Abstract
Objective:
Charcot-Marie-Tooth (CMT) disease is the most common inherited neuromuscular disorder, affecting 1 in 2,500 individuals. Mitochondrial DNA (mtDNA) mutations are not generally considered within the differential diagnosis of patients with uncomplicated inherited neuropathy, despite the essential requirement of ATP for axonal function. We identified the mtDNA mutation m.9185T>C in MT-ATP6, encoding the ATP6 subunit of the mitochondrial ATP synthase (OXPHOS complex V), at homoplasmic levels in a family with mitochondrial disease in whom a severe motor axonal neuropathy was a striking feature. This led us to hypothesize that mutations in the 2 mtDNA complex V subunit encoding genes, MT-ATP6 and MT-ATP8, might be an unrecognized cause of isolated axonal CMT and distal hereditary motor neuropathy (dHMN).
Methods:
A total of 442 probands with CMT type 2 (CMT2) (270) and dHMN (172) were screened for MT-ATP6/8 mutations after exclusion of mutations in known CMT2/dHMN genes. Mutation load was quantified using restriction endonuclease analysis. Blue-native gel electrophoresis (BN-PAGE) was performed to analyze the effects of m.9185T>C on complex V structure and function.
Results:
Three further probands with CMT2 harbored the m.9185T>C mutation. Some relatives had been classified as having dHMN. Patients could be separated into 4 groups according to their mutant m.9185T>C levels. BN-PAGE demonstrated both impaired assembly and reduced activity of the complex V holoenzyme.
Conclusions:
We have shown that m.9185T>C in MT-ATP6 causes CMT2 in 1.1% of genetically undefined cases. This has important implications for diagnosis and genetic counseling. Recognition that mutations in MT-ATP6 cause CMT2 enhances current understanding of the pathogenic basis of axonal neuropathy.
Mitochondrial ATP generation by oxidative phosphorylation (OXPHOS) underpins key molecular processes that are essential for normal central and peripheral nervous system axonal function. Axonal peripheral neuropathies are a well-recognized complication of primary mitochondrial DNA (mtDNA) mutations; however, the neuropathy is rarely the presenting or predominant clinical manifestation of the disease.1–8 In contrast, mutations in the nuclear-encoded mitochondrial genes MFN29 and GDAP1, 10 which encode outer mitochondrial membrane proteins, usually present with isolated peripheral neuropathy and are now recognized to be important causes of both the axonal and demyelinating forms of Charcot-Marie-Tooth (CMT) disease.
One of the major unresolved challenges in neuromuscular diseases, such as CMT type 2 (CMT2) and distal hereditary motor neuropathy (dHMN), is the determination of the genetic cause of inherited axonal neuropathy. We investigated an extended family in whom the index case presented with a pure motor neuropathy in childhood evolving into motor-predominant CMT2 in later life. The pathogenic missense mutation m.9185T>C in MT-ATP6, encoding the ATP6 subunit of the mitochondrial ATP synthase (OXPHOS complex V), was identified and shown to segregate with disease in affected members of the pedigree. In view of the striking neuropathic features observed in this family, we screened a large cohort of 442 unrelated probands with genetically undefined CMT2 and dHMN for mutations in MT-ATP6 and MT-ATP8, encoding 2 components of complex V. We show that mitochondrial mutations that impair the function and stability of complex V are an important but previously unreported cause of CMT2.
METHODS
Standard protocol approvals, registration, and patient consents.
The study was approved and performed under the ethical guidelines issued by our institutions for clinical studies, with written informed consent obtained from all subjects for genetic studies.
Patient cohort.
We selected a cohort of patients from the Medical Research Council Centre for Neuromuscular Diseases inherited neuropathy database. These were patients presenting with a clinical phenotype compatible with either CMT2 (n = 270) or dHMN (n = 172). Diagnosis was based on clinical and electrophysiologic phenotypic evaluation. Patients were excluded from the study if inheritance was consistent with paternal transmission.
Clinical assessment.
We performed detailed clinical assessments on all patients with pathogenic mutations. Data ascertained when possible included age of symptom onset, clinical history and examination findings, CMT examination and neuropathy scale, as an indicator of neuropathy severity,11 nerve conduction studies (NCS) and EMG, plasma creatine kinase and lactate, CSF lactate, central motor conduction times, and MRI studies.
Muscle histology and histochemistry.
Muscle biopsies were performed after informed consent. Standard histologic and histochemical stains were used on cryostat sections as described previously.12
Biochemical studies.
Spectrophotometric enzyme assays of mitochondrial respiratory chain complex I (NADH:ubiquinone reductase), complex II + III (succinate:cytochrome c reductase) and complex IV (cytochrome c oxidase) activities were performed and corrected for citrate synthase activities, and blue-native polyacrylamide gel electrophoresis (BN-PAGE) was used on available muscle tissue as described previously.13,14
Genetic analysis.
