OPA1 mutations cause autosomal dominant optic atrophy (DOA), a debilitating mitochondrial optic neuropathy characterized by irreversible loss of retinal ganglion cells (RGCs) and progressive visual failure starting in early childhood.1 Interestingly, ∼20% of OPA1 carriers will develop a more severe form of the disease, DOA+, where the optic atrophy is compounded by additional neurologic features.1 OPA1 is a multifunctional mitochondrial inner membrane protein, and rather unexpectedly, high levels of cytochrome c oxidase (COX)-negative muscle fibers were recently identified in biopsy specimens from patients manifesting both the pure and syndromal phenotypes.2,3 These COX-negative muscle fibers harbored high levels of somatically acquired mitochondrial DNA (mtDNA) deletions and marked mtDNA proliferation was also observed, clearly revealing OPA1 to be a novel disorder of mtDNA maintenance.4 To further investigate this key pathologic mechanism, mtDNA copy number was quantified in blood leukocytes from 3 independent patient cohorts with molecularly confirmed OPA1 mutations.
Methods.
Using different extraction protocols (Method e-1 on the Neurology® Web site at www.neurology.org), total genomic DNA was isolated from the leukocyte fraction of venous blood from 3 groups of patients harboring pathogenic OPA1 mutations: 1) British cohort (n = 61), 2) German cohort (n = 40), and 3) Danish cohort (n = 49). Relative mtDNA copy number was determined using the MyiQTM real-time PCR detection system (BioRad, Hercules, CA).5 The presence of multiple mtDNA deletions was investigated in a subgroup of 22 British OPA1-positive DNA samples using long-range PCR.1 Visual acuity was measured using a standard Snellen vision chart (Method e-2).
Standard protocol approvals, registrations, and patient consents.
This study had the relevant institutional ethical approval and complied with the Declaration of Helsinki.
Results.
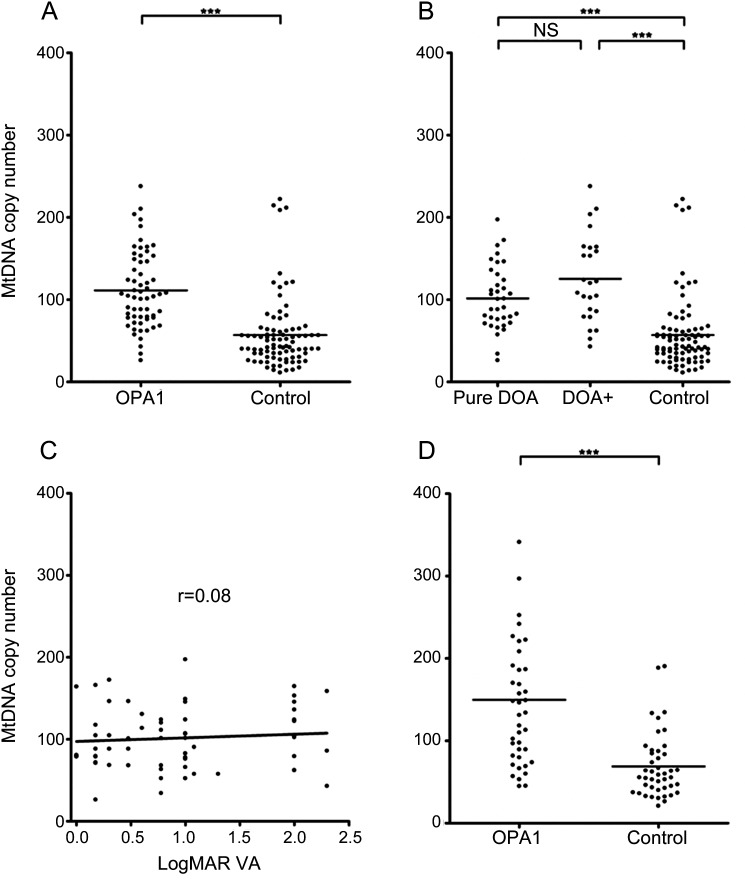
Blood leukocytes from British OPA1-positive patients had significantly higher mtDNA copy numbers compared with controls (figure 1A). In the British cohort, there was no significant difference in total mtDNA content between patients with pure DOA and DOA+ phenotypes (figure 1B). There was no significant correlation between patients' visual acuity and mtDNA copy number in blood leukocytes (figure 1C). There was evidence of significant mtDNA proliferation in blood leukocytes from German OPA1-positive patients (figure 1D) and Danish OPA1-positive patients (figure e-1). On subgroup analysis, there was no significant difference in total mtDNA content between patients with different OPA1 mutation subtypes (figure e-2). Multiple mtDNA deletions were not detected in blood leukocytes.
Figure 1. MtDNA copy number analysis.
(A) Comparison of total mtDNA content in blood leukocytes from the British cohort (OPA1-positive patients: mean = 111.3, SD = 46.1, n = 61; controls: mean = 56.9, SD = 43.3, n = 87). (B) Phenotype subgroup analysis of the British patient cohort (pure dominant optic atrophy subgroup: mean = 101.6, SD = 39.0, n = 36; DOA+ subgroup: mean = 125.3, SD = 52.6, n = 25, NS with Bonferroni correction at p value = 0.0471). (C) Correlation of logMAR visual acuity (VA) with total mtDNA content in blood leukocytes from British OPA1-positive patients (Spearman rank correlation coefficient = 0.08, NS at p value = 0.4081). (D) Comparison of total mtDNA content in blood leukocytes from the German cohort (OPA1-positive patients: mean = 149.8, SD = 95.0, n = 40; controls: mean = 68.7, SD = 39.4, n = 44). Statistical analysis was performed using GraphPadTM v.4 statistical software (San Diego, CA). Group comparisons were made with the unpaired t test. The relationship between logMAR VA and total mtDNA content level was assessed with Spearman rank correlation coefficient. ***p < 0.0001. NS refers to a nonsignificant p value, with p < 0.0167 being the threshold level for statistical significance after Bonferroni correction for multiple testing.
