Abstract
Objectives:
To determine continuous EEG (cEEG) patterns that may be unique to anti-NMDA receptor (NMDAR) encephalitis in a series of adult patients with this disorder.
Methods:
We evaluated the clinical and EEG data of 23 hospitalized adult patients with anti-NMDAR encephalitis who underwent cEEG monitoring between January 2005 and February 2011 at 2 large academic medical centers.
Results:
Twenty-three patients with anti-NMDAR encephalitis underwent a median of 7 (range 1−123) days of cEEG monitoring. The median length of hospitalization was 44 (range 2−200) days. Personality or behavioral changes (100%), movement disorders (82.6%), and seizures (78.3%) were the most common symptoms. Seven of 23 patients (30.4%) had a unique electrographic pattern, which we named “extreme delta brush” because of its resemblance to waveforms seen in premature infants. The presence of extreme delta brush was associated with a more prolonged hospitalization (mean 128.3 ± 47.5 vs 43.2 ± 39.0 days, p = 0.008) and increased days of cEEG monitoring (mean 27.6 ± 42.3 vs 6.2 ± 5.6 days, p = 0.012). The modified Rankin Scale score showed a trend toward worse scores in patients with the extreme delta brush pattern (mean 4.0 ± 0.8 vs 3.1 ± 1.1, p = 0.089).
Conclusions:
Extreme delta brush is a novel EEG finding seen in many patients with anti-NMDAR encephalitis. The presence of this pattern is associated with a more prolonged illness. Although the specificity of this pattern is unclear, its presence should raise consideration of this syndrome.
Anti-NMDA receptor (NMDAR) encephalitis is an increasingly recognized etiology of previously unexplained encephalopathy and encephalitis.1 The associated syndrome is usually severe, including neuropsychiatric symptoms, impaired consciousness, seizures, autonomic instability, and hypoventilation.2,3 Although seizures have been reported in 76% of adults and 77% of children, only a small percentage of patients have evidence of epileptiform activity on EEG. The majority of children with anti-NMDAR encephalitis demonstrate diffuse slowing or anteriorly predominant slowing.2,3 Thus far, no studies have attempted to systematically categorize the pattern of EEG abnormalities in adults.
In our efforts to better characterize these findings, we have identified several patients with an EEG pattern that we believe is unique to a subset of patients with this disorder. We refer to this pattern as “extreme delta brush” (EDB) because of its resemblance to the neonatal EEG pattern known as delta brush. We report here the clinical and electrographic features of 7 patients with anti-NMDAR encephalitis and EDB and 16 patients without this pattern.
METHODS
Standard protocol approvals, registrations, and patient consents.
The institutional review boards of both the Hospital of the University of Pennsylvania and Columbia University Medical Center approved this retrospective study and waived the need for informed consent.
Patients.
This case series includes 13 patients from the Hospital of the University of Pennsylvania and 10 from Columbia University Medical Center with either CSF or serum-confirmed NMDAR antibodies, who were hospitalized and underwent EEG monitoring between January 2005 and February 2011. Patients were identified using a clinical neurophysiology database of individuals older than 18 years.
EEG data.
We reviewed the EEG recordings and EEG reports of all patients. EEGs were evaluated for the presence or absence of electrographic seizures, diffuse slowing, focal slowing, rhythmic delta activity, excessive beta activity, and the extreme delta brush pattern. Rhythmic delta activity or periodic discharges that did not show clear evolution were not counted as electrographic seizures.
Patient data.
Information regarding age, gender, length of hospitalization, presence or absence of teratomas, past medical history, immunosuppressant treatment, clinical seizures, antiepileptic drug use, sedative use, neuropsychiatric features of disease, autonomic instability, neuroimaging findings, CSF findings, type of discharge and modified Rankin Scale score at discharge was collected for all subjects.
Statistical analysis.
Fisher exact tests allowed comparisons of categorical variables between patients with and without the extreme delta brush pattern. The Mann-Whitney U test was performed for comparison of nonparametric continuous data.
RESULTS
Patient characteristics.
