Abstract
Objectives:
Observational studies suggest reduced risk of Alzheimer disease (AD) in users of hormone therapy (HT), but trials show higher risk. We examined whether the association of HT with AD varies with timing or type of HT use.
Methods:
Between 1995 and 2006, the population-based Cache County Study followed 1,768 women who had provided a detailed history on age at menopause and use of HT. During this interval, 176 women developed incident AD. Cox proportional hazard models evaluated the association of HT use with AD, overall and in relation to timing, duration of use, and type (opposed vs unopposed) of HT.
Results:
Women who used any type of HT within 5 years of menopause had 30% less risk of AD (95% confidence interval 0.49–0.99), especially if use was for 10 or more years. By contrast, AD risk was not reduced among those who had initiated HT 5 or more years after menopause. Instead, rates were increased among those who began “opposed” estrogen-progestin compounds within the 3 years preceding the Cache County Study baseline (adjusted hazard ratio 1.93; 95% confidence interval 0.94–3.96). This last hazard ratio was similar to the ratio of 2.05 reported in randomized trial participants assigned to opposed HT.
Conclusions:
Association of HT use and risk of AD may depend on timing of use. Although possibly beneficial if taken during a critical window near menopause, HT (especially opposed compounds) initiated in later life may be associated with increased risk. The relation of AD risk to timing and type of HT deserves further study.
The association between estrogen hormone therapy (HT) and risk of Alzheimer disease (AD) is a subject of debate. In laboratory studies in vivo and in vitro, estrogens show positive neurobiological effects, which may provide a rationale for a neuroprotective role of HT.1,2 Observational studies of HT and AD risk have, in general, supported this idea, with well-designed, prospective studies showing a significant reduction in risk.3–5 Motivated by these studies, a large, multicenter, double-masked, randomized, prevention trial, the Women's Health Initiative Memory Study (WHIMS), tested HT in the form of conjugated equine estrogens (either alone or “opposed” with medroxyprogesterone acetate) vs placebo on dementia incidence. The trial was stopped prematurely after 6 years of follow-up because of concerns about adverse cardiovascular and cancer risks. Unexpectedly, the results of the trial showed an increased risk of dementia with HT.6,7 Arguably, the divergent results between observational and randomized studies might reflect differences in the effects of HT based on either type or timing of use.8
The Cache County Study (CCS) was one of the prospective, observational studies that suggested an association of HT use with reduced AD risk.5 Since its initial report, the CCS has accumulated 7 more years of follow-up and collected more detailed information on type and timing of HT use. We therefore examined the relationship of HT use and AD risk over an extended follow-up interval, testing whether there were differences in this relationship that depended on type of HT or timing of its use.
METHODS
Study overview.
The CCS was a population-based study of dementia among elderly residents of Cache County, Utah, aged 65 years or older as of January 1, 1995.9,10 Approximately 90% of an eligible population of 5,677 permanent residents (n = 5,092; 2,928 women) participated in a baseline assessment (wave I). Participants provided a buccal sample for APOE genotyping11,12 and were screened for dementia. They also completed a detailed questionnaire on potential risk and protective factors for dementia. Participants who did not have dementia at baseline were followed up using similar procedures in 1998–1999 (wave II), 2002–2003 (wave III), and 2005–2006 (wave IV).
Standard protocol approvals, registrations, and patient consents.
All procedures were approved by the Institutional Review Boards of Utah State University, Duke University, and Johns Hopkins University. Study participants provided informed consent at each stage of the study. Responsible spouses or next of kin gave consent when participants were unable to provide it.
Dementia evaluation.
Participants were evaluated at each wave for dementia using a multistage assessment procedure described previously.9,10 They were screened for cognitive impairments using the Modified Mini-Mental State Examination (3MS),13 or the Informant Questionnaire for Cognitive Decline in the Elderly,14 followed (in waves I and II) by the Dementia Questionnaire with a proxy informant.15 Participants with evidence of cognitive dysfunction received a clinical assessment including a clinical history and review of systems, brief physical examination, and 1-hour battery of neuropsychological tests.16 Based on this information, working diagnoses of cognitive disorders were assigned. Estimated age at onset of dementia was recorded as the year in which participants met DSM-III-R criteria. Participants with apparent cognitive disorders were reexamined by a study physician and underwent standard laboratory tests and a brain MRI. Finally, a panel of expert clinicians reviewed all available information and assigned differential diagnoses of dementia using National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association criteria17 for AD, modified National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherché et l'Enseignement en Neurosciences criteria18,19 for vascular dementia, and current research criteria for other illnesses. The diagnostic panel was unaware of participants' HT use. Examination of an independent 19% sample of the population enabled estimation of the sensitivity (88.9%) and specificity (95.6%) of this method for detection of incident dementia.20 Autopsy confirmation of clinical diagnoses was obtained in 85% of instances.21
Covariate assessments.
