Abstract
Objective:
Rapid-onset dystonia-parkinsonism (RDP) is caused by a variety of missense mutations in the ATP1A3 gene. Psychiatric comorbidity has been reported, although systematic examination of psychiatric disease in individuals with RDP is lacking. This study examines psychiatric morbidity for 23 patients with RDP in 10 families with family member control subjects and in 3 unrelated patients with RDP, totaling 56 individuals.
Methods:
Twenty-nine ATP1A3 mutation–positive individuals were examined; 26 exhibited motor symptoms (motor manifesting carrier [MMC]) and 3 did not (nonmotor manifesting carriers [NMC]). Twenty-seven ATP1A3 mutation-negative participants (noncarriers [NC]) were included. Rates of psychiatric illness for patients with RDP and related asymptomatic gene mutation carriers were compared with those for related nonmutation carriers. Outcome measures included the Unified Parkinson's Disease Rating Scale, Burke-Fahn-Marsden Dystonia Rating Scale, Instrumental Activities of Daily Living, Composite International Diagnostic Interview, Structured Clinical Interview for DSM-IV, Hamilton Depression Scale, Hamilton Anxiety Scale, and Yale-Brown Obsessive-Compulsive Scale.
Results:
NMC participants did not report any history of psychiatric disorder. Findings in MMC and NC groups included anxiety (MMC 48, NC 41%), mood (MMC 50%, NC 22%), psychotic (MMC 19%, NC 0%), and substance abuse/dependence (MMC 38%, NC 27%).
Conclusions:
ATP1A3 mutations cause a wide spectrum of motor and nonmotor features. Psychotic symptoms tended to emerge before or concurrent with motor symptom onset, suggesting that this could be another expression of the ATP1A3 gene mutation.
Rapid-onset dystonia-parkinsonism (RDP) was first reported in 1993 when Dobyns et al.1 discovered a large family presenting with abrupt onset of dystonia-parkinsonism, often after a trigger. Our group reported mutations in the ATP1A3 gene, a subunit of the Na,K-ATPase, as the cause of RDP.2 There are fewer than 50 estimated cases worldwide.3 RDP is characterized by abrupt onset of dystonic symptoms, often with bradykinesia and gait instability, and usually associated with speech and swallowing problems. Additional features of social phobia, anxiety, depression, and schizoid tendencies have been reported in one Irish family.4,5
Various forms of dystonia are associated with psychiatric disease, including major depression in DYT1 primary dystonia,6 whereas alcohol misuse, depression, anxiety, psychosis, and obsessive-compulsive disorder have been reported in myoclonus-dystonia (DYT11).7 Some studies have shown evidence for an association between ATP1A3 polymorphism and bipolar disorder.8 Low levels of dopamine, serotonin, and norepinephrine metabolites have been found in CSF of some, but not all, motor-manifesting and nonmotor-manifesting gene carriers,9 suggesting an alteration of neurotransmitter levels, possibly affecting psychiatric symptoms. Mouse studies localize ATP1A3 expression in neurons throughout the brain, including in the basal ganglia and cerebellum.10 Given widespread involvement of ATP1A3 in the brain and the association of other forms of genetic dystonia with psychiatric diseases, we hypothesized that psychiatric disease may be part of the RDP phenotype.
We report structured psychiatric interview data coupled with a standardized history and videotaped movement disorder examination.
METHODS
A total of 56 subjects (29 with ATP1A3 mutations present and 27 familial control subjects without the mutation) from 10 families and 3 unrelated subjects with RDP were included in this study. Two of the families have been reported previously,1,4,5 whereas data from another family included in this study are currently submitted for publication. The data reported here are newly collected as part of a broader longitudinal study of RDP. All participants underwent a structured clinical evaluation consisting of a videotaped neurologic examination, including the Burke-Fahn-Marsden Dystonia Rating Scale (BFMS) and Unified Parkinson's Disease Rating Scale (UPDRS). Participants also completed a standardized history questionnaire. To assess lifetime history of psychiatric disorders, all participants were interviewed and videotaped by a trained rater using multiple instruments, including the Composite International Diagnostic Interview (CIDI-Auto version), the Structured Clinical Interview for DSM-IV (SCID-I), the Hamilton Depression Scale (HAM-D), the Hamilton Anxiety Scale (HAM-A), and the Yale-Brown Obsessive Compulsive Scale (Y-BOCS). Discrepancies in diagnoses generated by the instruments were adjudicated by a psychiatrist (W.V.M.) blinded to mutation status, who rendered final diagnoses.
Standard protocol approvals, registrations, and patient consents.
All participants signed an informed consent form approved by the Wake Forest School of Medicine Institutional Review Board before contributing a blood or saliva sample for DNA screen for ATP1A3 mutations by direct sequencing as described elsewhere.2
Medical history/movement disorder assessment.
