The MAPK (mitogen-activated protein kinase) kinase (MEK1/2) signal transduction cascade plays a central role in the regulation of many cellular processes. Selumetinib (AZD6244, ARRY-142886), a selective non-ATP competitive small-molecule inhibitor of MEK1/2,1 has been used in clinical trials for various solid tumors. The most common adverse effects include dermatitis acneiform, diarrhea, nausea, and peripheral edema,2 with the most common being dermatologic side effects, possibly related to the inhibitory effect of selumetinib on MEK1/2, a component of epidermal growth factor receptor (EGFR) pathway.3 We describe 3 patients treated with selumetinib as part of an institutional review board–approved uveal melanoma monotherapy clinical trial (NCT01143402) who developed dropped head syndrome.
Case reports.
Case 1.
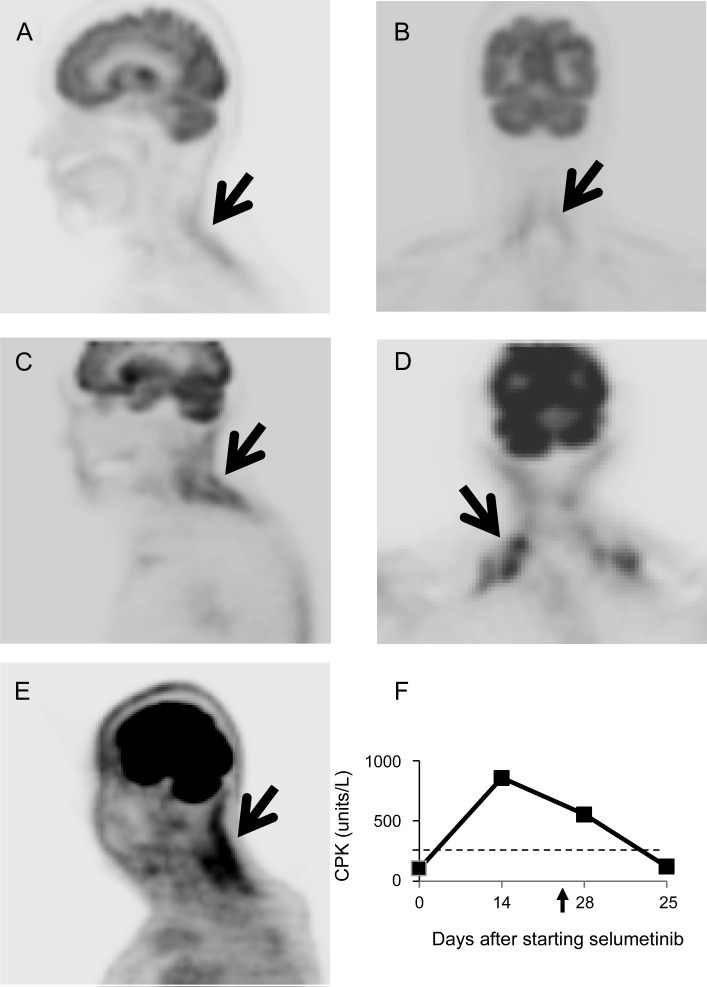
A 71-year-old woman with choroidal melanoma metastatic to the lung and breast was started on selumetinib (75 mg twice daily) in September 2010. In October she experienced pain and stiffness in the neck and shoulders, followed by neck extensor weakness. On examination, the neck extensor strength was 2/5. Creatine phosphokinase (CPK) was 402 units/L (normal range 38–174 units/L) and erythrocyte sedimentation rate (ESR) was 55 mm/hour (normal 0–20 mm/hour). EMG demonstrated mild axonal sensorimotor polyneuropathy and myopathic changes in the left biceps and cervical paraspinal muscles. MRI of the cervical spine showed disk bulging at C4/5 and C5/6 without abnormal signal changes or enhancement in the neck muscles. 18F-fluorodeoxyglucose PET (FDG-PET) scan was significant for intense uptake in the posterior neck muscles (figure, A and B). Selumetinib was discontinued with full recovery of neck extensor strength and normalization of the CPK approximately 1 month later.
Figure. Imaging.
18F-FDG-PET from case 1 (A, B), case 2 (C, D), and case 3 (E) demonstrated increased FDG uptake in neck extensors and trapezius as indicated by the arrows. (F) Serum creatine phosphokinase (CPK) level of case 3: the arrow indicates the day of stopping selumetinib and the dashed line indicates the upper limit of the normal value.
Case 2.