Between waves I and II, female participants were administered a supplemental telephone Women's Health Questionnaire (WHQ) that included questions about their reproductive history and use of HT, including types of HT and age at initiation and end of use. This information provided exposure variables for HT that captured 1) ever/never use of any agent; 2) ever/never use of specific types of HT; and 3) duration and timing of use relative to the age of menopause or to the baseline (to capture recent initiation). Other variables included parity, age at menarche, age at menopause, and history of hysterectomy or oophorectomy. Age at menopause was defined as the age the participant went more than 1 year without having a period, or the age when both ovaries were surgically removed, whichever came first. Information from the wave I baseline examination added self-reported covariates suggesting a tendency toward health-conscious behaviors that might have influenced HT use or confounded the relationship between HT use and AD risk. These included years of education; history of alcohol (ever regularly drank 2 or more alcoholic beverages a week) or tobacco use; self-rated health status during the past week (excellent, good, fair, or poor); body mass index (in kg/m2), both currently and at age 18 years; history of hypertension, high cholesterol, diabetes, stroke, heart attack, or coronary artery bypass graft surgery; and family history of AD in a first-degree relative. In addition, we used data from a supplemental mail-in questionnaire at wave I that obtained information on dietary patterns as well as physical and social activities. From this, variables were derived for reported numbers of vitamins or supplements regularly taken, regular physical activity (≥5 hours of light activity plus occasional moderate to vigorous activity), regular social activity (time with family/friends at least twice weekly), and dietary scores in quintiles that reflected adherence to either the Mediterranean or Dietary Approaches to Stop Hypertension diets.22
Analytic sample.
Of the 2,928 women enrolled at wave I, 2,090 (71.4%) completed the WHQ. Of these, 321 had no follow-up assessment (including 6 with dementia at the WHQ), leaving 1,769 eligible for analysis. Compared with the 838 women who did not complete the WHQ, respondents were younger at baseline (mean age, 75.3 years [SD 6.6 years] vs 81.2 years [8.0 years]; p < 0.001), more educated (mean years of education, 12.9 [2.2] vs 12.1 [2.5]; p < 0.01), and less likely to possess at least 1 APOE ε4 allele (30.3% vs 39.0%, p < 0.001). They did not differ meaningfully on any other covariates described above, or on baseline 3MS scores.
Participants were followed an average of 7 years, during which time 248 women developed incident dementia. Of these, 176 received diagnoses of definite, probable, or possible AD. Women who developed AD were older at baseline (mean age, 78.1 years [SD 6.3 years] vs 74.2 years [6.2 years]; p < 0.001), more often had an APOE ε4 allele (46.9% vs 28.8%, p < 0.001), and were more likely to have a history of diabetes (20.5% vs 11.6%, p < 0.001) than others.
Statistical analysis.
Characteristics of HT users and nonusers were compared on categorical variables using χ2 tests and on continuous variables by t tests. Survival analyses used Cox proportional hazard models to evaluate the association between HT use and incident AD. The time axis was participant age. Participants were followed from their age at study entry until AD onset or last assessment. Those with non-AD dementia were censored at their onset of dementia. Hazard ratios and 95% confidence intervals (CIs) were estimated from unadjusted models and from 2 sets of adjusted models. The first controlled for well-established AD risk factors including APOE status (≥1 ε4 allele), years of education, and age at baseline. The second set of models controlled for these variables and also for propensity to HT use, scored (in deciles) using predicted probabilities from a logistic regression model of any HT use related to the covariates described above in the “Covariate assessments” section (receiver operating characteristic area under the curve for the model was 0.56). The propensity score was intended to provide more efficient control on attributes that might confound the relationship between HT use and AD. Propensity scores included as continuous variables rather than in deciles provided indistinguishable results.
RESULTS
Among the participants eligible for analysis, 1,105 participants (62.5%) reported a history of HT use. Among the latter, 93.6% reported oral HT, 11% as a cream, and 6.0% as a patch (total was >100% because several participants reported using multiple forms). Because the overwhelming majority of HT use was oral, we considered all forms of HT together. We classified HT use as “opposed” if the participant used an agent that included a progestin in combination with estrogen (37.0%), “unopposed” (59.7%), or unknown (3.3%).
