 175% of the current wholesale price of dabigatran.33 Conversely, at a monthly cost of less than $170, apixaban was cost-saving compared with warfarin. Third, the cost-effectiveness of apixaban relative to that of warfarin varied with the starting age of our cohort. At age older than 87 years, the competing risk from background mortality overwhelmed the relative reductions in adverse events with apixaban. Last, apixaban was dominant if its relative risk of ischemic stroke compared with that of warfarin was less than 0.75 and remained cost-effective until its relative risk exceeded 1.09.
Figure 2. Incremental cost-effectiveness ratio of apixaban vs warfarin over plausible ranges of model inputs.
The solid vertical black lines represent values in the base case. ICH = intracerebral hemorrhage; INR = international normalized ratio; MI = myocardial infarction.
Varying other inputs across their plausible ranges did not significantly affect the ICER of apixaban compared with that of warfarin (figure 2). Changes in the utility of warfarin or the cost and number of INR checks throughout reasonable ranges had negligible effects on our results. Apixaban remained robustly cost-effective relative to warfarin across the spectrum of TTR. Plausible variations in the baseline risk of ICH or extracranial hemorrhage or their relative risks with apixaban vs warfarin did not negate the cost-effectiveness of apixaban. Similarly, apixaban remained cost-effective throughout a wide range of baseline stroke risk. Apixaban was cost-effective even if its discontinuation due to poor tolerability led to 4 times the short-term risk of stroke as discontinuation of warfarin. In addition, although missed doses would affect clinical outcomes more adversely with apixaban than with warfarin given the longer half-life of warfarin, imperfect compliance with twice daily dosing in a substantial proportion of patients did not significantly affect the ICER of apixaban.
Probabilistic sensitivity analysis.
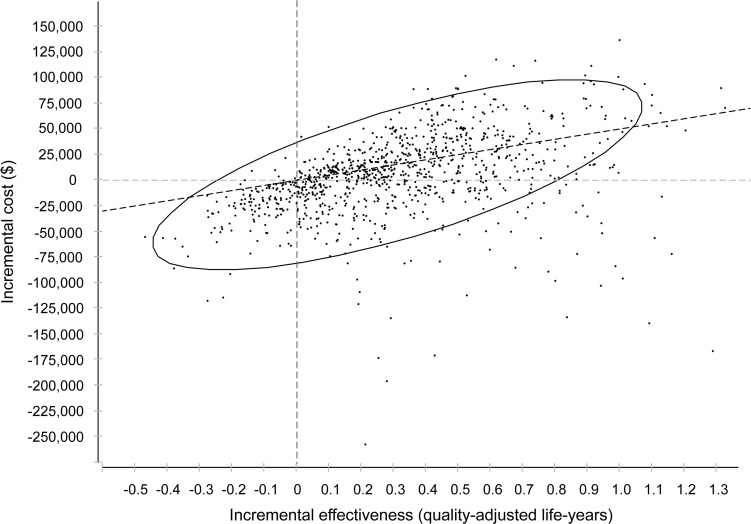
Given a willingness-to-pay threshold of $50,000 per QALY, apixaban was cost-effective in 62% of Monte Carlo simulations (figure 3). This proportion increased to 81% using a threshold of $100,000 per QALY.
Figure 3. Proportion of simulations in probabilistic sensitivity analysis resulting in a favorable incremental cost-effectiveness ratio of apixaban vs warfarin.
Points below the diagonal dotted line represent iterations in which apixaban was cost-effective at a threshold of $50,000 per quality-adjusted life-year. The ellipse encompasses 95% of all iterations.
DISCUSSION
Based on subgroup results from the ARISTOTLE trial, our analysis indicates that apixaban is cost-effective relative to VKAs for thromboprophylaxis in patients with AF and prior stroke or TIA. Our findings were robust across a reasonable range of model inputs.
Cost-effectiveness analyses of rivaroxaban or subgroup analyses of rivaroxaban in patients with prior stroke or TIA are not yet available. To ensure the comparability of this analysis with prior cost-effectiveness analyses of dabigatran, we used the same decision model as our analysis of dabigatran vs warfarin,6 which was broadly similar to that for other analyses of dabigatran.18,19 Our estimated ICER for apixaban ($11,400 per QALY) is lower than our previous estimate for dabigatran ($25,000 per QALY). Apixaban also appears more robustly cost-effective in sensitivity analyses than dabigatran. For example, although the relative cost-effectiveness of dabigatran depends on the quality of warfarin management in the comparator group,6,18 the cost-effectiveness of apixaban for secondary stroke prevention does not appear to be sensitive to the TTR of warfarin. However, whereas the baseline characteristics of patients with prior stroke or TIA in the Randomised Evaluation of Long-Term Anticoagulation Therapy (RE-LY) and ARISTOTLE trials appear broadly similar,5,34 caution should be used when estimates of the cost-effectiveness of these 2 drugs are compared, because directly comparative data are lacking.