Twenty-three patients with anti-NMDAR encephalitis underwent a median of 7 (range 1−123) days of cEEG monitoring. Indications for monitoring were seizures, altered mental status or both. The median age was 24 (range 18−55) years and 19 of 23 patients (83%) were female. All patients had a history of behavioral or personality changes before presentation and 14 (61%) were comatose with a Glasgow Coma Scale score <8 at admission. Clinical seizures before or during hospitalization occurred in 18 patients (78%), abnormal movements or dystonia in 19 patients (83%), and facial dyskinesias in 15 patients (65%). Hypoventilation was noted in 9 patients (39%). Eleven patients (48%) had unilateral or bilateral ovarian teratomas demonstrated by imaging or exploratory laparotomy. MRI T2/fluid-attenuated inversion recovery hyperintensities involving neocortex (occipital, frontal, temporal, and parietal), subcortical white matter, or mesial temporal lobes were identified in 16 patients; 7 patients (30%) had normal MRIs. CSF analysis was performed in 22 patients and was abnormal in 19. Median CSF white blood cell counts were 23 (range 0−243) per mm3 and median CSF protein levels were 39.5 (range 15−93) mg/dL. Oligoclonal bands were not systematically examined. The median length of hospitalization was 44 (range 2–200) days. The mean modified Rankin Scale score at hospital discharge was 3.4 ± 1.1. Four patients (17%) were discharged home, and the remaining patients were discharged to acute or subacute rehabilitation or skilled nursing facilities.
EEG findings.
The cEEG findings are shown in table 1. Two patients had normal cEEG with 1 and 3 days of monitoring. Diffuse slowing of the background was seen in 21 patients (91%) with severe diffuse slowing in 9 patients (39%). Eight patients (34%) had focal slowing in some of the recordings. Generalized rhythmic delta activity was seen in 11 patients (47.8%) and 12 