Discussion.
This study has revealed significant mtDNA proliferation in blood leukocytes harboring pathogenic OPA1 mutations in 3 independent patient cohorts. The degree of mtDNA proliferation in blood was comparable to the 2- to 4-fold increase in mtDNA content observed at the single fiber level in skeletal muscle biopsies from OPA1-positive patients.4 The OPA1 protein plays a key role in the assembly and stability of the respiratory chain complexes and these pathologic findings, in both blood and skeletal muscle, are consistent with the detrimental effect of OPA1 mutations on mitochondrial oxidative phosphorylation, the increased mtDNA copy number probably acting as a compensatory bioenergetic mechanism.4 OPA1 mediates mitochondrial membrane fusion in tandem with mitofusin 2 (MFN2), which is dysfunctional in the most common, autosomal dominant, axonal form of Charcot-Marie-Tooth disease (CMT2A).6 It is therefore noteworthy that MFN2 mutations also triggered a significant mtDNA proliferative response in blood, supporting our observation for OPA1 mutations.6 Unlike skeletal muscle, multiple mtDNA deletions were not identified in blood leukocytes from patients with OPA1 mutations. This is likely related to the relatively short half-life of those cells in the circulation, their rapid turnover precluding the formation and subsequent clonal expansion of somatic mtDNA abnormalities.
The mtDNA proliferative response in OPA1-positive blood leukocytes was not statistically different between patients with pure optic atrophy and those who developed additional neurologic complications. Furthermore, irrespective of whether or not OPA1 mutation carriers developed DOA+ features, there was no correlation between absolute mtDNA copy number and the severity of visual loss. Given the 3-fold increased risk of developing DOA+ with missense mutations and those mutations involving the GTPase catalytic domain, additional subgroup analysis was carried out based on these OPA1 mutational subtypes.1 No consistent significant difference in mtDNA copy number was identified, which suggests that this parameter of mitochondrial dysfunction is not associated with clinical severity per se, at least in blood leukocytes. Paradoxically, a previous study found relative mtDNA depletion in blood samples collected from patients with DOA. However, only 8 OPA1-positive cases were included and the observed reduction in mtDNA copy number was relatively small, ∼25% of the mean control value.7 Further work is needed to clarify the still poorly understood pathways linking OPA1 mutations with mtDNA instability and ultimately RGC loss in DOA.
Supplementary Material
Footnotes
See page 1517
Supplemental data at www.neurology.org
Author contributions: Kamil S. Sitarz: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, acquisition of data, statistical analysis. Gitte J. Almind: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, acquisition of data, statistical analysis. Rita Horvath: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, study supervision or coordination. Birgit Czermin: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data. Karen Grønskov: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data. Angela Pyle: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, acquisition of data. Robert W. Taylor: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data. Michael Larsen: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data. Patrick F. Chinnery: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, study supervision or coordination, obtaining funding. Patrick Yu-Wai-Man: drafting/revising the manuscript for content, including medical writing for content, analysis or interpretation of data, study supervision or coordination, obtaining funding.
Study funding: P.Y.-W.-M. is a Medical Research Council (MRC, UK) Clinician Scientist. R.H. is supported by the Academy of Medical Sciences (UK) and by the MRC (UK). R.W.T. is funded by the Wellcome Trust and the UK NHS Highly Specialised Services Group. P.F.C. is a Wellcome Trust Senior Fellow in Clinical Science and a UK National Institute of Health Senior Investigator who also receives funding from the MRC (UK), Parkinson's UK, the Association Française contre les Myopathies, and the UK NIHR Biomedical Research Centre for Ageing and Age-related disease award to the Newcastle upon Tyne Hospitals NHS Foundation Trust.
Disclosure: The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1. Yu-Wai-Man P, Griffiths PG, Gorman GS, et al. Multi-system neurological disease is common in patients with OPA1 mutations. Brain 2010; 133: 771– 786 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amati-Bonneau P, Valentino ML, Reynier P, et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy plus phenotypes. Brain 2008; 131: 338– 351 . [DOI] [PubMed] [Google Scholar]
- 3. Hudson G, Amati-Bonneau P, Blakely EL, et al. Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain 2008; 131: 329– 337 . [DOI] [PubMed] [Google Scholar]
- 4. Yu-Wai-Man P, Sitarz KS, Samuels DC, et al. OPA1 mutations cause cytochrome c oxidase deficiency due to loss of wild-type mtDNA molecules. Hum Molec Genet 2010; 19: 3043– 3052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pyle A, Burn DJ, Gordon C, et al. Fall in circulating mononuclear cell mitochondrial DNA content in human sepsis. Intens Care Med 2010; 36: 956– 962 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sitarz KS, Yu-Wai-Man P, Pyle A, et al. MFN2 mutations cause compensatory mitochondrial DNA proliferation. Brain 2012; 135: e219 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim JY, Hwang JM, Ko HS, et al. Mitochondrial DNA content is decreased in autosomal dominant optic atrophy. Neurology 2005; 64: 966– 972 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.