A standardized, videotaped movement disorder assessment was administered by a neurologist with expertise in dystonia (A.B.). Measurements included the UPDRS and BFMS. The BFMS is used to assess severity and frequency of dystonia in 9 body areas including eyes, mouth, speech or swallowing, neck, right and left arms, trunk, and right and left legs.11 A standardized medical history questionnaire was also administered to establish family history, age at onset, severity of symptoms, report of triggers, and second events.
Instrumental Activities of Daily Living.
The Instrumental Activities of Daily Living was administered to assess the impact of disease on activities of daily living. Self-report and proxy scores are highly correlated when patients are unable to respond, making it a useful tool for patients with RDP who have communication difficulty.12
CIDI-Auto version.
The CIDI-Auto was previously used in a study of psychiatric comorbidity in patients with DYT1 primary dystonia.6,7 The CIDI is a fully structured interview used by the World Health Organization as a standardized method of establishing diagnoses according to ICD-10 and DSM-IIIR definitions. It was intended to minimize clinical judgment and interviewer bias in establishing diagnoses. The CIDI-Auto is the computerized version of the CIDI items and scoring algorithms. For the present study, the lifetime version of the interview was conducted by a trained rater and videotaped (N.B.). The anxiety, depression, mania, psychosis, obsessive-compulsive disorder, panic disorder, alcohol, and substance modules were administered.
SCID-I.
Using the videotaped CIDI-Auto interview, the rater then completed a paper-and-pencil SCID-I.13 The SCID-I, a reliable and valid semistructured interview, is used to assess current and lifetime psychiatric diagnoses in medical patients and family members.
Rating scales.
To further assess psychiatric presentation, we administered various ratings scales. The 24-item version of the HAM-D was used to assess current depressive symptoms.14 The 14-item HAM-A was administered to assess current symptoms of anxiety.15 Patients also completed the 10-item Y-BOCS to assess obsessive-compulsive symptomatology.16
Statistical analysis.
Characteristics of the study participants were summarized using counts and percentages or means and SDs. p values for comparison of affective disorder status between motor manifesting carrier (MMC) and noncarrier (NC) individuals were derived from families having at least one MMC or NC individual with the affective disorder and having one or more NC family members. Family-specific p values were calculated first, based on the Fisher exact test for association in a 2 × 2 table, and then were multiplied across families. This method accounts for correlation of observations within families and conditions on the observed family-specific 2 × 2 table marginal totals and assumes independence between families. These p values represent the probability of the observed association or an association that is stronger (one-sided significance testing of the a priori hypotheses), between the affective disorder and ATP1A3 mutations. For the Hamilton and Y-BOCS scores, p values for comparisons between the MMC and NC groups (usual 2-sided significance testing) were derived from all individuals in these subgroups and were calculated under generalized linear mixed models. The scores were treated as continuous, and a random effect was used to account for correlation of scores among family members. p ≤ 0.05 was considered statistically significant. The modeling was performed using SAS (version 9.2; SAS Institute Inc., Cary, NC).
RESULTS
Twenty-nine ATP1A3 mutation–positive individuals were examined; 26 exhibited motor symptoms (MMC), and 3 did not exhibit any evidence of movement disorder (nonmotor manifesting carrier [NMC]). Twenty-seven ATP1A3 mutation–negative participants (NC) from the same RDP families were studied with the same battery. The data were examined in terms of disorder categories: mood, anxiety, psychosis, and substance use disorders.
Table 1 shows demographic characteristics of the 56 individuals interviewed for the present study. Among the MMCs, 77% had onset of motor symptoms by age 25, and 77% had initial symptom presentation in the upper body, mouth, and arm. UPDRS and Dystonia Rating Scale scores clearly indicate the absence of dystonic or parkinsonism features in the NMC and NC groups, with very distinct differences in mean scores. Those with motor symptoms reported more impact on daily living activities. In both carrier groups (MMC and NMC), 62% have smoked, not appreciatively different from the 67% who have smoked in the NC group.
Table 1.