patients (52.2%) had diffuse excess beta frequency activity, presumably related to benzodiazepine or barbiturate use. Fourteen patients (61%) had electrographic seizures during cEEG monitoring. Eight patients (34.8%) had nonconvulsive seizures, but no patients had exclusively nonconvulsive seizures.
Table 1.
Summary of cEEG findings
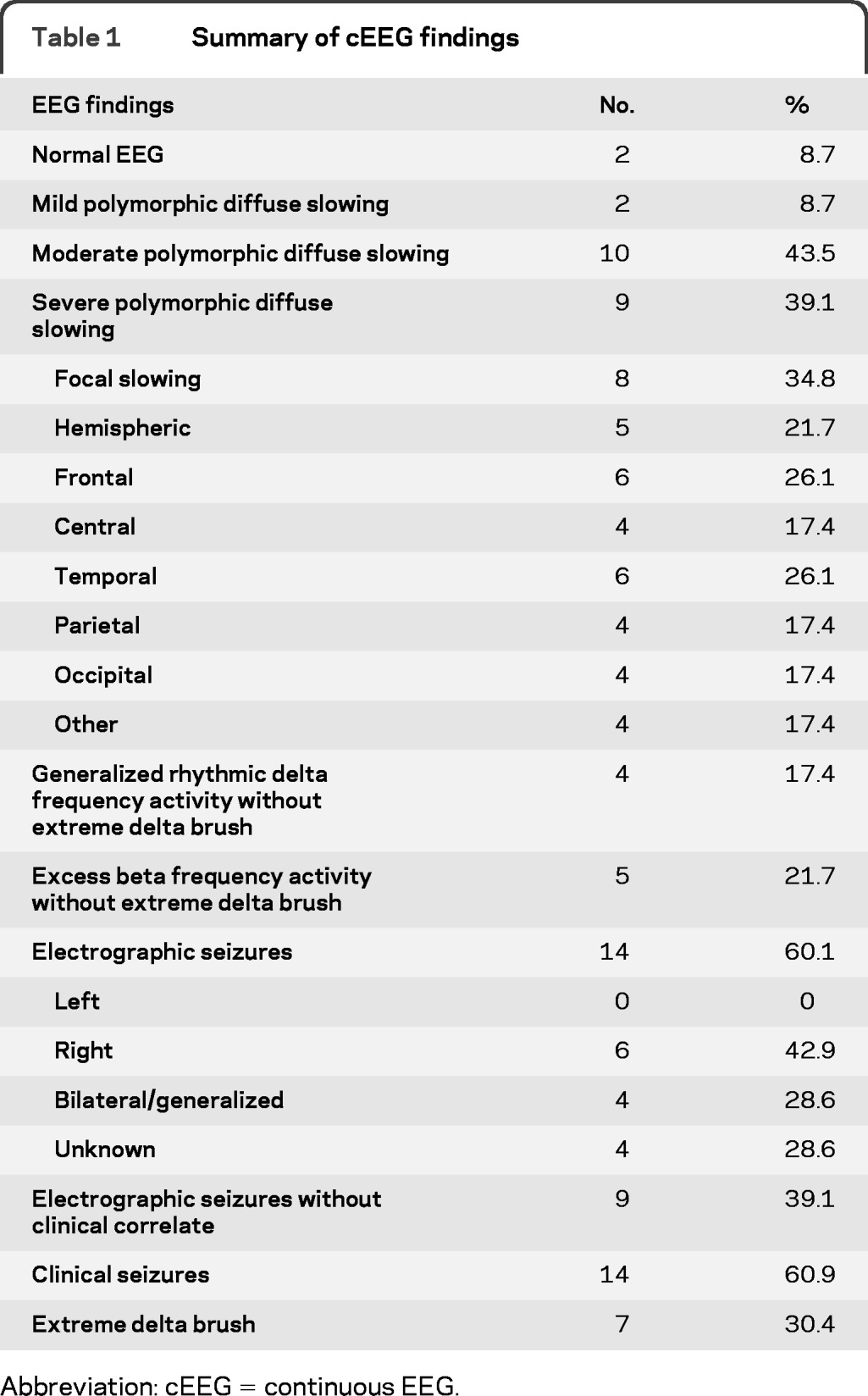
Abbreviation: cEEG = continuous EEG.
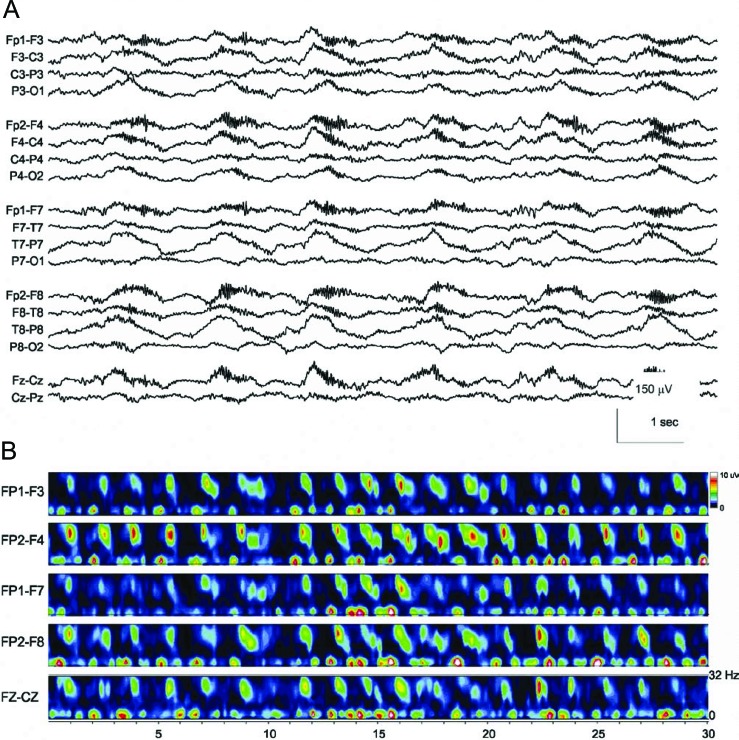
Seven patients (30%) had a unique electrographic pattern characterized by rhythmic delta activity at 1−3 Hz with superimposed bursts of rhythmic 20–30 Hz beta frequency activity “riding” on each delta wave (figures 1−3). We named this pattern extreme delta brush because of its resemblance to the delta brush EEG pattern seen in premature infants, also known as beta-delta complexes.4 Typical neonatal delta brushes are a combination of delta frequency transients with superimposed 8−20 Hz fast activity, which are commonly symmetric but are not typically synchronous; they can be seen in any head region but are less common in the frontal regions. They are present in both the awake and sleep states and typically disappear by 1 month postterm.4 In contrast, our EDB pattern typically appeared as a nearly continuous combination of delta activity with superimposed fast activity, usually in the beta range, which was most often symmetric and synchronous, typically seen broadly across all head regions. In all patients studied, the delta brush pattern was present continuously from the earliest available EEG. It did not vary with sleep-wake cycles and did not vary significantly with stimulation or level of arousal. The pattern occurred independent of episodes of dystonia, choreoathetosis, and orofacial dyskinesias and therefore was not felt to represent a movement-related artifact. One patient underwent neuromuscular paralysis and had no associated change in her EEG.