Total genomic DNA was extracted from peripheral blood leukocytes, cultured skin fibroblasts, urinary tract epithelial cells, and muscle tissue using standard extraction protocols. Amplification of fragments for sequencing was performed using specific overlapping primers, Amplitaq Gold 360 Master Mix (Applied Biosystems), and a BigDye Terminator v.1.1 cycle sequencing kit (Applied Biosystems). The samples were run on a 3730xl DNA Analyzer, were assembled and analyzed using Seqscape v.2.5 software (Applied Biosystems) and were compared with the rCRS reference sequence (National Center for Biotechnology Information accession number NC_012920). Full mitochondrial genome sequencing (primers available on request) was performed in one patient (family A, patient III-6). Targeted sequencing of MT-ATP6/8 was performed in all remaining patients (see table e-1 on the Neurology® Web site at www.neurology.org for primer sequences). Restriction endonuclease analysis was used to quantitate mutant load in the m.9185T>C-positive cases as described previously.15 The experiment was performed in triplicate and the mean mutant mtDNA level was calculated. All patients with CMT2 were negative for mutations in MFN2. The majority of patients with CMT2 were also screened for mutations in MPZ, HSPB1, HSPB8, TRPV4, and GJB1 where appropriate. Patients with dHMN were negative for mutations in HSPB1, HSPB8, and TRPV4, and selected patients were negative for mutations in BSCL2 and GARS.
RESULTS
Disease-causing m.9185T>C mutations were detected in 3 further unrelated probands (family B, C, and D discussed below) from the 270 CMT2 index cases analyzed, representing a specific mutation frequency for this variant in MT-ATP6 of 1.1%. No pathogenic MT-ATP6 variants were found in patients with dHMN (172), and no pathogenic mutations in MT-ATP8 were found in either the CMT2 or dHMN groups. All synonymous and nonsynonymous single nucleotide variants detected in MT-ATP6 and MT-ATP8 are outlined in table e-2. The potential pathogenicity of each variant was evaluated using the following databases: mitowheel (http://mitowheel.org/mitowheel.html); mtDB (http://www.mtdb.igp.uu.se); and HmtDB (http:ki//www.hmtdb.uniba.it:8080/hmdb). One patient with CMT2 had a novel variant m.8828A>G, causing a missense change from asparagine with serine at amino acid position 101 of the ATP6 protein (Asn101Ser). Unfortunately, there was no muscle tissue available from the patient for BN-PAGE; thus, the potential pathogenic nature of this variant remains uncertain.
Clinical and electrophysiologic characteristics of the index family harboring m.9185T>C in MT-ATP6.
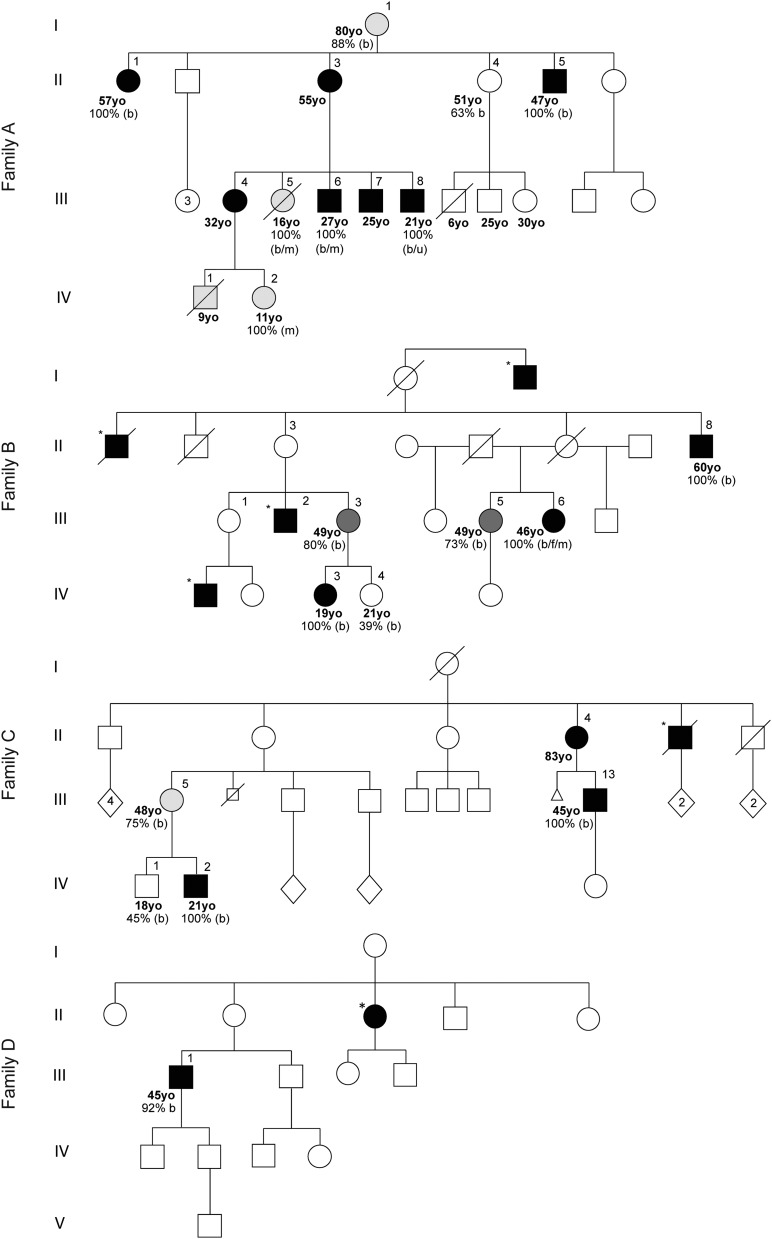
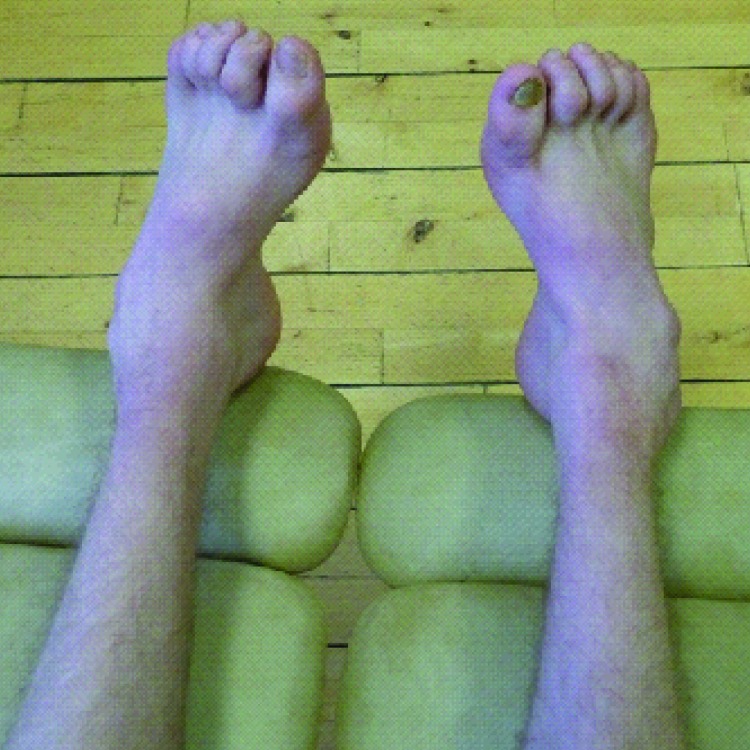