A 76-year-old woman with uveal melanoma arising from the ciliary body with liver metastases was started on selumetinib in April 2011. One month later, she developed pain in the right occipital and posterior neck after a fall. The pain ultimately resolved; however, she then developed neck extensor weakness a few days later. On examination, strength of the sternocleidomastoid and trapezius muscles were 4+/5, neck extensors 3−/5, and neck flexors 4/5. EMG showed myopathic units in cervical paraspinal muscles. MRI of the cervical spine only demonstrated moderate degenerative spondylosis. PET scan was significant for intense uptake in the muscles of the shoulder girdle and posterior neck (figure, C and D). Serum CPK was 335 units/L and the ESR was 38 mm/hour. The patient was initiated on prednisone 60 mg for 1 week and subsequently tapered off while selumetinib was continued. Despite the steroid, neck weakness persisted and later resolved after stopping selumetinib.
Case 3.
A 73-year-old man with uveal melanoma with lung metastases was initiated on selumetinib in August 2011. Within weeks he developed neck soreness extending to both shoulders, followed by difficulty lifting his head. On examination, neck extensors were 4−/5 with easy fatigability. The sternocleidomastoid and trapezius muscles were 5−/5 bilaterally, with other muscles intact. CPK was 857 units/L (figure, E). Selumetinib was stopped and a muscle biopsy of the right trapezius was performed 10 days later, showing abnormal fiber size variation and rare myofibers with regenerative changes. There was no evidence of inflammation, denervation, metastasis, or mitochondrial abnormalities. The neck muscle weakness resolved approximately 2 weeks after stopping selumetinib with normalization of CPK.
Discussion.
Dropped head syndrome, an uncommon progressive weakness of neck extensor muscles, has been associated with various neurologic disorders. The most commonly reported etiology is focal myopathy. This syndrome was recently reported in a phase I study in patients with advanced cancers treated with PD-0325901, another MEK inhibitor.4 Our cases further demonstrated a possible link between MEK inhibition and dropped head syndrome which is clinically characterized by focal noninflammatory myopathy, moderately elevated serum CPK, no response to corticosteroids, and a full recovery after discontinuation of the offending agent.
An unusual finding in our patients was the increased FDG uptake in the affected muscles on PET scan. Nonmalignant, abnormal uptake of FDG in muscles has been observed in patients with graft vs host disease–associated polymyositis, dermatomyositis, and statin-induced necrotizing myositis, but not in low-grade myositis and inclusion body myositis.5 MEK1/2 activation is an important upstream signal for fatty acid uptake by skeletal muscles.6 The energy failure due to selective inhibition of MEK1/2 by selumetinib might contribute to the neck extensor weakness. The affected muscles increase glucose uptake as an alternate energy source, which might lead to the observed increased FDG uptake. Based on these observations, a frequent neurologic examination with particular attention to assessment of neck muscle strength might be warranted in patients treated with MEK inhibitors.
Acknowledgments
Acknowledgment: The authors thank Dr. Jerome B. Posner for advice and support from AstraZeneca.
Footnotes
Author contributions: Dr. Chen: drafting/revising manuscript, analysis and acquisition of data. Dr. Schwartz: providing the patients and revising manuscript. Dr. DeAngelis: revising manuscript. Dr. Kaley: providing the patients and revising manuscript. Dr. Carvajal: the principal investigator of clinical trial (NCT01143402), providing the patients, and revising manuscript.
Disclosure: The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
References
- 1.Yeh TC, Marsh V, Bernat BA, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res 2007; 13: 1576– 1583 [DOI] [PubMed] [Google Scholar]
- 2.Kirkwood JM, Bastholt L, Robert C, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res 2012; 18: 555– 567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balagula Y, Barth Huston K, Busam KJ, Lacouture ME, Chapman PB, Myskowski PL. Dermatologic side effects associated with the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886). Invest New Drugs 2011; 29: 1114– 1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boasberg PD, Redfern CH, Daniels GA, Bodkin D, Garrett CR, Ricart AD. Pilot study of PD-0325901 in previously treated patients with advanced melanoma, breast cancer, and colon cancer. Cancer Chemother Pharmacol 2011; 68: 547– 552 [DOI] [PubMed] [Google Scholar]
- 5.Al-Nahhas A, Jawad AS. PET/CT imaging in inflammatory myopathies. Ann NY Acad Sci 2011; 1228: 39– 45 [DOI] [PubMed] [Google Scholar]
- 6.Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol 2007; 103: 388– 395 [DOI] [PubMed] [Google Scholar]