Figure 1. Continuous EEG recording in a 23-year-old woman with anti-NMDA receptor encephalitis, seizures, and coma.
(A) The initial EEG tracing demonstrates generalized rhythmic and semirhythmic delta frequency activity at 1 Hz with superimposed, frontally predominant bursts of rhythmic beta frequency activity. High pass filter 0.5 Hz; low pass filter 70 Hz; notch filter off. (B) Time-frequency analysis of the EEG tracing. A compressed spectral density array over 30 seconds of recording demonstrates increased low-frequency power with rhythmic bursts of 18−32 Hz power, which is characteristic of the extreme delta brush pattern.
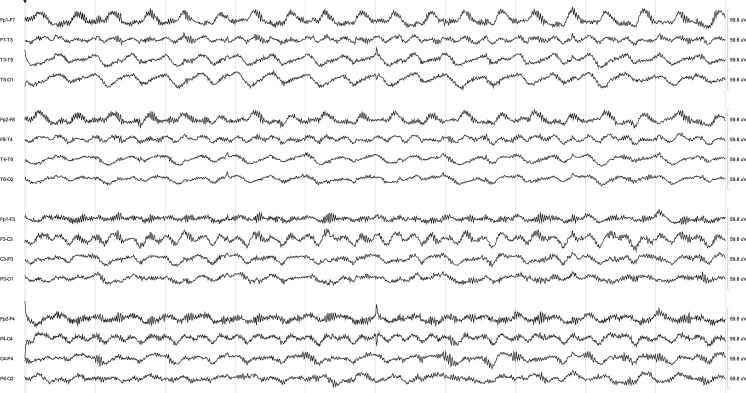
Figure 3. Continuous EEG from the patient in figure 2, 7 months after initiation of aggressive immunotherapy, including rituximab, cyclophosphamide, IV methylprednisolone, and IV immunoglobulin.
The extreme delta brush pattern has resolved, and background EEG activity has largely normalized.
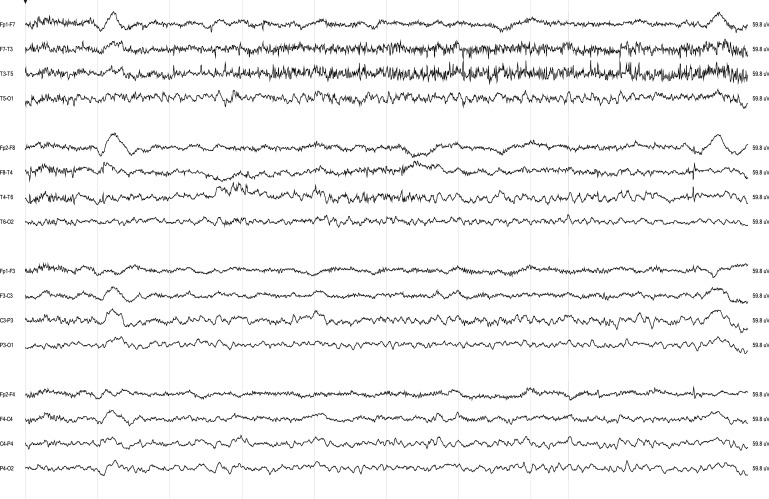
Figure 2. Continuous EEG recording in a 19-year-old man with anti-NMDA receptor encephalitis associated with dyskinesias, seizures, and coma.