Table 1 compares key characteristics of HT users vs nonusers. Users were significantly younger and better educated, and typically had fewer live births, younger age at menopause, and more frequent history of partial or bilateral oophorectomy. They also had a shorter period of time between the age at menopause and baseline. They also developed AD at a lower crude rate than nonusers (7.9% vs 13.4%, p = 0.001).
Table 1.
Baseline characteristics by HT use (n = 1,768)
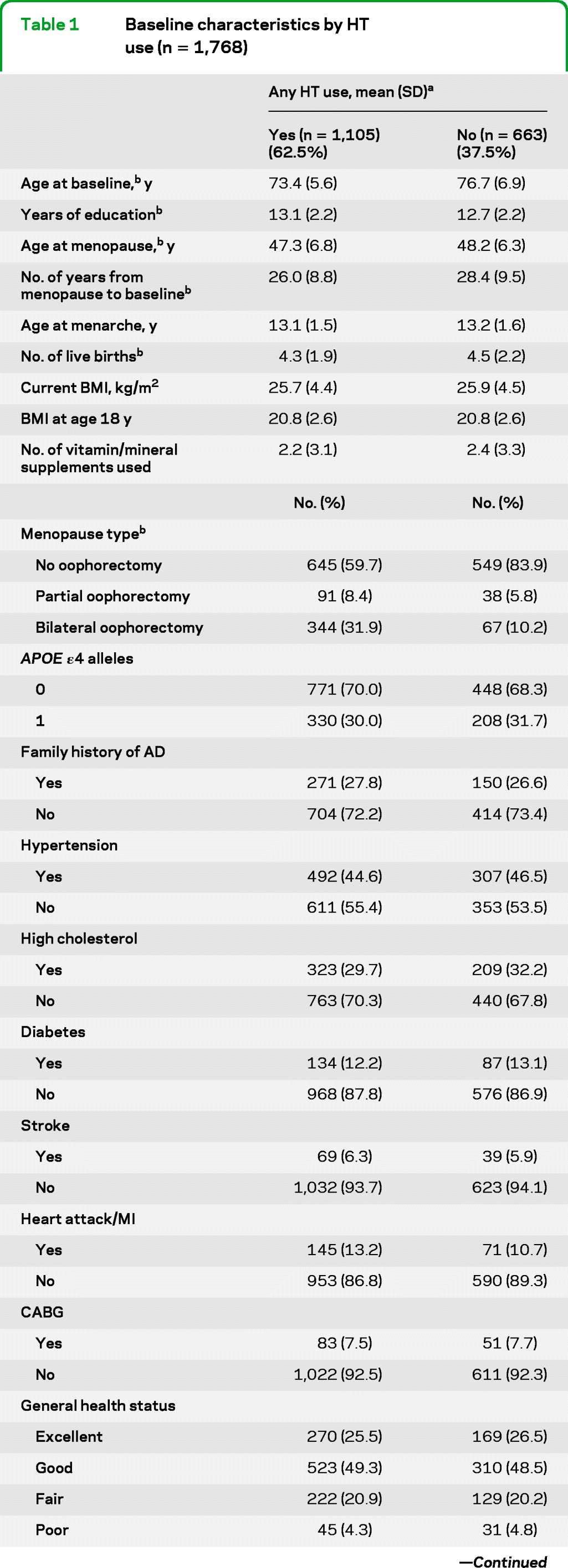
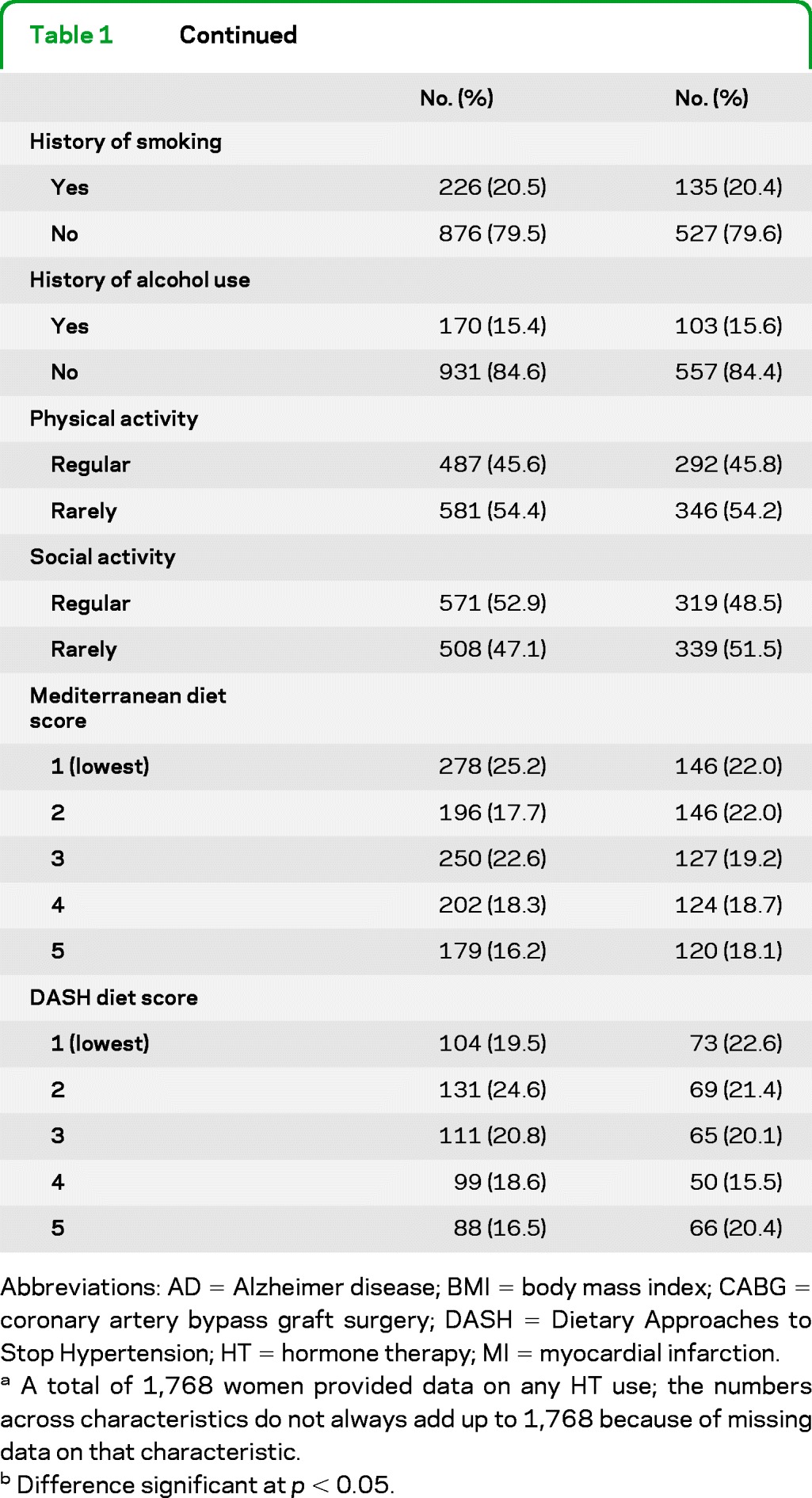
Abbreviations: AD = Alzheimer disease; BMI = body mass index; CABG = coronary artery bypass graft surgery; DASH = Dietary Approaches to Stop Hypertension; HT = hormone therapy; MI = myocardial infarction.
A total of 1,768 women provided data on any HT use; the numbers across characteristics do not always add up to 1,768 because of missing data on that characteristic.
Difference significant at p < 0.05.
Table 2 shows how the relationship between HT use and incident AD varied with timing, duration, and type of HT. The results of the 2 sets of adjusted models were similar, so only the fully adjusted models are discussed below. Overall, HT users showed a reduction of 20% in their AD risk that was suggestive but not statistically significant (model 1: adjusted hazard ratio [aHR] 0.80; 95% CI 0.58–1.09). However, HT initiated within 5 years of menopause was associated with significantly lower AD risk (model 2: aHR 0.70; 95% CI 0.49–0.99), whereas HT use initiated later showed no such association (aHR 1.03; 95% CI 0.68–1.55). There was some evidence of a duration effect (model 3): HT initiated within 5 years of menopause, but used for less than 10 years, was associated with a trend toward AD risk reduction, but use for 10 or more years was associated with a further reduction that was statistically significant (aHR 0.63; 95% CI 0.41–0.98). By contrast, there was no association with HT initiated more than 5 years after menopause, regardless of duration.
Table 2.