Demographic characteristics and motor symptoms
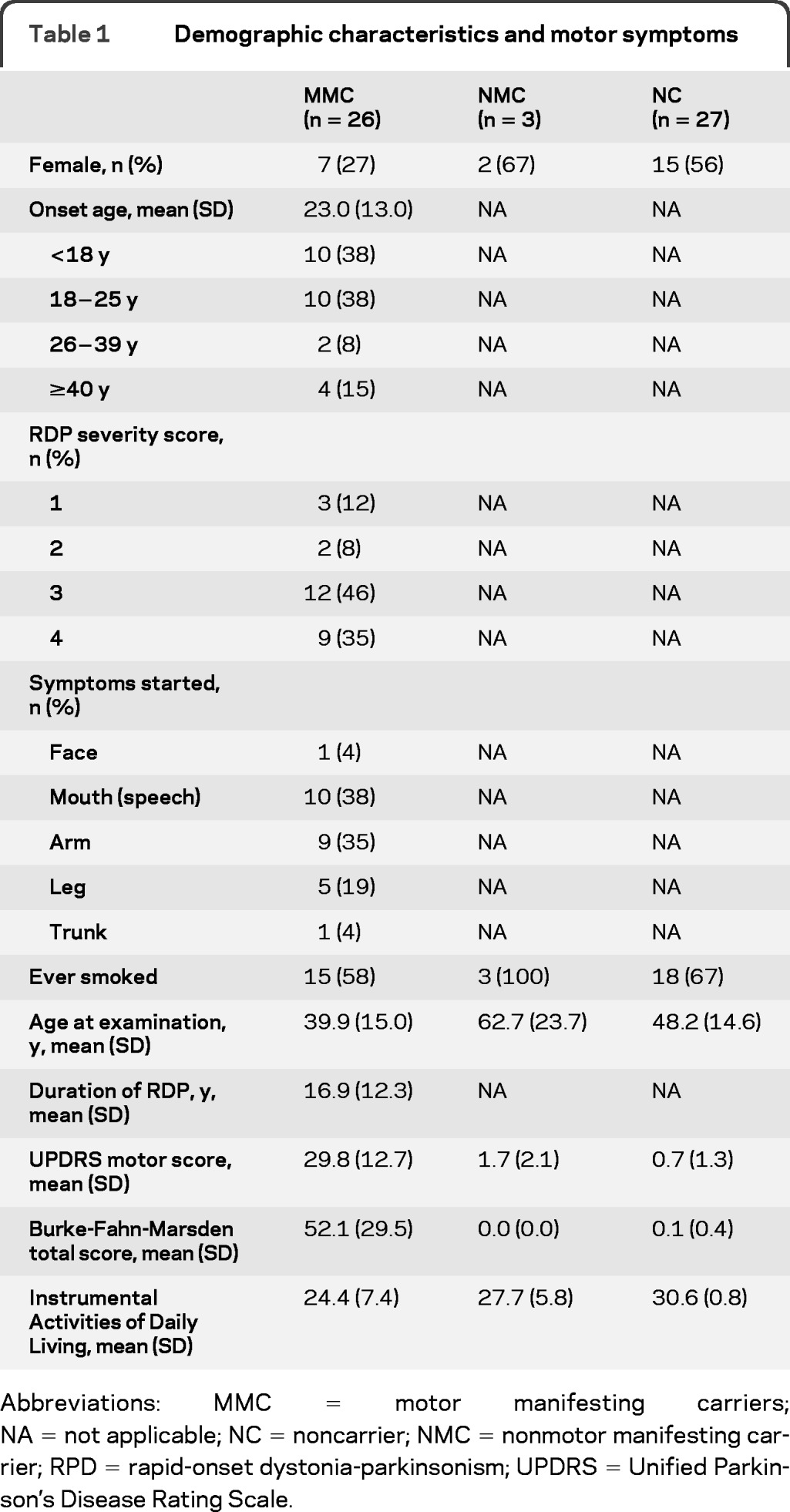
Abbreviations: MMC = motor manifesting carriers; NA = not applicable; NC = noncarrier; NMC = nonmotor manifesting carrier; RPD = rapid-onset dystonia-parkinsonism; UPDRS = Unified Parkinson's Disease Rating Scale.
Table 2 shows rates of mood, anxiety, psychotic, and substance use disorders among MMC, NMC, and NC groups. The 3 NMC participants did not report any history of psychiatric disorder. Subsequent comparisons between MMC and NC groups indicated no appreciable difference in rates of anxiety (48% vs 41%), although substance abuse/dependence (38% vs 27%) and mood disorders (50% vs 22%) appeared more to be prevalent in the MMC group. Exactly half of the MMC group had a mood disorder compared with only 20.8% in the general population.17 Most striking, however, were the rates of psychotic disorder seen in the MMC group. No participant in the noncarrier group was diagnosed with a psychotic disorder, but 19% (5 participants) in the MMC group exhibited some form of psychotic disorder (p = 0.02 in case-control families having one or more members with psychosis), although none of those met the criteria for schizophrenia. Three patients from 3 separate families with 3 distinct ATP1A3 mutations received a diagnosis of psychotic disorder not otherwise specified (NOS). Two patients from 2 of the 3 families mentioned above received a diagnosis of brief psychotic disorder. Psychotic disorder NOS is characterized by psychotic symptomatology with inadequate information to make a specific diagnosis or about which there is contradictory information.18 Psychotic disorder NOS also includes patients with psychotic symptoms who do not meet the criteria for any specific psychotic disorder. Brief psychotic disorder is characterized by the presence of delusions, hallucinations, disorganized speech, or grossly disorganized or catatonic behavior for at least 1 day, but lasting less than 1 month.18 In addition, symptoms eventually remit to premorbid functioning.