The initial EEG demonstrates generalized rhythmic delta frequency activity at 2–2.5 Hz with superimposed rhythmic beta frequency activity.
This pattern was unlikely to be due to superimposed benzodiazepine- or barbiturate-induced beta activity because 2 patients demonstrated this pattern on the initial EEG before receiving benzodiazepines or barbiturates. Phenobarbital was used in only one patient with the EDB pattern, and, for that patient, the presence of the EDB pattern preceded the use of phenobarbital by almost 2 weeks. The presence of this pattern was not altered by administration of nonsedative antiepileptic medications. However, the use of pentobarbital in one patient led to a transient burst-suppression pattern. After pentobarbital was stopped, the EDB pattern resumed. We compared the clinical characteristics of patients with EDB and those without EDB to determine whether this pattern can provide additional clinical information (table 2). Patients with EDB had more protracted hospital courses, with a median of 126 (range 70−200) days in the hospital and 14 (5−123) days undergoing cEEG monitoring compared with to 36 (2−142) hospital days and 3.5 (1−20) cEEG days in patients without the pattern (Mann-Whitney two-tailed U test, p = 0.0008 and p = 0.012, respectively). There was a trend toward worse outcomes at discharge in patients with EDB with a mean modified Rankin Scale score of 4.0 ± 0.8 compared with 3.1 ± 1.1 in patients without EDB (p = 0.089). In addition, patients without EDB were more likely to have an abnormal MRI than those with EDB (87.5% vs 28.6%, Fisher exact test, p = 0.01).
Table 2.
Comparison of patients with and without the EDB pattern on cEEG
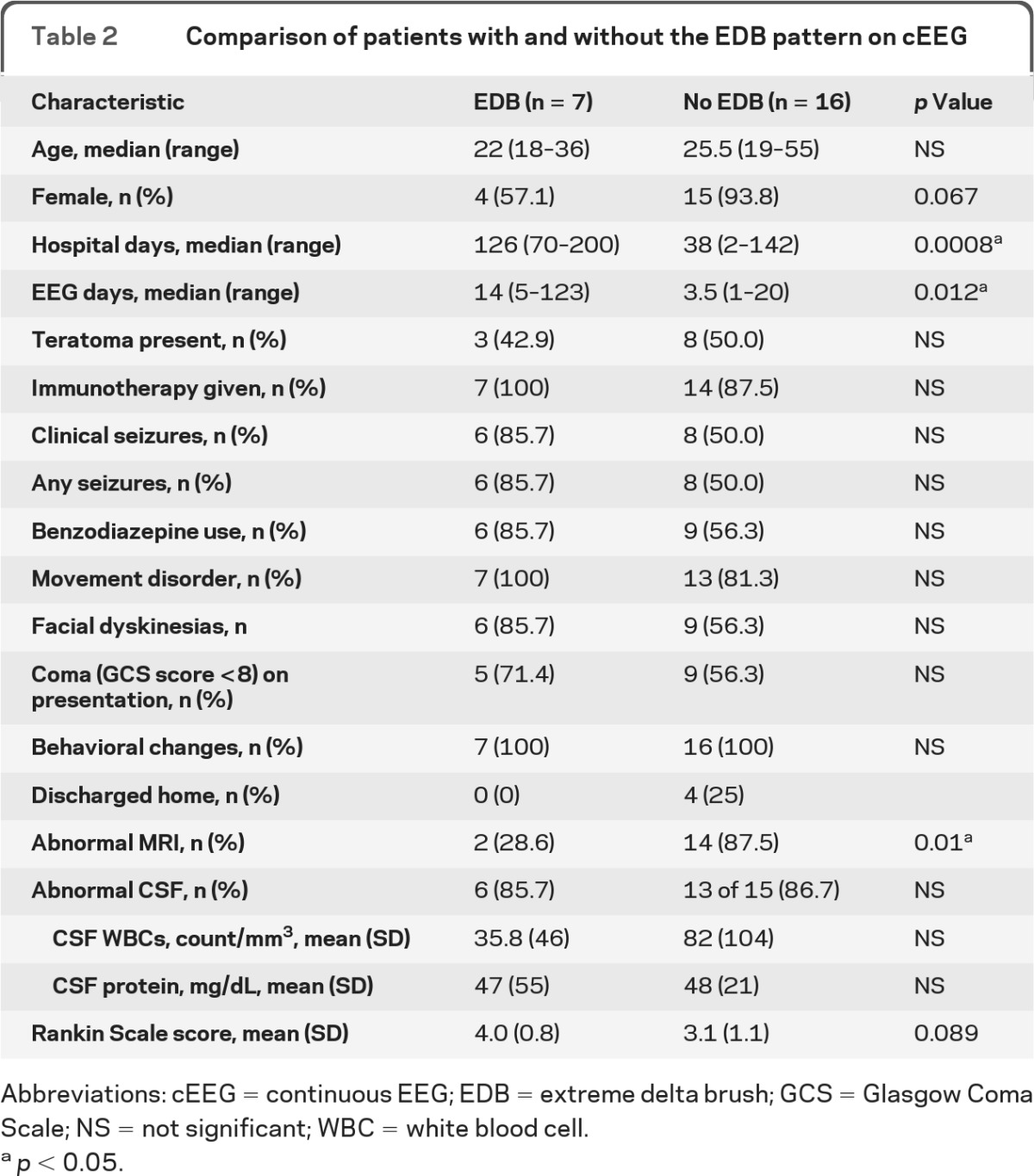
Abbreviations: cEEG = continuous EEG; EDB = extreme delta brush; GCS = Glasgow Coma Scale; NS = not significant; WBC = white blood cell.