Cox proportional hazard models of association with incident AD by timing, duration, and type of HT
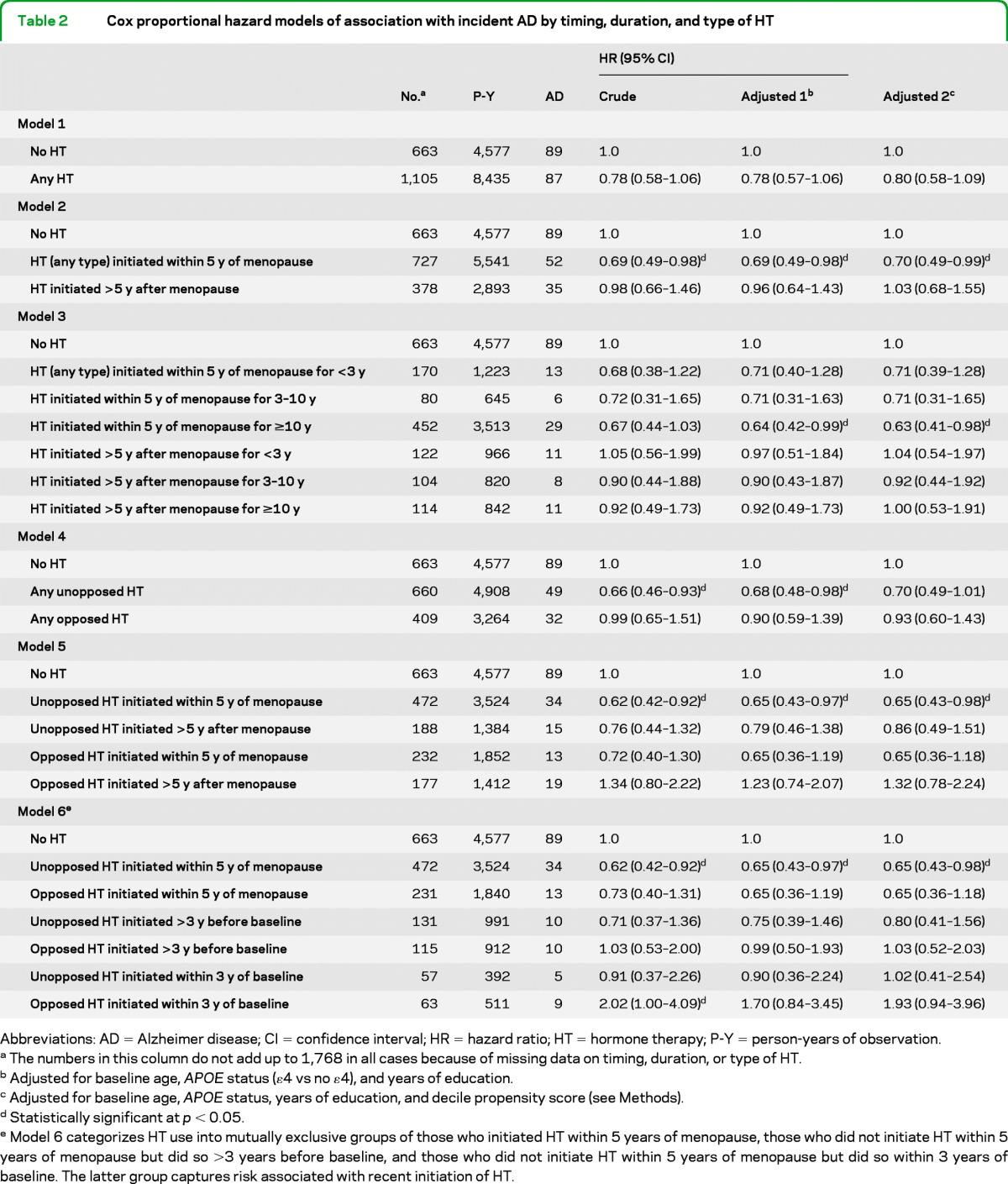
Abbreviations: AD = Alzheimer disease; CI = confidence interval; HR = hazard ratio; HT = hormone therapy; P-Y = person-years of observation.
The numbers in this column do not add up to 1,768 in all cases because of missing data on timing, duration, or type of HT.
Adjusted for baseline age, APOE status (ε4 vs no ε4), and years of education.
Adjusted for baseline age, APOE status, years of education, and decile propensity score (see Methods).
Statistically significant at p < 0.05.
Model 6 categorizes HT use into mutually exclusive groups of those who initiated HT within 5 years of menopause, those who did not initiate HT within 5 years of menopause but did so >3 years before baseline, and those who did not initiate HT within 5 years of menopause but did so within 3 years of baseline. The latter group captures risk associated with recent initiation of HT.