Table 2.
Affective disorders by mutation statusa
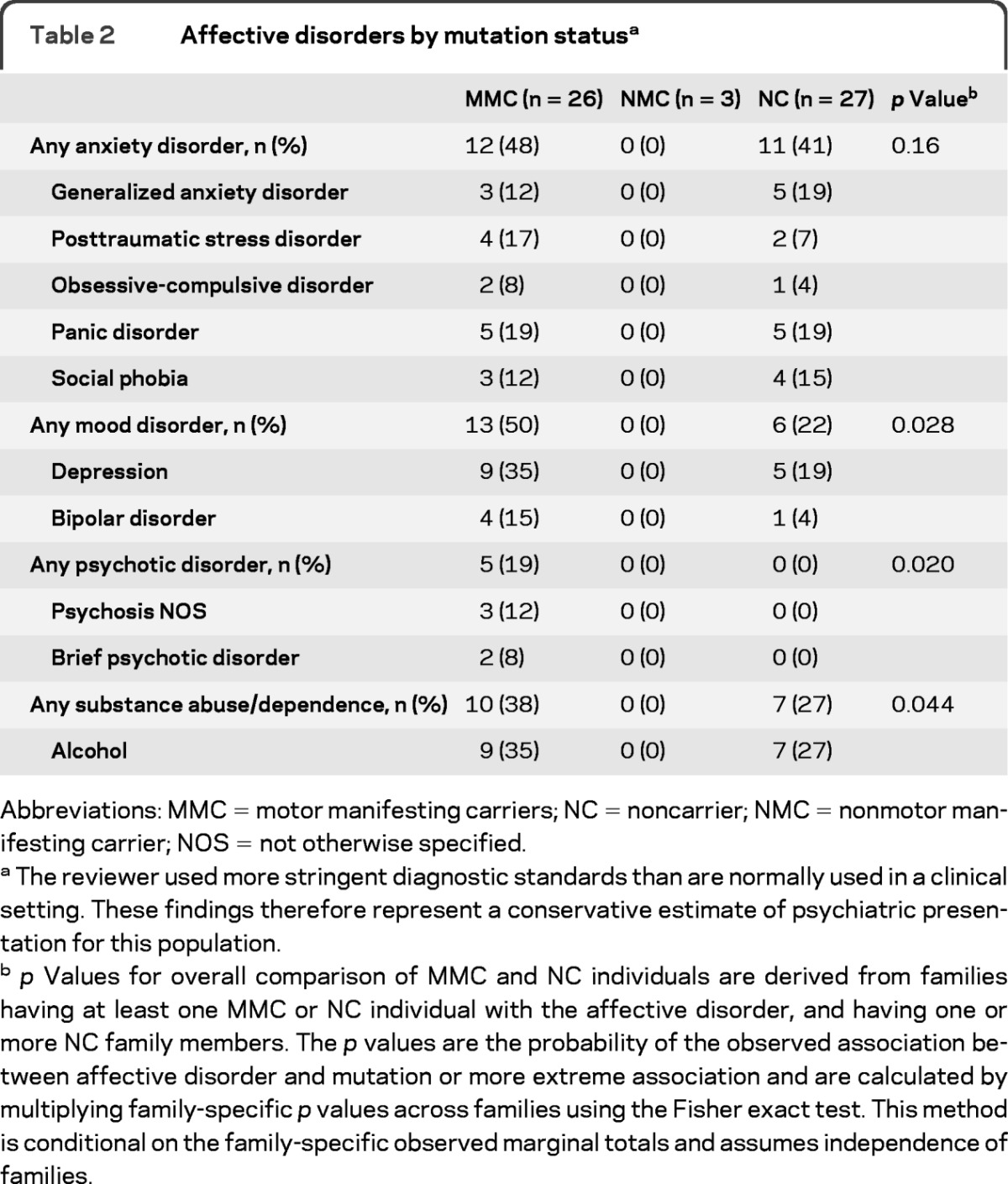
Abbreviations: MMC = motor manifesting carriers; NC = noncarrier; NMC = nonmotor manifesting carrier; NOS = not otherwise specified.
The reviewer used more stringent diagnostic standards than are normally used in a clinical setting. These findings therefore represent a conservative estimate of psychiatric presentation for this population.
p Values for overall comparison of MMC and NC individuals are derived from families having at least one MMC or NC individual with the affective disorder, and having one or more NC family members. The p values are the probability of the observed association between affective disorder and mutation or more extreme association and are calculated by multiplying family-specific p values across families using the Fisher exact test. This method is conditional on the family-specific observed marginal totals and assumes independence of families.
