Abstract
Objective:
To study the incidence and pattern of neurologic disorders in a large cohort of HIV-positive men, compared with HIV-negative men, in the era of highly active antiretroviral therapy (HAART).
Methods:
The Multicenter AIDS Cohort Study is a prospective study of men who have sex with men enrolled in 4 cities in the United States. We compared HIV-positive vs HIV-negative men for incidence and category of neurologic diagnoses in the HAART era (July 1, 1996, to last known follow-up or death, on or before July 1, 2011).
Results:
There were 3,945 participants alive during the HAART era (2,083 HIV negative, 1,776 HIV positive, and 86 who became infected with HIV during the study period) including 3,427 who were older than 40 years of age. Median age at first neurologic diagnosis among all participants alive in the HAART era was lower in HAART-treated HIV-positive vs HIV-negative men (48 vs 57 years of age, p < 0.001). Incidence of neurologic diagnoses was higher in HAART-treated HIV-positive vs HIV-negative men (younger than 40 years: 11.4 vs 0 diagnoses per 1,000 person-years [p < 0.001]; 40–49 years: 11.6 vs 2.0 [p < 0.001]; 50–60 years: 15.1 vs 3.0 [p < 0.001]; older than 60 years: 17.0 vs 5.7 [p < 0.01]). Excess neurologic disease was found in the categories of nervous system infections (p < 0.001), dementia (p < 0.001), seizures/epilepsy (p < 0.01), and peripheral nervous system disorders (p < 0.001), but not stroke (p = 0.60).
Conclusions:
HIV-positive men receiving HAART have a higher burden of neurologic disease than HIV-negative men and develop neurologic disease at younger ages.
More than 30 million adults are infected with HIV globally.1 The introduction of highly active antiretroviral therapy (HAART) has improved survival among people infected with HIV and leads to long life expectancies among those who have access to and regularly take HAART. This decade is the first opportunity to study a large cohort of HIV-infected individuals of all age groups who are expected to approximate the life expectancy of those who are uninfected.2,3
In the early AIDS epidemic, neurologic disorders were a major cause of morbidity and mortality among people with HIV because of direct infection of the nervous system and susceptibility to opportunistic infections of the nervous system. Later, effects of antiretroviral therapy led to concerns of neurotoxicity among people treated for HIV. In the current era of HAART, other neurologic disorders, such as ischemic stroke, are becoming increasingly important to assess among people with chronic, treated HIV infection. An increased risk of neurologic disorders in the adult HIV-positive population receiving HAART would have important scientific and policy implications in many countries.
Using data from the Multicenter AIDS Cohort Study (MACS), a large, ongoing, prospective study of men who have sex with men (MSM) in 4 cities in the United States, we report the distribution and incidence of neurologic diagnoses in HIV-1–positive men during 15 years of the HAART era. We compare the incidence of neurologic diagnoses by decade of life in HIV-positive men compared with HIV-negative men of similar lifestyle, behaviors, and age in the same cohort. We also expand the time period over which the mean incidence of key HIV-1–associated neurologic diseases has been reported in the MACS (previously 1985–19924 and 1990–19985) including HIV dementia, cryptococcal meningitis, toxoplasmosis, progressive multifocal leukoencephalopathy (PML), and CNS lymphoma (CNSL).
METHODS
Ethics approval.
The MACS was approved by the Institutional Review Board at each site, and informed consent was obtained from each participant. The analysis of neurologic diagnoses was approved by The Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Cohort.
Since 1984, the MACS enrolled 6,992 men in Baltimore, Chicago, Los Angeles, and Pittsburgh. The MACS was designed to understand early pathophysiologic events in the course leading to AIDS and AIDS-related conditions, including infections, sexual practices, and specific psychoactive drugs.6 In the early 1980s, MSM represented the optimal population in which rates were high enough to provide a meaningful longitudinal inquiry into the study of AIDS with sufficient long-term follow-up.6 HIV-negative MSM in the MACS were considered as a control group in this study.
MACS participants were enrolled in various ways. This included active recruitment through media, personal connections with activists and enrolled study participants (for recruitment after 1984), communication with physicians and clinics with high numbers of MSM, and drawing from preexisting cohorts of MSM.
MACS participants continue to undergo a standardized in-person interview, clinical evaluation, neurologic screening tests, and laboratory tests every 6 months. Participants also provide information on preselected reportable diseases including seizure and stroke, current medications including HAART, medication adherence, interim medical history, health behaviors, and activities of daily living. Each visit involves testing of HIV-negative men for possible HIV seroconversion, ensuring continued enrollment in the HIV-negative group. All HIV-positive men are tested for T-cell subsets and HIV viral load at each visit, using Roche ultrasensitive assay (<50 copies/mL; Roche Molecular Systems, Somerville, NJ), and standardized and quality-controlled flow cytometry.7
Ascertainment of neurologic diagnoses.