The index case (family A, patient III-8; figure 1, table 1, table e-3) presented with recurrent falls and foot drop at age 6 years after normal early development. Clinical examination at age 21 years showed distal muscle wasting of the legs, pes cavus, and clawing of the toes (figure 2). Formal manual muscle strength testing (Medical Research Council graded) was normal in the upper limbs with mild proximal lower limb weakness (hip flexion 4+ bilaterally) and moderate distal lower limb weakness (ankle dorsiflexion 3, plantarflexion 4+, inversion 5, and eversion 2 bilaterally). Ankle jerks were absent. Plantar responses were extensor. Pinprick sensation was normal, but vibration detection was reduced to the knees. Mitochondrial DNA sequencing in an older sibling (family A, patient III-6; figure 1) revealed m.9185T>C, a homoplasmic (i.e., mutant load 100%) pathogenic mutation in MT-ATP6, causing a missense change from leucine to proline at amino acid position 220 of the ATP6 protein (Leu220Pro). The presence of the m.9185T>C mutation was confirmed in the index case and was shown to segregate with disease within the family. All affected individuals were homoplasmic, and the mutational load was present at much lower levels in unaffected relatives (family A; figure 1, table e-3). Clinical features in addition to a pure motor/motor-predominant axonal neuropathy included learning difficulties (patients II-3, II-5, III-4, III-5, III-6, III-7, III-8, IV-1, and IV-2), sensorineural hearing loss (patient III-7), and retinal degeneration (patient III-8). Early proximal lower limb weakness was evident in 3 patients despite a relatively mild neuropathy (patients III-6, III-7, and III-8). Two patients had rapid decompensation after a febrile illness (patient III-5 and patient IV-1). The available clinical data suggest a sudden onset Leigh syndrome (LS)-like illness, with cortical blindness documented in one patient (patient III-5). Electrophysiologically, the neuropathy was a pure motor neuropathy/neuronopathy in 3 individuals (patients II-1, III-7, and III-8); however, sensory signs have since developed in one patient, making the diagnosis clinically compatible with motor-predominant CMT2 (patient III-8). The neuropathy was particularly severe in 2 adults with wheelchair dependence in the third decade (patients II-3 and III-4).
Figure 1. Pedigrees of 4 unrelated families harboring the m.9185T>C mutation in MT-ATP6.
Filled symbols indicate individuals with Charcot-Marie-Tooth type 2 (CMT2), dark gray shaded symbols indicate individuals with upper motor neuron signs only, light gray shaded symbols indicate individuals with unknown phenotype, square symbols indicate male gender, round symbols indicate female gender, diamond symbols indicate gender unknown, numbers in symbols indicate multiple individuals, symbols with slashes indicate deceased, a small square with a slash indicates still birth, a small triangle indicates miscarriage, asterisks indicate affected individuals not examined by the authors, and % = percentage m.9185T>C mutant load detected in each patient. b = blood; u = urine; f = fibroblasts; m = muscle; yo = year old.
Table 1.