To explore current severity, the Hamilton scores were compared across carrier and noncarrier groups (table 3). The results suggested slightly greater severity of current anxiety (p = 0.068) and depressive symptoms (p = 0.025) in the MMC group relative to that in the NC control group. The NC group did not report or exhibit appreciable levels of current depression, although the MMC group, as a whole, reported mild levels of current depressive symptoms on average. Groups did not differ in scores on the Y-BOCS.
Table 3.
Hamilton Anxiety and Depression and Y-BOCS scores
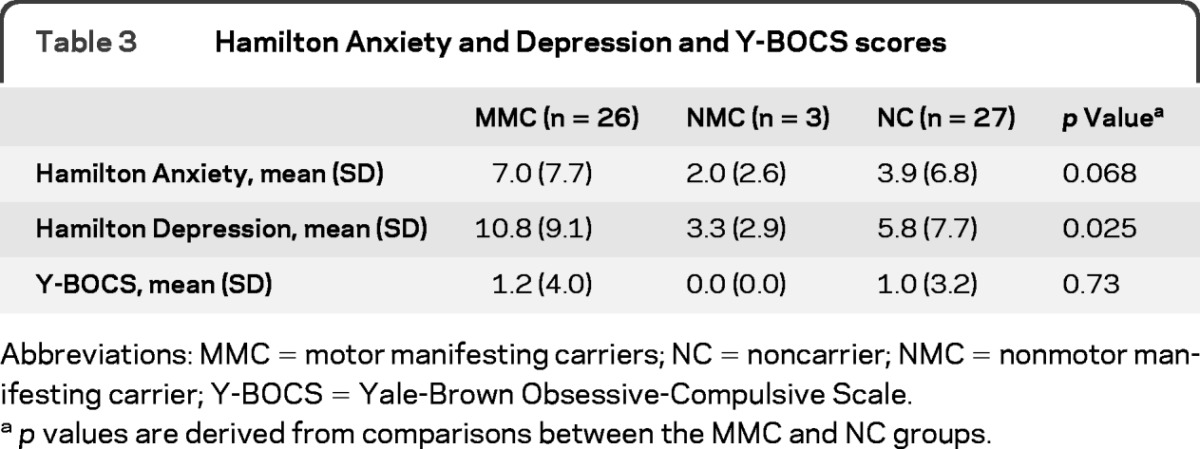
Abbreviations: MMC = motor manifesting carriers; NC = noncarrier; NMC = nonmotor manifesting carrier; Y-BOCS = Yale-Brown Obsessive-Compulsive Scale.
p values are derived from comparisons between the MMC and NC groups.
Although we are unable to distinguish whether mood or psychotic disorders are an independent expression of ATP1A3 mutation or result from other sources (familial or the disease itself), age at onset of psychotic disorder in 2 individuals preceded that of motor disease (table 4), suggesting the possibility that psychotic symptoms may be expressed independently from the motor disease in some cases. Two participants reported onset of psychotic symptoms occurring in the same year of movement symptoms, whereas the age at onset is not known for the fifth participant. The finding across 3 families decreases the likelihood that environment played a role.
Table 4.
Age of psychiatric disorder onset for participants with RDP who met reviewer diagnostic criteriaa
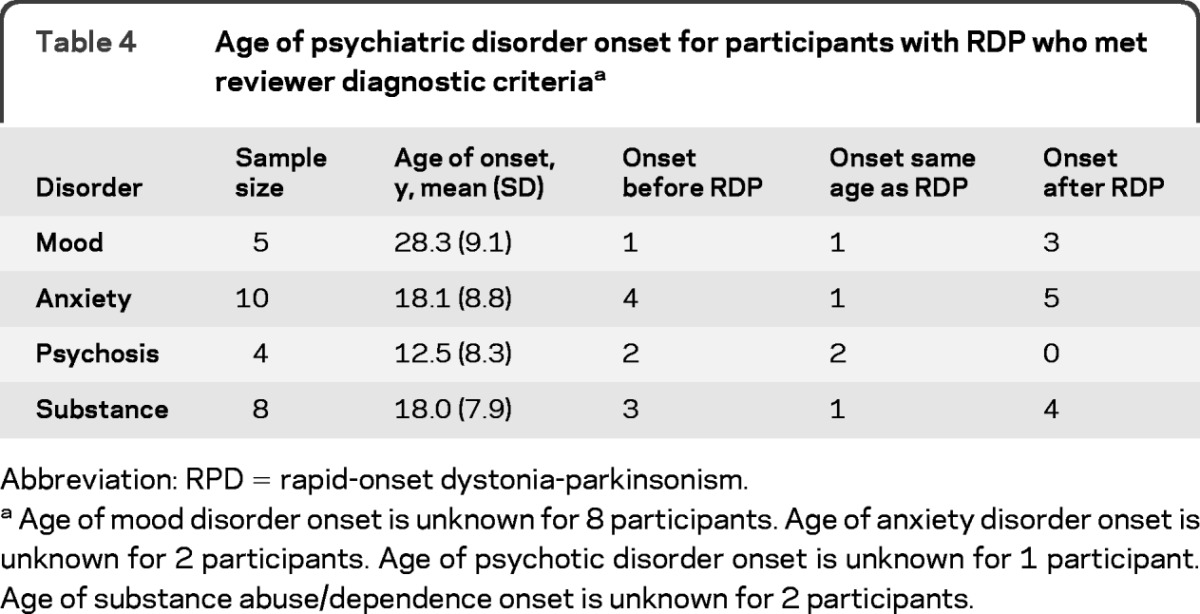
Abbreviation: RPD = rapid-onset dystonia-parkinsonism.