Neurologic diagnoses were preselected by the authors using ICD-9 codes and searched among all participants in the MACS. Additional searches were performed by selecting neurologic conditions from the MACS status form, which includes Centers for Disease Control and Prevention–defined diagnoses such as PML, CNSL, and 24 other neurologic conditions of relevance including primary HIV-related and non–HIV-related dementia, neuropathies, myelopathies, myopathies, and meningitides.
Self-report forms were searched to ascertain whether the participant reported a stroke or seizure since his last study visit. Each chart of the person who self-reported a neurologic event was verified by request of medical records, and 1 neurologist (F.J.M.), blinded to the participant's HIV serostatus, reviewed all available MACS participants' files for accuracy and confirmation of neurologic diagnoses.
Neurologic diagnoses that could be confirmed by receipt of records from medical providers are included in the analysis presented in this report. Self-report data that could not be confirmed by review of available medical records were deemed “unconfirmed” and reported separately in the Results and in table e-1 on the Neurology® Web site at www.neurology.org.
In the case of seizures, if a participant was reported to have the diagnosis “seizure” on multiple occasions, only the first seizure was considered to be a neurologic diagnosis. Subsequent seizures were excluded as events and the diagnosis was considered to be a “seizure disorder.” Data on injuries were not routinely collected in the MACS; traumatic injuries of the nervous system were therefore excluded from this analysis. Diagnoses related to asymptomatic or incidental findings on neuroimaging, such as carotid stenosis, were also excluded.
Deaths are continuously monitored by the MACS and all deaths are attempted to be recorded by the study. All death certificate diagnoses were searched for neurologic diagnoses. Death due to a neurologic disease was considered as a neurologic diagnosis in this study. Participants who died were no longer considered at risk after death.
Categorization of neurologic diagnoses.
Neurologic diagnoses were categorized as follows: 1) infectious diseases, including opportunistic and comorbid nervous system infections; 2) dementia and neurodegenerative conditions, including HIV-associated dementia and dementia related to alcohol and other causes; 3) seizures and epilepsy; 4) stroke; 5) peripheral neuropathies and myopathies; 6) myelopathies; and 7) other neurologic diagnoses, such as multiple sclerosis, neurofibromatosis, and other diseases not categorized in the above groups. Diagnoses were based on the medical charts, neurologic examinations, and neuropsychological testing where appropriate. Consensus diagnoses were made by neurologists and neuropsychologists from all MACS sites. Dementia diagnoses conformed to the criteria for HIV dementia established by the American Academy of Neurology in 1991.8
Definition of the HAART era.
HAART became available to more than 50% of MACS participants by July 1, 1996.9 The HAART era in this study is defined as July 1, 1996, to present, although the main medications that comprise antiretroviral therapy have changed over time. All neurologic diagnoses reported from July 1, 1996, until the participant's last known study visit or death before July 1, 2011, were considered within the HAART era and included in this analysis.
Statistical analysis.
Statistical calculations were implemented using R (version 2.12.0, 2011; R Foundation for Statistical Computing, Vienna, Austria). Missing values were excluded on a study visit basis for both exploratory analyses and incidence estimation. Incidence of disease, stratified by age group, HIV status, and HAART status, was modeled using Poisson regression models of subject-specific periods of person-time contributions. Estimates of model parameters were obtained using generalized estimating equations.10 Robust standard errors were used when performing two-sided hypothesis testing and constructing confidence intervals. Similar Poisson models fitted via generalized estimating equations were used to compare the diagnostic category-specific incidence rates between HIV-positive and HIV-negative individuals. The distribution of age at neurologic diagnosis was compared between HIV-positive and -negative subjects using the Wilcoxon rank sum test. For descriptive purposes, men over the age of 40 were also reported as a subgroup in some cases. The participant's 40th birthday represented a cutoff point, decided by the MACS team a priori, that could be used to depict participants in middle and older age groups yet still have an adequate sample size to represent a large group of MACS participants.
RESULTS
Cohort characteristics.