Clinical and electrophysiologic findings in patients with the m.9185T>C mutation in MT-ATP6
Abbreviations: ADHD = attention deficit hyperactivity disorder; CMTES2 = Charcot-Marie-Tooth examination score 2 (total score 28); CMTNS2 = Charcot-Marie-Tooth neuropathy score 2 (total score 36, mild neuropathy: 0−10; moderate neuropathy: 11−20; severe neuropathy: >20); DM = diabetes mellitus; LD = learning difficulties; LL = lower limbs; mod = moderate; N = no; NCS = nerve conduction studies; ND = not done; OCD = obsessive compulsive disorder; prox = proximal; SAPs = sensory nerve action potentials; SNHL = sensorineural hearing loss; U = unknown; UL = upper limbs; UMN = upper motor neuron; Y = yes.
Figure 2.
Photograph demonstrating distal lower limb muscle wasting and pes cavus in a patient with the m.9185T>C mutation in MT-ATP6 (family A, patient III-8)
Clinical and electrophysiologic characteristics of the 3 further families harboring m.9185T>C in MT-ATP6.
In family B (figure 1, table 1, table e-3), patients with CMT2 (patients II-8, III-6, and IV-3, all with 100% mutant load) presented in their first and second decades with typical features of inherited neuropathy. Patient III-6 had a pure motor neuropathy/neuronopathy electrophysiologically at age 29, but repeat studies at age 45 revealed reduced sensory nerve action potentials compatible with motor-predominant CMT2. Despite a gradually progressive clinical course, 2 patients experienced rapid decline in mobility in their fifth and sixth decades with wheelchair dependence from unaided walking over a 5-year period (patients II-8 and III-6). In these patients with severe disease, there was upper limb and proximal lower limb weakness without evidence of myopathy on EMG; however, early proximal lower limb weakness was also present in a mildly affected individual (patient IV-3) without upper limb involvement.
In family C (figure 1, table 1, table e-3), symptom onset was in the first and second decades (patients III-13 and IV-2, both with 100% mutant load). Electrophysiologically, patients with homoplasmic mutant loads had a pure motor neuropathy/neuronopathy (patient IV-2) or sensorimotor axonal neuropathy (patient III-13). Those with lower mutant levels were asymptomatic (patient III-5 with 75% mutant load and patient IV-1 with 45% mutant load). Patient IV-2 had a severe neuropathic phenotype associated with learning difficulties, behavioral problems, and wheelchair dependence by early adulthood. Patient III-13 had motor-predominant CMT2 with pyramidal tract signs and proximal muscle weakness, which developed in later life.
In family D (figure 1, table 1, table e-3), unlike in family A, B, and C, patient III-1 appeared to have a sporadic case of motor-predominant CMT2 (92% mutant load). He presented in the first decade with recurrent ankle sprains and falls and was diagnosed with CMT2 at age 11 years. NCS demonstrated a length-dependent sensorimotor axonal neuropathy and EMG showed proximal and distal lower limb denervation with no evidence of myopathy.
Laboratory, muscle histologic, and biochemical findings in patients harboring m.9185T>C in MT-ATP6.
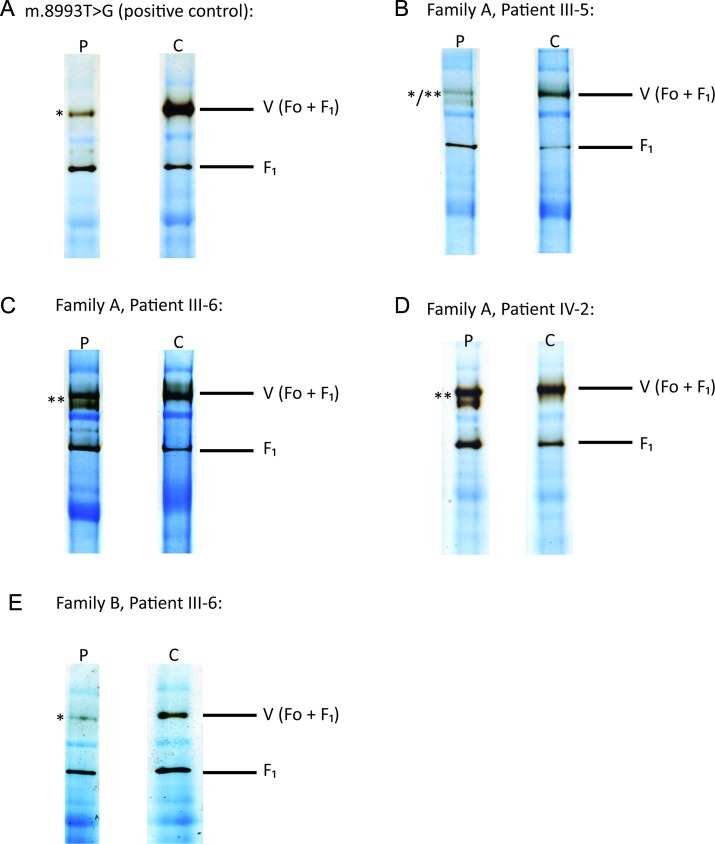
Investigations for all 4 families are summarized in table 1 and table e-3. BN-PAGE (figure 3) was performed on 4 muscle samples and revealed both impaired assembly (demonstrated by multiple bands indicative of abnormal assembly intermediates) or activity (demonstrated by reduced band intensity) of complex V when muscle tissue of patients was compared with control muscle tissue (family A, patients III-5, III-6, and IV-2 and family B, patient III-6).