Age of mood disorder onset is unknown for 8 participants. Age of anxiety disorder onset is unknown for 2 participants. Age of psychotic disorder onset is unknown for 1 participant. Age of substance abuse/dependence onset is unknown for 2 participants.
Table 5 further characterizes the 5 patients with a psychotic disorder. Mean age at onset of psychosis within the MMC group (12.5 years) appears to be early compared with age for prototypical psychosis, which tends to occur between 15 and 24 years.19 Two siblings had onset of psychosis symptoms at ages 4 and 8 years. The other patients with known age at onset come from separate families and have a more typical onset of 15 and 23 years. Co-occurring disorders including anxiety, mood disorders, and substance abuse/dependence appear in all but 1 of the 5 patients.
Table 5.
Characteristics of RDP psychosis
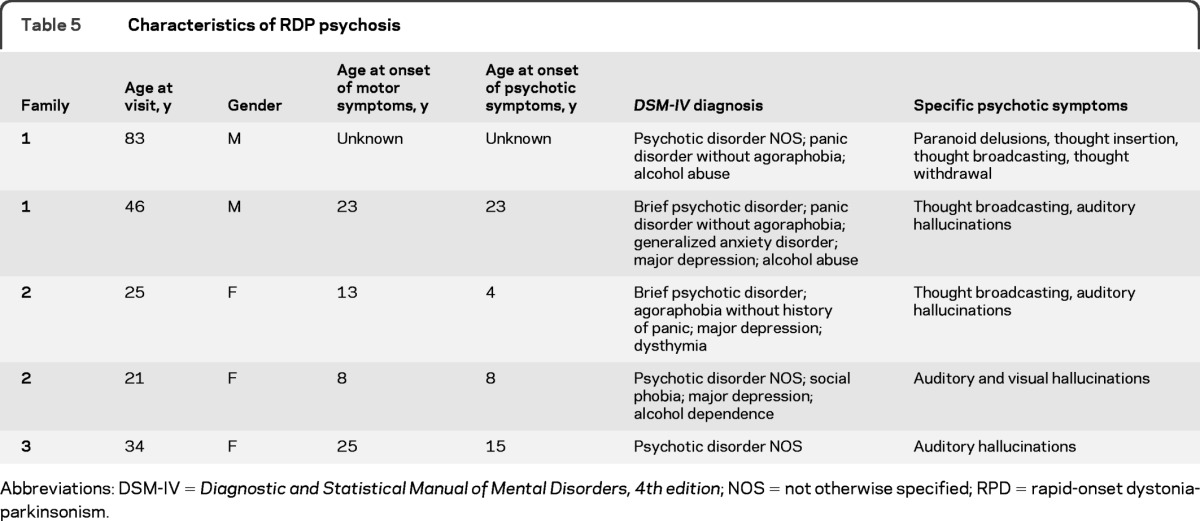
Abbreviations: DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, 4th edition; NOS = not otherwise specified; RPD = rapid-onset dystonia-parkinsonism.
DISCUSSION
Our finding of significantly higher prevalence of mood disorders and psychosis in patients with RDP who have motor symptoms supports our hypothesis that ATP1A3 mutations present with a wide spectrum of features, both motor and nonmotor. These findings are observed across multiple families with distinct ATP1A3 mutations and are consistent with previous reports of depression in individuals with motor-manifesting ATP1A3 mutations.4,5,20 We were unable to discriminate higher rates of psychiatric disorders in a group of nonmanifesting carrier control subjects. Further, mood state–related decreases in Na,K-ATPase activity have been found in both manic and depressed patients with bipolar disorder compared with euthymic patients,21 suggesting a shared pathway for motor and mood symptoms in patients with RDP.