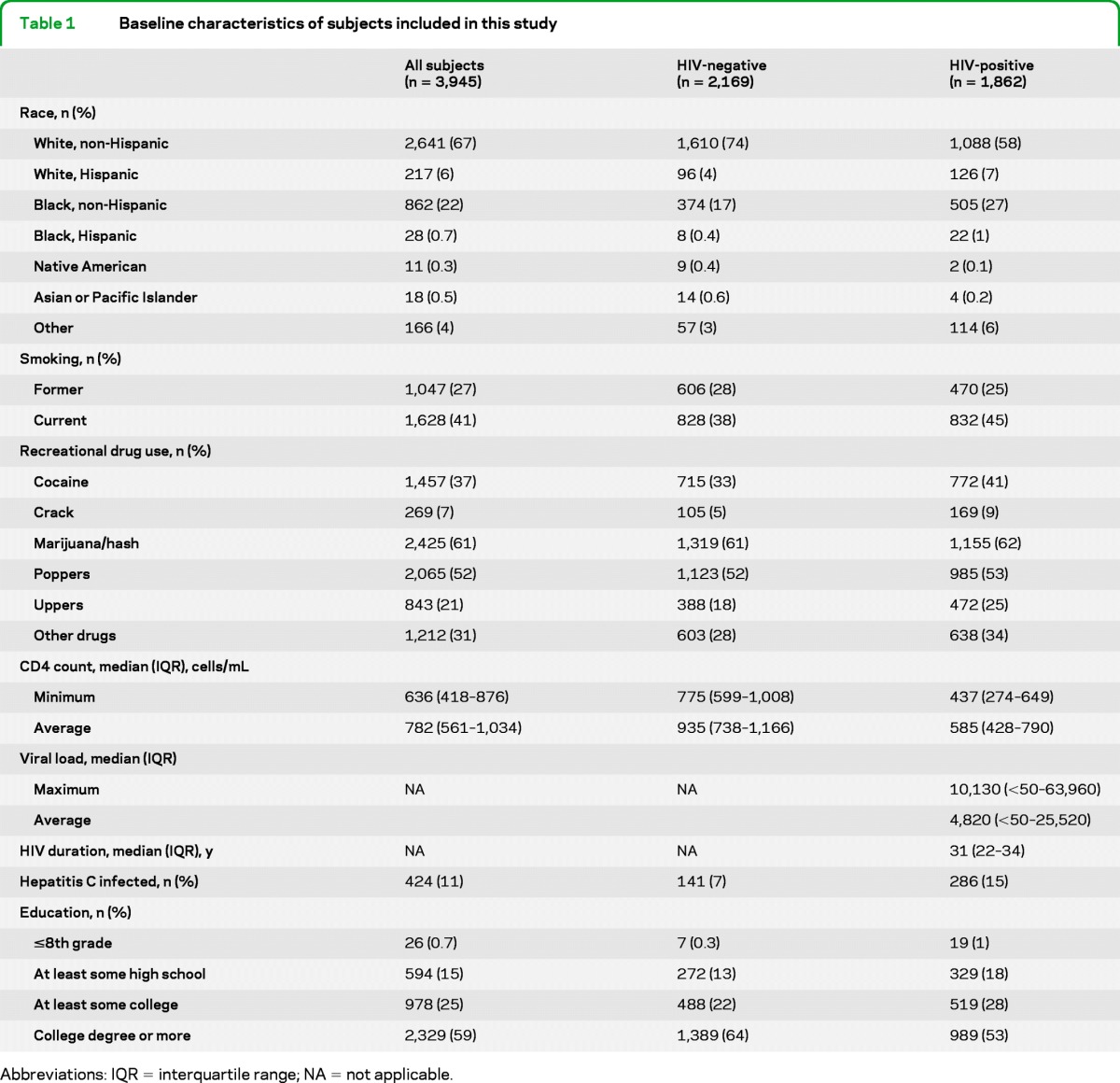
There were 3,945 MACS participants alive during the HAART era including 2,083 HIV-negative men, 1,776 HIV-positive men, and 86 men who converted from HIV-negative to HIV-positive status during the period (table 1). The total time at risk was 38,780 person-years (21,621 HIV negative and 17,159 HIV positive). By age group, the number of persons contributing person-time by age was as follows: n = 1,584 for younger than 40 years of age (754 HIV negative and 859 HIV positive), n = 2,739 for 40 to 49 years old (1,406 HIV negative and 1,374 HIV positive), n = 2,091 for 50 to 60 years old (1,226 HIV negative and 879 HIV positive), and n = 886 for older than 60 years (607 HIV positive and 281 HIV negative). The average duration of HAART was 10.6 years for all HIV-positive participants.
Table 1.
Baseline characteristics of subjects included in this study
Abbreviations: IQR = interquartile range; NA = not applicable.
Men 40 years of age and older.
There were 3,427 individuals 40 years and older (age range, 40–88 years) alive during the HAART era. Among HIV-positive participants 40 years and older, 593 men (39%) turned 40 years old while enrolled in the MACS and 946 (61%) entered after their 40th birthday. Among men older than 40 years in the MACS, the average duration of HAART was similar (11.4 years).
Confirmed neurologic diagnoses.