p < 0.05.
In contrast, rhythmic delta activity without a frank EDB pattern was seen in 4 patients (17%). Unlike the EDB pattern, this pattern was not associated with a statistically significant increased length of hospital stay, with a median hospital stay of 47.5 (range 34–68) days in patients with rhythmic delta activity without EDB and a mean stay of 37.5 (range 7–142) days in patients without rhythmic delta or EDB (Mann-Whitney 2-tailed U-test, p = 0.38). Excess beta activity was also a common finding in patients without EDB (5 patients [21.7%]) and was not associated with a longer length of stay (median 38 [range 13–142] days) with excess beta and no EDB vs 38 [2–132] days without excess beta and no EDB, p = 0.44).
DISCUSSION
In this series of 23 adult patients with anti-NMDAR encephalitis and cEEG monitoring, 30% had the EDB pattern early on cEEG. To our knowledge, this pattern has not been described in other neurologic conditions, suggesting that it may be unique for a subset of patients with this disorder. Patients with EDB were hospitalized and monitored with cEEG longer and demonstrated a trend toward a worse outcome. These findings suggest that the EDB pattern may be a marker of more severe disease and perhaps worse outcome. At least 2 patients with this pattern demonstrated gradual EEG improvement over the course of their hospitalization after aggressive immunotherapy. In both patients, resolution of EDB was associated with clinical improvement. In addition, patients with this pattern were more likely to have a normal MRI. Therefore, although patients with EDB were syndromically similar to those without EDB, the differences in disease severity and neuroimaging suggest that detection of EDB predicts a more aggressive phenotype.