There were apparent differences in the association with AD risk by type of HT. Unopposed HT was associated with a trend toward reduced AD risk (model 4: aHR 0.70; 95% CI 0.49–1.01) whereas opposed HT was not. If initiated within 5 years after menopause, however, both unopposed and opposed HT were associated with a similar reduction in AD risk (model 5), although only the former was statistically significant. There was no association of AD risk with either type of HT use initiated more than 5 years after menopause. Interestingly, opposed HT initiated within 3 years of the baseline was associated with an increased, albeit nonsignificant, risk of AD (model 6: aHR 1.93; 95% CI 0.94–3.96) that was not observed with unopposed HT. Similar results were obtained in analyses of all-cause dementia (n = 248) as an outcome (table e-1 on the Neurology® Web site at www.neurology.org), or after stratifying the data by history of natural vs surgical menopause (after partial or bilateral oophorectomy), although some of the models could not be reliably estimated due to the smaller number of women with surgical menopause (data not shown).
DISCUSSION
We report new results from the CCS on the relationship between HT use and incident AD. Our findings extend earlier findings by inclusion of up to 7 years of additional follow-up and more detailed data on the timing, duration, and type of HT. Consistent with the hypothesis of a critical window around the time of menopause during which HT may protect against AD, we observed a 30% reduction in AD risk in HT users who initiated treatment within 5 years after menopause. This reduction was greater with sustained use of 10 years or more. We saw no differences in these associations with use of opposed vs unopposed. If HT was initiated later, there was no such association and there was some suggestion that opposed HT was associated with increased AD risk if initiated shortly before the study's baseline.
The nature of the relationship between HT and AD risk remains controversial. Although findings of previous observational studies have not always been consistent, 2 meta-analyses estimated the risk reduction at 29% to 44%.23,24 By contrast, the randomized WHIMS6,7 found that HT use was associated with a significant increase in dementia risk. The divergence of results between observational and randomized studies has been a source of puzzlement. One explanation posits existence of a critical time window around menopause during which HT is protective. Many observational studies might capture this association but the WHIMS trial, which enrolled women older than 65 years, would not.8
Our original report from the CCS5 provided limited evidence in support of the critical window hypothesis. It noted that reduction in AD risk was greatest with prior HT use, whereas current HT users showed reduced risk only if the duration of treatment was for more than 10 years. Presumably, the last group included some women who had initiated treatment around the time of menopause. At the time, we were unable to test this notion directly, but a large subsequent retrospective study provided further circumstantial evidence in support of it,25 showing an inverse association between HT and AD risk for younger women who used HT near the time of menopause but not for older women who used HT at other times. More recently, a large prospective study found that women who used HT only in midlife had a 26% lower risk of dementia compared with nonusers.26 The CCS findings reported here show point estimates (0.74) for AD risk with HT initiated around menopause (i.e., in midlife) that are nearly identical to those of the last study.
We also found a suggestive increase in AD risk associated with HT initiated later in life, i.e., close to the CCS baseline when all participants were 65 years or older (average age at study entry was approximately 75 years). This increase was similar to findings from a recent large prospective study26 and the WHIMS trial. Interestingly, our finding of an increase specific to opposed HT also echoed the results of the WHIMS trial, which found that the increase in AD risk was greater with opposed (HR 2.05; 95% CI 1.21–3.48) than with unopposed treatment (HR 1.49; 95% CI 0.83–2.66).
Our study has several limitations. Women who used HT may have differed from nonusers in ways that explain their lower AD risk. For example, women who took HT were younger and better educated, younger at menopause, and had fewer live births. Control on these and other potential confounders in our analyses may have been incomplete, or there may have been other unmeasured characteristics that we did not consider. We note, however, that adjustment for potential measured confounders that might be associated with HT use or AD risk did not change the present results meaningfully. Alternatively, HT initiated around menopause could have increased mortality among women who were more vulnerable to develop AD in later life, thus creating a misleading impression of reduced risk in these women. This explanation seems unlikely given that mortality during the follow-up interval was lower, if anything, among HT users, including in particular those who initiated HT within 5 years of menopause (data not shown). There was also a significantly longer lag between age at menopause and baseline for nonusers compared with users. AD events among women occurring during this period of time might be undetected by the current study, potentially biasing the results. However, one might expect a greater number of events undetected among nonusers given their longer lag time, tending to bias the results toward a lower AD risk among nonusers. This is the opposite of what we found. Finally, the observed inverse association might reflect recall bias if women who developed AD during the follow-up interval had experienced prodromal memory problems that made them less likely to report HT use decades earlier, around menopause (note that 37 women had a nondementia cognitive impairment at the baseline visit). To explore this possibility, we repeated our analyses after controlling for baseline 3MS as a measure of cognitive status, but results were virtually identical (data not shown).