Figure 3. Blue-native polyacrylamide gel electrophoresis was performed on muscle tissue from 4 patients with the m.9185T>C mutation and 1 patient with the m.8993T>G mutation.
P = patient; C = control; V (Fo + F1) = complex V holoenzyme; F1 = F1 catalytic site of complex V only. *Reduced complex V activity in patient compared with control muscle tissue (demonstrated by reduced band density). **Impaired complex V assembly in patient compared with control muscle tissue (demonstrated by multiple bands indicative of abnormal assembly intermediates). */**Both impaired complex V activity and assembly in patient compared to control muscle tissue (demonstrated by coexistence of reduced band density and multiple bands indicative of abnormal assembly intermediates). (A) Patient with the pathogenic mutation m.8993T>G (positive control). (B–D) Family A, patients III-5, III-6, and IV-2, respectively. (E) Family B, patient III-6.
Quantitation of m.9185T>C mutant load.
Quantitation of the m.9185T>C mutant load showed segregation with disease severity and phenotype (table 1, table e-3) and allowed patients to be broadly classified into 4 clinical groups according to m.9185T>C mutant load: group 1, unaffected individuals (<64% mutant load); group 2, asymptomatic individuals with upper motor neuron (UMN) signs detectable on examination only (64%−79% mutant load); group 3, affected individuals with clinical symptoms and signs of UMN involvement without neuropathy (80%−91% mutant load); and group 4, affected individuals with symptoms and signs of motor-predominant CMT2 (92%−100% mutant load).
DISCUSSION
In our genetically undefined CMT2 cohort, we identified the disease-causing MT-ATP6 m.9185T>C variant in 3 unrelated probands in addition to the index family in which we originally found the mutation. Some affected family members were also classified as having dHMN. That 1.1% of our genetically undefined CMT2 cohort harbored the m.9185T>C mutation in MT-ATP6 is important, given that molecular defects are currently only detected in approximately 35% of patients with CMT2 and in 15% of patients with dHMN.16–19 Patient m.9185T>C mutant load correlated with disease severity within each pedigree.
It is notable that all patients with m.9185T>C had previously been assessed and considered to have a CMT2 phenotype based on clinical and electrophysiologic evaluation by experienced clinicians. All but one of these patients was homoplasmic for the m.9185T>C mutation. Although each of the pedigrees was compatible with matrilineal transmission, the underlying mitochondrial basis for disease had not been considered because of the isolated peripheral nerve-specific phenotype in the absence of multisystem involvement.
The m.9185T>C variant was originally reported in a 7-year-old patient who developed sudden onset ptosis, ophthalmoparesis, and fatigue.20 Although isolated axonal neuropathy is documented at 85% m.9185T>C heteroplasmy, higher mutant loads have been reported to cause a more severe neurologic phenotype, including neurogenic muscle weakness, ataxia, and retinitis pigmentosa (NARP) and early- and late-onset LS.15,20–22 Families B, C, and D exhibit surprising tissue specificity of disease expression, considering that they had homoplasmic m.9185T>C mutant levels detected in blood, urine, skin, and muscle.
BN-PAGE was used in muscle tissue to demonstrate the deleterious effects of the m.9185T>C;p.Leu220Pro mutation on complex V structure and function. The complex V assay measures ATP synthase activity in the reverse direction. ATP is hydrolyzed to produce ADP and inorganic phosphate. In the presence of inorganic phosphate, the lead ions used in the buffer [lead(II) nitrate] form a precipitate, which correlates with the degree of ATP hydrolysis. This technique has not been used to assess complex V in patients with m.9185T>C previously. It has been shown that the m.8993T>G mutation associated with LS and NARP not only impairs assembly and stability of complex V but also reduces intrinsic activity of the ATP synthase holoenzyme.23 We confirmed impaired complex V assembly (family A, patients III-6 and IV-2; figures 1 and 3, table e-3), reduced complex V activity (family B, patient III-6; figures 1 and 3, table e-3), or both (family A, patient III-5; figures 1 and 3, table e-3) in patients who were homoplasmic for m.9185T>C. These data support the pathogenicity of m.9185T>C and indicate that this mutation impairs both complex V assembly and activity. In view of the previously published data documenting impaired assembly and stability of complex V associated with the NARP m.8993T>G mutation,23 the m.9185T>C mutation may also be expected to result in a poorly assembled and unstable complex V holoenzyme. The variation in both complex V activity and assembly observed on BN-PAGE for patients with identical m.9185T>C mutation loads may, therefore, be attributable to holoenzyme disassembly occurring during sample preparation. In our patients with the m.9185T>C mutation, muscle histology and histochemistry were either normal or exhibited subtle nonspecific mitochondrial abnormalities on electron microscopy only, as is frequently the case for mutations involving mtDNA protein encoding genes.