The etiology of the EDB pattern remains unclear and certainly requires further investigation. Excess beta activity is commonly seen in patients receiving barbiturates and benzodiazepines, the latter of which were administered to 15 of our patients. However, for at least 2 patients, the EDB pattern was observed before the documented initial administration of either barbiturates or benzodiazepines, suggesting that beta activity occurred independently of these medications. In 2 other patients with EDB, the EDB pattern persisted for at least 2 weeks after benzodiazepines were stopped. Furthermore, the beta activity tended to occur in bursts synchronized with diffuse, frontally predominant rhythmic delta activity, which would not be explained by sedative use alone. The ictal vs interictal nature of this pattern is also unclear. None of our patients with EDB responded clinically or electrographically to trials of either benzodiazepines or other IV antiepileptic drugs. Although the pattern does not qualify as ictal by proposed definitions of nonconvulsive seizures,5,6 it may lie on the ictal-interictal continuum.5
Modulation of NMDAR-mediated currents can alter rhythmic neuronal activity and lead to unique electrographic patterns. Ketamine, an NMDAR antagonist, can enhance gamma frequency oscillations and attenuate theta activity in mouse CA37 and in computational models of hippocampus.8 In patients with anti-NMDAR encephalitis, antibodies reduce the levels of synaptic NMDAR and NMDAR-mediated currents, which could perhaps explain the electrographic pattern we saw in our patients with more severe disease who had increased delta activity with superimposed faster frequencies.9 Owing to the retrospective nature of the current study, we could not appropriately address antibody titers because the antibody data were only available in a qualitative manner in most patients' charts, and the antibodies was not systematically determined by the time cEEG monitoring was performed (i.e., some patients were tested several weeks before or after the stage in which they had cEEG monitoring and before and after immunotherapy).
To our knowledge, this is the largest series of cEEG recordings in patients with anti-NMDAR encephalitis. An important practical implication is that detection of EDB may be a unique electrographic marker for a subset of patients with more severe disease. Although it is not yet known whether the EDB pattern is specific for anti-NMDAR encephalitis, its presence in the correct clinical context should raise a strong suspicion of the diagnosis. For example, in one of the patients included in this study, results of initial serum anti-NMDAR antibody testing at an outside hospital were negative; however, the presence of the EDB pattern on cEEG monitoring prompted repeated serum and CSF testing, results of which were positive. Since the completion of this series, one of the authors noted the pattern on a routine EEG performed on a 30-year-old woman who presented to the hospital with a 2-week course of agitation, personality changes, and low-grade fever. The EEG, performed on the third day of her hospitalization, demonstrated periods of generalized rhythmic delta activity intermixed with EDB. Based on the prior observations reported here, the patient underwent additional diagnostic testing including a pelvic ultrasound and then MRI, which revealed abnormal fat stranding in the right ovary. She was treated with a course of IV methylprednisolone followed by IV immunoglobulin and was scheduled for an exploratory laparotomy all before positive results for NMDAR antibodies came back from the laboratory. She was ultimately found to have a mature ovarian teratoma in her right ovary, which was removed. The rapid identification and resection of a teratoma, if present, may improve recovery times in anti-NMDAR encephalitis.2 Because the return of antibody testing results from clinical laboratories may take weeks, the presence of EDB on an EEG, which can be obtained at the time of presentation, can potentially improve the speed of discovery of associated teratomas and reduce delays in diagnosis and treatment.
In the current study, the EDB pattern was identified in 30% of patients, but a prospective analysis of patients with systemic cEEG monitoring at different stages of the disease may reveal a higher incidence. We are aware of 3 case reports of patients with anti-NMDAR encephalitis and nonconvulsive seizures that included EEG tracings similar to the EDB pattern.10–12 One author10 suggested that this pattern, which they termed “burst and slow complexes” was a novel manifestation of nonconvulsive status epilepticus. Further studies are needed to examine the frequency, specificity, and sensitivity of EDB in anti-NMDAR encephalitis and its implications for outcome and whether there is a relationship between this pattern and antibody titers in serum and CSF.
GLOSSARY
- cEEG
continuous EEG
- NMDAR
NMDA receptor
AUTHOR CONTRIBUTIONS
Sarah E. Schmitt was responsible for study conceptualization and design, data collection, data analysis, interpretation of data, drafting the manuscript, and revising the manuscript for intellectual content. Kimberly Pargeon was responsible for study conceptualization and design, data collection, drafting portions of the manuscript, and revising the manuscript for intellectual content. Eric S. Frechette was responsible for study conceptualization, data collection, and revising portions of the manuscript for intellectual content. Lawrence J. Hirsch was responsible for study conceptualization and revising portions of the manuscript for intellectual content. Josep Dalmau was responsible for analysis and interpretation of portions of the data and revising the manuscript for intellectual content. Daniel Friedman was responsible for study conceptualization, data analysis and interpretation, and drafting portions of the manuscript.