Our study should be interpreted in the context of several important limitations. First, many of our model inputs were derived from a substudy of a single randomized clinical trial. This resulted in a fair amount of uncertainty in our model, as reflected in the fact that only 62% of Monte Carlo simulations supported the base case finding that apixaban is cost-effective at a threshold of $50,000 per QALY. Larger sample sizes would provide more precise parameter estimates and greater certainty, as can be seen in the analysis of Freeman et al.19 of the entire RE-LY cohort, in which dabigatran was cost-effective (at $50,000 per QALY) in >80% of simulations. Therefore, although apixaban appears to be cost-effective for secondary stroke prevention based on currently available evidence, further data would allow this to be assessed more definitively. Second, our model represented a population of patients with prior stroke or TIA, and therefore our results cannot necessarily be extrapolated to all patients with AF. Third, our model quantified costs and QALYs over a 20-year horizon, but many of our inputs were derived from studies with much shorter follow-up, such as the median 1.8 years of follow-up achieved in the ARISTOTLE trial. Last, we used the European price of apixaban to estimate its US cost if it were to be approved, and therefore our results may change depending on the final US price of apixaban.
In this dynamic area of medicine, further data about the real-world patterns of use, effectiveness, and adverse event rates of new oral anticoagulant drugs are anticipated, and such data will allow more refined estimates of the relative cost-effectiveness of these agents. In the meantime, however, questions about these drugs' costs already factor into the decisions of physicians, patients, insurers, and health systems, and therefore our results may be helpful in determining optimal strategies of anticoagulation to prevent stroke from AF. Based on available data, apixaban appears to be a cost-effective alternative to VKAs for secondary stroke prevention in patients with AF.
Supplementary Material
GLOSSARY
- AF
atrial fibrillation
- ARISTOTLE
Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation
- CPT
Current Procedural Terminology
- DRG
diagnosis-related group
- FDA
US Food and Drug Administration
- ICER
incremental cost-effectiveness ratio
- ICH
intracerebral hemorrhage
- INR
international normalized ratio
- MI
myocardial infarction
- QALY
quality-adjusted life-years
- RE-LY
Randomised Evaluation of Long-Term Anticoagulation Therapy
- TTR
time in a therapeutic INR range
- VKA
vitamin K antagonist
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Study concept and design: H. Kamel, J.D. Easton, S.C. Johnston, A.S. Kim. Drafting/revising the manuscript for content: H. Kamel, J.D. Easton, S.C. Johnston, A.S. Kim. Analysis or interpretation of data: H. Kamel, A.S. Kim. Principal investigator/guarantor: H. Kamel.
DISCLOSURE
H. Kamel serves as an Associate Editor for Journal Watch Neurology. J.D. Easton is funded by NIH grant 1U01 NS062835; is a consultant for Bristol-Myers Squibb; is on an Advisory Board for AstraZeneca; is on Data Monitoring Boards for Daiichi-Sankyo, Schering-Plough Research Institute, and Novartis; and is on the Adjudication Committee for the WARCEF trial (NIH). S.C. Johnston is funded by NIH grants 1U01 NS062835 and UL1 RR024131; has received research support from the American Heart Association; is coeditor of Journal Watch Neurology and Vice Editor of the Annals of Neurology; is a coholder of a patent on an RNA panel to identify and risk stratify TIA; receives research support from sanofi-aventis (drug and placebo for an NIH-sponsored trial), Stryker Neurovascular, and Boston Scientific; holds an appointment as a Kaiser Permanente Division of Research adjunct investigator; and has served as a consultant for AstraZeneca. A.S. Kim receives research support from the American Heart Association, the National Stroke Association, and SanBio Inc. and has received research support from NIH grant UL1 RR024131. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367: 1903– 1912 [DOI] [PubMed] [Google Scholar]
- 2. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883– 891 [DOI] [PubMed] [Google Scholar]
- 3. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139– 1151 [DOI] [PubMed] [Google Scholar]
- 4. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981– 992 [DOI] [PubMed] [Google Scholar]
- 5. Easton JD, Lopes RD, Bahit MC. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol 2012; 11: 503– 511 [DOI] [PubMed] [Google Scholar]
- 6. Kamel H, Johnston SC, Easton JD, Kim AS. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in patients with atrial fibrillation and prior stroke or transient ischemic attack. Stroke 2012; 43: 881– 883 [DOI] [PubMed] [Google Scholar]
- 7. Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses: Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996; 276: 1339– 1341 [DOI] [PubMed] [Google Scholar]
- 8.Consumer Price Index [online]. [Accessed April 24, 2012]. Available at: http://www.bls.gov/CPI/.