It is not clear why some patients harboring homoplasmic levels of m.9185T>C develop a tissue-specific peripheral neuropathic phenotype, whereas other reported patients and families with similar mutant loads develop a multisystem neurologic syndrome such as LS. Variable clinical severity in patients with similar mutant loads has been reported with a number of known pathogenic mtDNA mutations. It is possible that this variability relates to subtle differences in tissue heteroplasmy; however, no correlation has been shown between the presence of neuropathy and muscle m.3243A>G mutant levels despite the disease phenotype being more severe in the patients with neuropathy.6 This finding suggests that there may be additional factors that increase the susceptibility of certain patients to neuropathy, such as activation of nuclear-encoded genes that enhance pathways to partially compensate for aberrant complex V function or reduced ATP production. This hypothesis requires further study.
The precise molecular mechanisms linking respiratory chain dysfunction to axonal degeneration have not been determined. One possibility is that axonal degeneration relates to loss of the axon membrane potential as a consequence of failure of the energy-dependent Na+/K+-ATPase pump, comparable to ischemia. However, a recent study did not show detectable changes in nerve excitability using the Trond protocol in patients with mitochondrial disease, as would be expected if there was significant axonal depolarization due to failure of energy-dependent systems.24
The findings reported here add to increasing evidence that mitochondrial dysfunction is an important cause of CMT2. Mutations in MFN2, a critical nuclear gene that regulates mitochondrial fusion, have been identified as a cause of CMT2. Evidence is emerging that MFN2 has a distinct role aside from mitochondrial fusion in mitochondrial axonal transport and is an important component of the linker/adaptor complex between mitochondria and kinesin/microtubules.25 Disruption of this complex may explain the length-dependent nature of the neuropathy seen with MFN2 mutations. More recently, a novel MFN2 mutation causing optic atrophy, axonal neuropathy, and mitochondrial myopathy was found to cause multiple mtDNA deletions in skeletal muscle. This finding was thought to result from mtDNA instability, caused by the variability of repair protein content across the mitochondrial population as a consequence of impaired mitochondrial fusion, and expands current knowledge of the pathogenic basis of MFN2-related neurologic disease.26 It has also been shown that nerves expressing mutant forms of neurofilament (which causes CMT2E) can also alter mitochondrial dynamics, suggesting that dysfunctional axonal transport might precipitate axonal degeneration in a number of other CMT subtypes.27 These observations imply that impaired ATP-dependent axonal transport is a candidate for axonal damage in other mitochondrial diseases.
We suggest that MT-ATP6 should be considered in the molecular diagnostic evaluation of patients with CMT2, together with MFN2, MPZ, RAB7, HSPB1, HSPB8, GDAP1, TRPV4, NEFL, and GARS, especially in pedigrees in which there appears to be no male-to-male transmission.17,19 These findings have important clinical implications, particularly for genetic counseling. Diagnosis can be made in DNA extracted from blood, using restriction fragment length polymorphism genetic analysis, because m.9185T>C mutant load appears to be high in peripheral blood leukocytes in all affected patients reported to date. An invasive muscle biopsy is not required for diagnostic purposes, and histologic analysis is usually unhelpful; however, if muscle tissue is available, BN-PAGE demonstrates both the structural and functional impairment caused by the effects of m.9185T>C;p.Leu220Pro on the ATP6 subunit.
The clinical and electrophysiologic phenotype in our families was typical of CMT2/dHMN; however, the following features should be used to help prioritize MT-ATP6 for mutation analysis in patients with neuropathy: 1) disease onset in the first or second decade; 2) variable clinical severity with wheelchair dependence as early as age 19 years; 3) an initial slowly progressive neuropathy, which may accelerate in the fifth and sixth decades from unaided walking to wheelchair dependence within a relatively short time frame; 4) an evolving clinical phenotype from a pure motor to a motor-predominant axonal neuropathy in the third and fourth decades; 5) early proximal lower limb muscle involvement despite mild distal weakness; 6) multisystem involvement in the patient or relatives; 7) UMN signs in affected or asymptomatic relatives; 8) rapid clinical decompensation in affected individuals after viral or septic illness.
The data presented here add to the increasing evidence that mitochondrial dysfunction is an important cause of CMT2. We suggest that MT-ATP6 mutations should be considered early in the diagnostic evaluation of patients with inherited axonal neuropathies.
Supplementary Material
GLOSSARY
- BN-PAGE
blue-native polyacrylamide gel electrophoresis
- CMT
Charcot-Marie-Tooth
- CMT2
Charcot-Marie-Tooth type 2
- dHMN
distal hereditary motor neuropathy
- LS
Leigh syndrome
- mtDNA
mitochondrial DNA
- NARP
neurogenic muscle weakness, ataxia, and retinitis pigmentosa
- NCS
nerve conduction studies
- OXPHOS
oxidative phosphorylation
- UMN
upper motor neuron
Footnotes
Supplemental data at www.neurology.org
AUTHOR AFFILIATIONS
From the MRC Centre for Neuromuscular Diseases (R.D.S.P., S.M.M., E.C., J.L.H., M.P.L., S.R., M.M.R., M.G.H.) and Department of Clinical Neurophysiology (J.B.), UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, London, UK; Neurometabolic Unit (A.C., I.H., S.H., J.L.) and Neurogenetics Unit (M.G.S., C.W., E.E.M.), National Hospital for Neurology and Neurosurgery, London, UK; Department of Molecular Neuroscience (J.L.H., H.H., M.P.L., M.M.R., M.G.H.), UCL Institute of Neurology, London, UK; Department of Clinical Neurophysiology (J.B.), Norfolk and Norwich University Hospital, Norwich, UK; Department of Inherited Metabolic Disease (M.C.), Evelina Children's Hospital, St Thomas' Hospital, London, UK; Clinical Genetics Department (F.F.), Guy's & St Thomas' NHS Foundation Trust, London, UK; Dubowitz Neuromuscular Centre (S.A.R.), UCL Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust, London, UK; Dorset Epilepsy Service (R.P.), Poole Hospital NHS Foundation Trust, Poole, Dorset, UK; Department of Neurology (M.R.), King's College Hospital NHS Foundation Trust, London, UK; Department of Clinical Neurology (J.P.), University of Oxford, John Radcliffe Hospital, Oxford, UK; Division of Clinical Genetics (C.C.), MetroHealth Medical Center, Cleveland, OH; Ferguson Smith Centre for Clinical Genetics (C.L.), Yorkhill Hospital, Glasgow, UK; and Mitochondrial Research Group (S.R.), Clinical and Molecular Genetics Unit, UCL Institute of Child Health, London, UK.