Our study also has several strengths. It is one of only a few studies with sufficient data to examine the long-term relationship between HT use around menopause and subsequent AD risk decades later. Although our results were similar to the aforementioned recent observational study,26 the latter was limited by its reliance on clinical databases of patient encounters with community-based physicians for case identification. Instead, we used standardized methods of case detection based on direct contact and validated for research purposes.20,27,28 Our study was also differentiated by availability of specific data on the timing and type of HT used, whereas others (e.g., reference 26) could only contrast “midlife” vs “late-life” use. Our study's reliance on a relatively homogeneous population in rural Utah might conceivably limit the generalizability of our findings, but there are also strengths in comparisons conducted within restricted populations that offer reduced chance of selection or other biases producing spurious results.
Whereas HT was once thought to offer promise for AD prevention, the WHIMS trial cast serious doubts on its utility and effectively discouraged continued research in this area. Importantly, however, the results reported here add to a nascent body of findings suggesting that HT may have neuroprotective effects that depend on when it is initiated. Our findings therefore provide a rationale for continued research into the varied effects of HT. Practical concerns make it unlikely that new randomized trials of HT can examine the timing effects of HT (because of the long periods of follow-up necessary to properly test the critical window hypothesis). Such trials could also raise ethical concerns, given the current view that HT use would pose confirmed risks to trial participants. We suggest that other forms of new evidence will be needed before findings such as the current CCS results should reasonably result in any clinical recommendation of HT use for the prevention of AD.29
Supplementary Material
ACKNOWLEDGMENT
The authors thank the study participants for their contribution to the study; the neurogenetics laboratory of the Bryan AD Research Center at Duke University for APOE genotyping; and Nancy Sassano, PhD (Utah State University), Linda Sanders, MPH, and Michelle McCart, BA (Duke University), and Cara Brewer, BA, Tony Calvert, BSC, Tiffany Newman, BA, Roxane Pfister, MS, Russell Ray, MS, Sarah Schwartz, MS, Leslie Toone, MS, and Joslin Werstak, BA (Utah State University), for their technical assistance in study coordination and data collection, entry, and analysis.
GLOSSARY
- AD
Alzheimer disease
- aHR
adjusted hazard ratio
- CCS
Cache County Study
- CI
confidence interval
- DSM-III-R
Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised
- HT
hormone therapy
- 3MS
Modified Mini-Mental State Examination
- WHIMS
Women's Health Initiative Memory Study
- WHQ
Women's Health Questionnaire
Footnotes
Cache County Study Investigators are listed on the Neurology® Web site at www.neurology.org.
Editorial, p 1840
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Drafting/revising the manuscript for content, including medical writing for content: H. Shao, Dr. Breitner, Dr. Whitmer, Dr. Wang, Dr. Hayden, Dr. Wengreen, Dr. Corcoran, Dr. Tschanz, Dr. Norton, Dr. Munger, Dr. Welsh-Bohmer, Dr. Zandi. Study concept or design: Dr. Breitner, Dr. Welsh-Bohmer, Dr. Hayden, Dr. Tschanz, Dr. Norton, Dr. Munger, Dr. Corcoran, Dr. Whitmer, Dr. Zandi. Analysis or interpretation of data: H. Shao, Dr. Breitner, Dr. Welsh-Bohmer, Dr. Tschanz, Dr. Norton, Dr. Munger, Dr. Hayden, Dr. Whitmer, Dr. Zandi. Acquisition of data: Dr. Breitner, Dr. Welsh-Bohmer, Dr. Hayden, Dr. Tschanz, Dr. Norton, Dr. Munger, Dr. Zandi. Statistical analysis: H. Shao, Dr. Zandi. Study supervision or coordination: Dr. Breitner, Dr. Welsh-Bohmer, Dr. Zandi. Obtaining funding: Dr. Breitner, Dr. Welsh-Bohmer, Dr. Hayden, Dr. Tschanz, Dr. Norton, Dr. Munger, Dr. Corcoran, Dr. Zandi.
DISCLOSURE
H. Shao reports no disclosures. J.C.S. Breitner and R. Whitmer are funded by grants from the National Institutes of Health. J. Wang reports no disclosures. K. Hayden, H. Wengreen, C. Corcoran, J. Tschanz, M. Norton, R. Munger, K. Welsh-Bohmer, and P. Zandi are funded by grants from the National Institutes of Health.