Age at onset of psychotic symptoms is atypical within a single family, suggesting that these disorders are distinct from schizophrenia for these patients. Although other patients did not have an earlier than expected onset of psychosis, no patient met the criteria for schizophrenia. Symptoms for 3 of the 5 patients, although clinically relevant, did not fit any specific classification for psychosis. Given the unique symptom presentation and a trend for psychotic symptoms to emerge before or concurrent with motor symptom onset, psychosis could potentially be another expression of mutation of the ATP1A3 gene.
Shared environment is an important feature of this study. MMC, NMC, and NC participants were all recruited from the same families. The environment was similar for participants irrespective of genetic status, suggesting that environmental factors have less influence over differences in psychiatric status for these patients.
One limitation of the current study is the relatively few participants, particularly NMC control subjects. We suspect that psychotic disorder may be more common because diagnosis assessed by the blinded psychiatrist was stricter than typically used in practice. In this study, for a patient to be identified with psychosis, the psychiatrist needed to diagnose the disorder using both the CIDI and the SCID-I. Given our experience in the field, we suspect that this strict criterion underestimates the psychosis in patients with ATP1A3 mutations. For example, an additional individual in our sample acknowledged what seemed to be auditory hallucinations, although the nature of the hallucinations was unclear and did not meet diagnostic criteria. Another individual in one of our familial studies had probable psychosis and has been reported as having RDP.4 Although he did consent to participate in the study, because of his RDP symptoms, this individual was unable to complete a psychiatric interview. His data are not included in this study. His mother did report that he appears to have conversations with others while alone in his room at night. Therefore, psychosis in patients with ATP1A3 mutations may be even more prevalent than reported here.
The results of this study of 29 patients from 10 families and 3 unrelated patients with RDP with ATP1A3 mutations and their mutation-negative family members suggest the presence of psychiatric disease, particularly psychosis, in those with ATP1A3 mutations and motor manifestations. The suggestion that psychosis may be an independent manifestation of ATP1A3 mutations needs to be confirmed in a larger sample of NMCs. In addition, these findings should prompt those who deal with patients with psychosis, particularly in families with a history of dystonia-parkinsonism, to consider ATP1A3 mutation as a possible contributor to the mental illness.
GLOSSARY
- BFMS
Burke-Fahn-Marsden Dystonia Rating Scale
- CIDI
Composite International Diagnostic Interview
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- HAM-A
Hamilton Anxiety Scale
- HAM-D
Hamilton Depression Scale
- MMC
motor manifesting carrier
- NC
noncarrier
- NMC
nonmotor manifesting carrier
- NOS
not otherwise specified
- RPD
rapid-onset dystonia-parkinsonism
- UPDRS
Unified Parkinson's Disease Rating Scale
- Y-BOCS
Yale-Brown Obsessive-Compulsive Scale
AUTHOR CONTRIBUTIONS
Dr. Brashear: primary investigator, data collector, and lead clinician on the study. J.F. Cook: analysis and interpretation of the data, drafting and revising the manuscript. D.F. Hill: data collector and contributed to the drafting of the manuscript. A. Amponsah: Drafting and revising the manuscript. Dr. Snively, L. Light, and C.K. Suerken performed the statistical analysis of the data. N. Boggs: data collector and contributed to interpretation of data. Dr. Stacy: reviewed and provided interpretation of the clinical data. Dr. Ozelius: provided input on the design of the study and interpretation of data. Dr. Sweadner: provided input on the design of the study and interpretation of the data. Dr. McCall: reviewed and provided interpretation of the clinical data.