AUTHOR CONTRIBUTIONS
Robert D.S. Pitceathly: drafting/revising manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, acquisition of data. Sinéad M. Murphy: drafting/revising manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, acquisition of data. Ellen Cottenie: analysis or interpretation of data, acquisition of data. Annapurna Chalasani: analysis or interpretation of data, acquisition of data. Mary G. Sweeney: drafting/revising manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data. Cathy Woodward: analysis or interpretation of data, acquisition of data. Ese E. Mudanohwo: analysis or interpretation of data, acquisition of data. Iain Hargreaves: drafting/revising manuscript for content, including medical writing for content, analysis or interpretation of data, acquisition of data. Simon Heales: drafting/revising manuscript for content, including medical writing for content, analysis or interpretation of data. John Land: drafting/revising manuscript for content, including medical writing for content, analysis or interpretation of data. Janice L. Holton: analysis or interpretation of data, acquisition of data. Henry Houlden: drafting/revising manuscript for content, including medical writing for content, analysis or interpretation of data, study supervision or coordination. Julian Blake: analysis or interpretation of data, acquisition of data. Michael Champion: analysis or interpretation of data, acquisition of data. Frances Flinter: analysis or interpretation of data, acquisition of data. Stephanie Robb: analysis or interpretation of data, acquisition of data. Rupert Page: analysis or interpretation of data, acquisition of data. Michael Rose: analysis or interpretation of data, acquisition of data. Jacqueline Palace: analysis or interpretation of data, acquisition of data. Carol Crowe: analysis or interpretation of data, acquisition of data. Cheryl Longman: analysis or interpretation of data, acquisition of data. Michael P. Lunn: drafting/revising manuscript for content, including medical writing for content, acquisition of data. Shamima Rahman: drafting/revising manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, study supervision or coordination. Mary M. Reilly: drafting/revising manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, study supervision or coordination. Michael G. Hanna: drafting/revising manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, study supervision or coordination.
STUDY FUNDING
Supported by the NIHR UCLH/UCL Comprehensive Biomedical Research Centre and undertaken at University College London Hospitals/University College London, which received a proportion of funding from the Department of Health National Institute for Health Research Biomedical Research Centres funding scheme.
DISCLOSURE
R.D.S. Pitceathly is funded by MRC grant G0800674. S.M. Murphy is supported by NINDS/ORD (1U54NS065712–01). E. Cottenie is funded by MRC Centre grant G0601943. A. Chalasani, M.G. Sweeney, C. Woodward, E.E. Mudanohwo, I. Hargreaves, S. Heales, and J. Land work at University College London Hospitals/University College London, which received a proportion of funding from the Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme. J.L. Holton is supported by The Myositis Support Group and the Reta Lila Weston Institute for Neurological Studies. H. Houlden is supported by the MRC and Wellcome Trust. J. Blake, M. Champion, F. Flinter, S. Robb, R. Page, M. Rose, J. Palace, C. Crowe, and C. Longman report no disclosures. M. P. Lunn is supported by MRC Centre grant G0601943. S. Rahman is supported by Great Ormond Street Hospital Children's Charity. M.M. Reilly is supported by MRC Centre grant G0601943, the Muscular Dystrophy Campaign, and NINDS/ORD (1U54NS065712–01). M.G. Hanna is supported by an MRC Centre grant G0601943 and by The Myositis Support Group. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Pezeshkpour G, Krarup C, Buchthal F, et al. Peripheral neuropathy in mitochondrial disease. J Neurol Sci 1987;77:285–304 [DOI] [PubMed] [Google Scholar]
- 2. Holt IJ, Harding AE, Petty RK, Morgan-Hughes JA. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet 1990;46:428–433 [PMC free article] [PubMed] [Google Scholar]
- 3. Coker SB. Leigh disease presenting as Guillain-Barré syndrome. Pediatr Neurol 1993;9:61–63 [DOI] [PubMed] [Google Scholar]
- 4. Rahman S, Blok RB, Dahl HH, et al. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann Neurol 1996;39:343–351 [DOI] [PubMed] [Google Scholar]