DISCLOSURE
S. Schmitt, K. Pargeon, and E. Frechette report no disclosures. L. Hirsch has received research support from Eisai, Pfizer, UCB-Pharma, Upsher-Smith, and Lundbeck and consultation fees from Lundbeck, Upsher-Smith, and GlaxoSmithKline. J. Dalmau receives royalties from patents for the use of Ma2 and NMDAR as an autoantibody test. Dr. Dalmau has received a research grant from Euroimmun, and his contribution to the current work was supported in part by grants from the National Institutes of Health (R01-NS077851, R01-MH094741), the National Cancer Institute (R01CA89054), Fundació la Marató de TV3, and a McKnight Neuroscience of Brain Disorders award. D. Friedman receives salary support from The Epilepsy Study Consortium, a nonprofit organization dedicated to improving the lives of epilepsy patients, and devotes 10% of his time to work done for the Consortium. The Consortium receives payments from a large number of pharmaceutical companies for consulting activities. All payments are made to The Consortium and not to Dr. Friedman directly. Because there are so many companies contributing, the amount from each company towards Dr. Friedman's salary is minimal. Within the past year, the Epilepsy Study Consortium received payments from the 21 companies: Cyberonics, Cypress Biosience, Inc., Eisai Medical Research, Entra Pharmaceuticals, GlaxoSmithKline, Icagen, Inc., Intranasal/Ikano, Johnson & Johnson, Marinus, Neurotherapeutics, NeuroVista Corporation, Ono Pharma USA, Inc., Ovation/Lundbeck, Pfizer, Sepracor, SK Life Science, Supernus Pharmaceuticals, Taro, UCB Inc./Schwarz Pharma, Upsher Smith, and Valeant. All payments are reported annually and reviewed by NYU's Conflict of Interest Committee. Dr. Friedman has served on an advisory board for GlaxoSmithKline. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Pruss H, Dalmau J, Harms L, et al. Retrospective analysis of NMDA receptor antibodies in encephalitis of unknown origin. Neurology 2010;75:1735–1739 [DOI] [PubMed] [Google Scholar]
- 2. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol 2009;66:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clancy RR, Bergqvist AGC, Dlugos DJ. Neonatal Electroencephalography. In: Ebersole JS, Pedley TA, eds. Current Practice of Clinical Electroencephalography, 3rd ed Philadelphia: Lippincott Williams & Wilkins; 2003:160−234 [Google Scholar]
- 5. Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol 22:79–91, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology 1996;47:83–89 [DOI] [PubMed] [Google Scholar]
- 7. Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci 2010;22:1452–1464 [DOI] [PubMed] [Google Scholar]
- 8. Neymotin SA, Lazarewicz MT, Sherif M, Contreras D, Finkel LH, Lytton WW. Ketamine disrupts theta modulation of gamma in a computer model of hippocampus. J Neurosci 2011;31:11733–11743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 2010;30:5866–5875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ikeda A, Matsui M, Hase Y, et al. “Burst and slow complexes” in nonconvulsive epileptic status. Epileptic Disord 2006;8:61–64 [PubMed] [Google Scholar]
- 11. Johnson N, Henry C, Fessler AJ, Dalmau J. Anti-NMDA receptor encephalitis causing prolonged nonconvulsive status epilepticus. Neurology 2010;75:1480–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirkpatrick MP, Clarke CD, Sonmezturk HH, Abou-Khalil B. Rhythmic delta activity represents a form of nonconvulsive status epilepticus in anti-NMDA receptor antibody encephalitis. Epilepsy Behav 2011;20:392–394 [DOI] [PubMed] [Google Scholar]