DISCLOSURE
A. Brashear has salary support from NINDS (this project); performs research for Allergan, Merz USA, and Ipsen; and consults for Allergan, Merz USA and Ipsen. Her conflict of interest is being managed by Wake Forest University School of Medicine. J. Cook, D. Hill, A. Amponsah, B. Snively, L. Light, N. Boggs, and C. Suerken receive salary support from NINDS 5R01-NS058949-04. M. Stacy has received a grant/research support from Ceregene, IMPAX, Neuraltus, Novartis, Schering-Plough, and Parkinson Study Group; has acted as a consultant for Allergan, Biogen, GE, Novartis, Osmotica, TEVA, and Synosia; has received honoraria from Allergan, Boeringher-Ingelheim, Novartis, and TEVA; and is also on the Safety Monitoring Board for Biogen and Neurologix. His conflict of interest is being managed by Duke University. He is a consultant on this project. L. Ozelius receives salary support from the NIH [NS046340, NS058949, NS037409, RR026123] and has received previous grant support from the Bachmann-Strauss Dystonia and Parkinson Foundation and The Dystonia Medical Research Foundation. She is a current member of the scientific advisory boards of the National Spasmodic Dysphonia Association and the Benign Essential Blepharospasm Research Foundation and a past member of the scientific advisory boards of the Bachmann-Strauss Dystonia and Parkinson Foundation and The Dystonia Medical Research Foundation. Dr. Ozelius receives royalty payments from Athena Diagnostics related to a patent. Athena has supported one Grand Rounds given by Dr. Ozelius. K. Sweadner has salary support from NINDS (R01 NS050696 [PI] and R01 NS058949 [co-investigator]) and the DoD (PR100747) and has received research support from the Bachmann Strauss Dystonia and Parkinson Foundation. W. McCall's research has been supported by NIMH, Cephalon, and Sealy. He has been on the speaker's bureau for Merck and Sepracor and is a scientific advisor for Sepracor, Sealy, and Merck. His conflict of interest is being managed by Wake Forest University School of Medicine. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Dobyns WB, Ozelius LJ, Kramer PL, et al. Rapid-onset dystonia-parkinsonism. Neurology 1993;43:2596–2602 [DOI] [PubMed] [Google Scholar]
- 2. de Carvalho Aguiar P, Sweadner KJ, Penniston JT, et al. Mutations in the Na+/K+-ATPase α3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 2004;43:169–175 [DOI] [PubMed] [Google Scholar]
- 3. Brashear A, Hill DF, Snively B, Sweadner KJ, Ozelius L. De novo and recurrent mutations in ATP1A3 are common in rapid-onset dystonia-parkinsonism. Neurology 2010;74(suppl 2):A204 [Google Scholar]
- 4. Pittock SJ, Joyce C, O'Keane V, et al. Rapid-onset dystonia-parkinsonism: a clinical and genetic analysis of a new kindred. Neurology 2000;55:991–995 [DOI] [PubMed] [Google Scholar]
- 5. McKeon A, Ozelius LJ, Hardiman O, Greenway MJ, Pittock SJ. Heterogeneity of presentation and outcome in the Irish rapid-onset dystonia-parkinsonism kindred. Mov Disord 2007;22:1325–1327 [DOI] [PubMed] [Google Scholar]
- 6. Heiman GA, Ottman R, Saunders-Pullman RJ, Ozelius LJ, Risch NJ, Bressman SB. Increased risk for recurrent major depression in DYT1 dystonia mutation carriers. Neurology 2004;63:631–637 [DOI] [PubMed] [Google Scholar]
- 7. Hess CW, Raymond D, Aguiar PC, et al. Myoclonus-dystonia, obsessive-compulsive disorder, and alcohol dependence in SGCE mutation carriers. Neurology 2007;68:522–524 [DOI] [PubMed] [Google Scholar]
- 8. Goldstein I, Lerer E, Laiba E, et al. Association between sodium and potassium activated adenosine triphosphatase α isoforms and bipolar disorders. Biol Psychiatry 2009;11:985–991 [DOI] [PubMed] [Google Scholar]
- 9. Brashear A, Butler IJ, Hyland K, Farlow MR, Dobyns WB. Cerebrospinal fluid homovanillic acid levels in rapid-onset dystonia-parkinsonism. Ann Neurol 1998;43:521–526 [DOI] [PubMed] [Google Scholar]
- 10. McGrail KM, Phillips JM, Sweadner KJ. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na,K-ATPase. J Neurosci 1991;11:381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krystkowiak P, du Montcel ST, Vercueil L, et al. Reliability of the Burke-Fahn-Marsden scale in a multicenter trial for dystonia. Mov Disord 2007;22:685–689 [DOI] [PubMed] [Google Scholar]
- 12. McCall WV, Dunn AG, Rosenquist P, Hughes D. Proxy validation of patient self-reports of ADL and IADL function before and after electroconvulsive therapy. J ECT 2002;18:74–79 [DOI] [PubMed] [Google Scholar]
- 13. First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID). Washington, DC: American Psychiatric Association; 1995 [Google Scholar]
- 14. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol 1959;32:50–55 [DOI] [PubMed] [Google Scholar]
- 16. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale: I: development, use, and reliability. Arch Gen Psychiatry 1989;46:1006–1011 [DOI] [PubMed] [Google Scholar]
- 17. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 2005;62:593–602 [DOI] [PubMed] [Google Scholar]
- 18. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 19. Messias EL, Chen C, Eaton WW. Epidemiology of schizophrenia: a review of findings and myths. Psychiatr Clin N Am 2007;30:323–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brashear A, DeLeon D, Bressman SB, Thyagarajan D, Farlow MR, Dobyns WB. Rapid-onset dystonia-parkinsonism in a second family. Neurology 1997;48:1066–1069 [DOI] [PubMed] [Google Scholar]
- 21. Looney SW, El-Mallakh RS. Meta-analysis of erythrocyte Na,K-ATPase activity in bipolar illness. Depress Anxiety 1997;5:53–65 [DOI] [PubMed] [Google Scholar]