- 5. Chu CC, Huang CC, Fang W, et al. Peripheral neuropathy in mitochondrial encephalomyopathies. Eur Neurol 1997;37:110–115 [DOI] [PubMed] [Google Scholar]
- 6. Kärppä M, Syrjälä P, Tolonen U, Majamaa K. Peripheral neuropathy in patients with the 3243A>G mutation in mitochondrial DNA. J Neurol 2003;250:216–221 [DOI] [PubMed] [Google Scholar]
- 7. Kaufmann P, Pascual JM, Anziska Y, et al. Nerve conduction abnormalities in patients with MELAS and the A3243G mutation. Arch Neurol 2006;63:746–748 [DOI] [PubMed] [Google Scholar]
- 8. Erol I, Alehan F, Horvath R, et al. Demyelinating disease of central and peripheral nervous systems associated with a A8344G mutation in tRNALys. Neuromuscul Disord 2009;19:275–278 [DOI] [PubMed] [Google Scholar]
- 9. Chung KW, Kim SB, Park KD, et al. Early onset severe and late-onset mild Charcot-Marie-Tooth disease with mitofusin 2 (MFN2) mutations. Brain 2006;129:2103–2118 [DOI] [PubMed] [Google Scholar]
- 10. Baxter RV, Ben Othmane K, Rochelle JM, et al. Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot-Marie-Tooth disease type 4A/8q21. Nat Genet 2002;30:21–22 [DOI] [PubMed] [Google Scholar]
- 11. Murphy SM, Herrmann DN, McDermott MP, et al. Reliability of the CMT neuropathy score (second version) in Charcot-Marie-Tooth disease. J Peripher Nerv Syst 2011;16:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor RW, Schaefer AM, Barron MJ, et al. The diagnosis of mitochondrial muscle disease. Neuromuscul Disord 2004;14:237–245 [DOI] [PubMed] [Google Scholar]
- 13. Hargreaves P, Rahman S, Guthrie P, et al. Diagnostic value of succinate ubiquinone reductase activity in the identification of patients with mitochondrial DNA depletion. J Inherit Metab Dis 2002;25:7–16 [DOI] [PubMed] [Google Scholar]
- 14. Schägger H. Tricine-SDS-PAGE. Nat Protoc 2006;1:16–22 [DOI] [PubMed] [Google Scholar]
- 15. Castagna AE, Addis J, McInnes RR, et al. Late onset Leigh syndrome and ataxia due to a T to C mutation at bp 9,185 of mitochondrial DNA. Am J Med Genet A 2007;143A:808–816 [DOI] [PubMed] [Google Scholar]
- 16. Reilly MM, Shy ME. Diagnosis and new treatments in genetic neuropathies. J Neurol Neurosurg Psychiatry 2009;80:1304–1314 [DOI] [PubMed] [Google Scholar]
- 17. Reilly MM, Murphy SM, Laurá M. Charcot-Marie-Tooth disease. J Peripher Nerv Syst 2011;16:1–14 [DOI] [PubMed] [Google Scholar]
- 18. Dierick I, Baets J, Irobi J, et al. Relative contribution of mutations in genes for autosomal dominant distal hereditary motor neuropathies: a genotype-phenotype correlation study. Brain 2008;131:1217–1227 [DOI] [PubMed] [Google Scholar]
- 19. Saporta ASD, Sottile SL, Miller LJ, et al. Charcot-Marie-Tooth disease subtypes and genetic testing strategies. Ann Neurol 2011;69:22–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moslemi A-R, Darin N, Tulinius M, et al. Two new mutations in the MTATP6 gene associated with Leigh syndrome. Neuropediatrics 2005;36:314–318 [DOI] [PubMed] [Google Scholar]
- 21. Childs A-M, Hutchin T, Pysden K, et al. Variable phenotype including Leigh syndrome with a 9185T>C mutation in the MTATP6 gene. Neuropediatrics 2007;38:313–316 [DOI] [PubMed] [Google Scholar]
- 22. Saneto RP, Singh KK. Illness-induced exacerbation of Leigh syndrome in a patient with the MTATP6 mutation, m. 9185 T>C. Mitochondrion 2010;10:567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nijtmans LG, Henderson NS, Attardi G, Holt IJ. Impaired ATP synthase assembly associated with a mutation in the human ATP synthase subunit 6 gene. J Biol Chem 2001;276:6755–6762 [DOI] [PubMed] [Google Scholar]
- 24. Ng K, Winter S, Sue C, Burke D. Preserved motor axonal membrane potential in mitochondrial disease. J Neurol Neurosurg Psychiatry 2010;81:844–846 [DOI] [PubMed] [Google Scholar]
- 25. Misko A, Jiang S, Wegorzewska I, et al. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci 2010;30:4232–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rouzier C, Bannwarth S, Chaussenot A, et al. The MFN2 gene is responsible for mitochondrial DNA instability and optic atrophy “plus” phenotype. Brain 2012;135:23–34 [DOI] [PubMed] [Google Scholar]
- 27. Tradewell ML, Durham HD, Mushynski WE, Gentil BJ. Mitochondrial and axonal abnormalities precede disruption of the neurofilament network in a model of Charcot-Marie-Tooth disease type 2E and are prevented by heat shock proteins in a mutant-specific fashion. J Neuropathol Exp Neurol 2009;68:642–652 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.