- 9. Wallentin L. for the ARISTOTLE Investigators. Efficacy and safety of apixaban compared with warfarin at different levels of INR control for stroke prevention in atrial fibrillation. Presented at the European Society of Cardiology Congress; August 28, 2011; Paris, France [Google Scholar]
- 10. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994; 154: 1449– 1457 [PubMed] [Google Scholar]
- 11. Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke 2003; 34: 2060– 2065 [DOI] [PubMed] [Google Scholar]
- 12. Connolly SJ, Pogue J, Hart RG, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360: 2066– 2078 [DOI] [PubMed] [Google Scholar]
- 13. van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA 2002; 288: 2441– 2448 [DOI] [PubMed] [Google Scholar]
- 14. Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med 2002; 347: 969– 974 [DOI] [PubMed] [Google Scholar]
- 15. Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486– 2497 [DOI] [PubMed] [Google Scholar]
- 16. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010; 362: 2155– 2165 [DOI] [PubMed] [Google Scholar]
- 17. Gage BF, Cardinalli AB, Albers GW, Owens DK. Cost-effectiveness of warfarin and aspirin for prophylaxis of stroke in patients with nonvalvular atrial fibrillation. JAMA 1995; 274: 1839– 1845 [PubMed] [Google Scholar]
- 18. Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 2011; 123: 2562– 2570 [DOI] [PubMed] [Google Scholar]
- 19. Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011; 154: 1– 11 [DOI] [PubMed] [Google Scholar]
- 20.Scottish Medicines Consortium. National Health Service Scotland [online]. [Accessed April 24, 2012]. Available at: http://www.scottishmedicines.org.uk/files/advice/apixaban_Eliquis_FINAL_November_2011_for_website.pdf.
- 21. Red Book. Montvale, NJ: Thomson Healthcare; 2009 [Google Scholar]
- 22. American Medical Association. CPT Code search [online]. Available at: https://commerce.ama-assn.org/store/. Accessed April 24, 2012
- 23. Point-of-care reimbursement FAQs [online]. http://www.poc.roche.com/en_US/pdf/44156_Coag2009Handbook_FINAL_APPROVED.pdf. Accessed April 24, 2012
- 24. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project [online]. Available at: http://hcupnet.ahrq.gov. Accessed April 24, 2012
- 25. Tsevat J, Kuntz KM, Orav EJ, Weinstein MC, Sacks FM, Goldman L. Cost-effectiveness of pravastatin therapy for survivors of myocardial infarction with average cholesterol levels. Am Heart J 2001; 141: 727– 734 [DOI] [PubMed] [Google Scholar]
- 26. Mark DB, Knight JD, Cowper PA, Davidson-Ray L, Anstrom KJ. Long-term economic outcomes associated with intensive versus moderate lipid-lowering therapy in coronary artery disease: results from the Treating to New Targets (TNT) Trial. Am Heart J 2008; 156: 698– 705 [DOI] [PubMed] [Google Scholar]
- 27. Kauf TL, Velazquez EJ, Crosslin DR, et al. The cost of acute myocardial infarction in the new millennium: evidence from a multinational registry. Am Heart J 2006; 151: 206– 212 [DOI] [PubMed] [Google Scholar]
- 28. Holloway RG, Witter DM, Jr, Lawton KB, Lipscomb J, Samsa G. Inpatient costs of specific cerebrovascular events at five academic medical centers. Neurology 1996; 46: 854– 860 [PubMed] [Google Scholar]
- 29. Leibson CL, Hu T, Brown RD, Hass SL, O'Fallon WM, Whisnant JP. Utilization of acute care services in the year before and after first stroke: a population-based study. Neurology 1996; 46: 861– 869 [PubMed] [Google Scholar]
- 30. Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation 2004; 110: 2287– 2292 [DOI] [PubMed] [Google Scholar]
- 31. Cotton BA, McCarthy JJ, Holcomb JB. Acutely injured patients on dabigatran. N Engl J Med 2011; 365: 2039– 2040 [DOI] [PubMed] [Google Scholar]
- 32. Fleming TR, Emerson SS. Evaluating rivaroxaban for nonvalvular atrial fibrillation–regulatory considerations. N Engl J Med 2011; 365: 1557– 1559 [DOI] [PubMed] [Google Scholar]
- 33. Boehringer Ingelheim Pharmaceuticals. Pradaxa prescribing information [online]. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022512s000lbl.pdf. Accessed April 24, 2012
- 34. Diener HC, Connolly SJ, Ezekowitz MD, et al. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol 2010; 9: 1157– 1163 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.