REFERENCES
- 1.Henderson VW. The epidemiology of estrogen replacement therapy and Alzheimer's disease. Neurology 1997; 48: S27– S35. [DOI] [PubMed] [Google Scholar]
- 2.Henderson VW. Estrogen-containing hormone therapy and Alzheimer's disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience 2006; 138: 1031– 1039. [DOI] [PubMed] [Google Scholar]
- 3.Tang MX, Jacobs D, Stern Y, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 1996; 348: 429– 432. [DOI] [PubMed] [Google Scholar]
- 4.Kawas C, Resnick S, Morrison A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology 1997; 48: 1517– 1521. [DOI] [PubMed] [Google Scholar]
- 5.Zandi PP, Carlson MC, Plassman BL, et al. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 2002; 288: 2123– 2129. [DOI] [PubMed] [Google Scholar]
- 6.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289: 2651– 2662. [DOI] [PubMed] [Google Scholar]
- 7.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004; 291: 2947– 2958. [DOI] [PubMed] [Google Scholar]
- 8.Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: a critical time. JAMA 2002; 288: 2170– 2172. [DOI] [PubMed] [Google Scholar]
- 9.Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology 1999; 53: 321– 331. [DOI] [PubMed] [Google Scholar]
- 10.Miech RA, Breitner JC, Zandi PP, et al. Incidence of AD may decline in the early 90s for men, later for women: the Cache County study. Neurology 2002; 58: 209– 218. [DOI] [PubMed] [Google Scholar]
- 11.Richards B, Skoletsky J, Shuber AP, et al. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Hum Mol Genet 1993; 2: 159– 163. [DOI] [PubMed] [Google Scholar]
- 12.Saunders AM, Roses AD. Apolipoprotein E4 allele frequency, ischemic cerebrovascular disease, and Alzheimer's disease. Stroke 1993; 24: 1416– 1417. [DOI] [PubMed] [Google Scholar]
- 13.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987; 48: 314– 318. [PubMed] [Google Scholar]
- 14.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med 1994; 24: 145– 153. [DOI] [PubMed] [Google Scholar]
- 15.Silverman JM, Breitner JC, Mohs RC, Davis KL. Reliability of the family history method in genetic studies of Alzheimer's disease and related dementias. Am J Psychiatry 1986; 143: 1279– 1282. [DOI] [PubMed] [Google Scholar]
- 16.Tschanz JT, Welsh-Bohmer KA, Skoog I, et al. Dementia diagnoses from clinical and neuropsychological data compared: the Cache County study. Neurology 2000; 54: 1290– 1296. [DOI] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34: 939– 944. [DOI] [PubMed] [Google Scholar]
- 18.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993; 43: 250– 260. [DOI] [PubMed] [Google Scholar]
- 19.Tatemichi TK, Sacktor N, Mayeux R. Dementia associated with cerebrovascular disease, other degenerative diseases, and metabolic disorders. In: Terry RD, Katzman R, Bick KL. eds. Alzheimer Disease. New York: Raven Press; 1994: 123– 166. [Google Scholar]
- 20.Hayden KM, Khachaturian AS, Tschanz JT, et al. Characteristics of a two-stage screen for incident dementia. J Clin Epidemiol 2003; 56: 1038– 1045. [DOI] [PubMed] [Google Scholar]
- 21.Fearing MA, Bigler ED, Norton M, et al. Autopsy-confirmed Alzheimer's disease versus clinically diagnosed Alzheimer's disease in the Cache County Study on Memory and Aging: a comparison of quantitative MRI and neuropsychological findings. J Clin Exp Neuropsychol 2007; 29: 553– 560. [DOI] [PubMed] [Google Scholar]
- 22.Wengreen HJ, Nelson C, Munger R, Corcoran C. DASH diet adherence scores and cognitive decline and dementia among aging men and women: Cache County Study of Memory Health and Aging. Alzheimers Dement 2009; 5: 128. 19328441 [Google Scholar]
- 23.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA 1998; 279: 688– 695. [DOI] [PubMed] [Google Scholar]
- 24.Hogervorst E, Williams J, Budge M, et al. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience 2000; 101: 485– 512. [DOI] [PubMed] [Google Scholar]
- 25.Henderson VW, Benke KS, Green RC, et al. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J Neurol Neurosurg Psychiatry 2005; 76: 103– 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitmer RA, Quesenberry CP, Zhou J, Yaffe K. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol 2011; 69: 163– 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khachaturian AS, Gallo JJ, Breitner JC. Performance characteristics of a two-stage dementia screen in a population sample. J Clin Epidemiol 2000; 53: 531– 540. [DOI] [PubMed] [Google Scholar]
- 28.Plassman BL, Khachaturian AS, Townsend JJ, et al. Comparison of clinical and neuropathologic diagnoses of Alzheimer's disease in 3 epidemiologic samples. Alzheimers Dement 2006; 2: 2– 11. [DOI] [PubMed] [Google Scholar]
- 29. North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause 2010; 17: 242– 255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