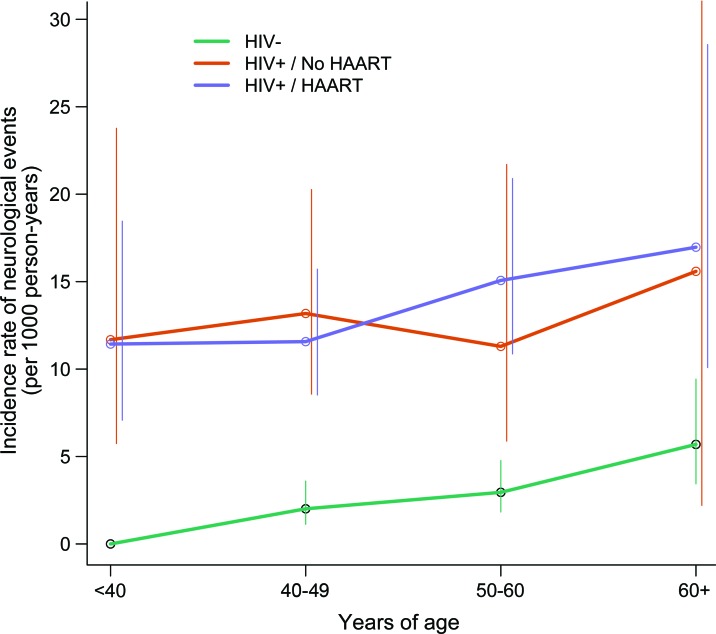
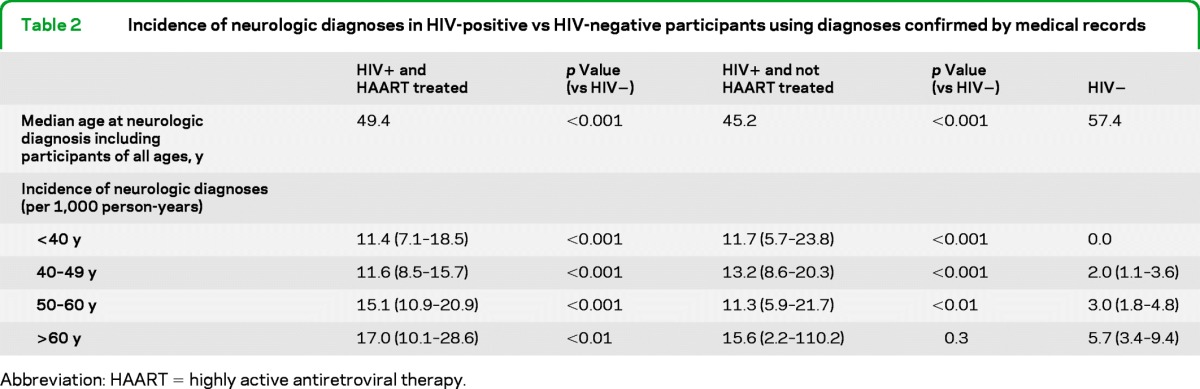
There were 279 neurologic diagnoses: 49 among HIV-positive men never taking HAART, 221 among HIV-positive men taking HAART, and 58 among HIV-negative men. Among MACS participants aged 40 years and older (age range, 40–82 years), there were 241 neurologic diagnoses: 36 among HIV-positive men never taking HAART, 147 among HIV-positive men taking HAART, and 58 among HIV-negative men (figure). One participant could have more than 1 neurologic diagnosis. Overall, 21% of HIV-positive participants with a neurologic diagnosis were not receiving HAART at the time of the neurologic diagnosis. The incidence of all confirmed neurologic events by HIV status and decade of adult life is provided in table 2. Median age at first neurologic diagnosis among all participants alive in the HAART era was lower in HAART-treated HIV-positive vs HIV-negative men (48 vs 57 years old, p < 0.001).
Figure. Incidence of neurologic events in the Multicenter AIDS Cohort Study by HIV and highly active antiretroviral therapy (HAART) status.
Table 2.
Incidence of neurologic diagnoses in HIV-positive vs HIV-negative participants using diagnoses confirmed by medical records
Abbreviation: HAART = highly active antiretroviral therapy.
Combined confirmed and unconfirmed neurologic diagnoses.
The incidence of neurologic diagnoses, when including self-reported cases that could be neither confirmed nor refuted by medical chart review, is higher (total number of participants with neurologic diagnoses, n = 367; HIV negative, n = 97 diagnoses; HIV positive receiving HAART, n = 217 diagnoses; and HIV positive not receiving HAART, n = 53 diagnoses). For unconfirmed and confirmed neurologic diagnoses combined, the median age at first neurologic diagnosis among all participants alive in the HAART era was 50 years (48 years for HIV-negative vs 56 years for HIV-positive participants, p < 0.001). Table e-1 provides the incidence estimation for combined confirmed and unconfirmed neurologic diagnoses for HIV-positive vs HIV-negative participants.
Pattern of neurologic diagnoses.
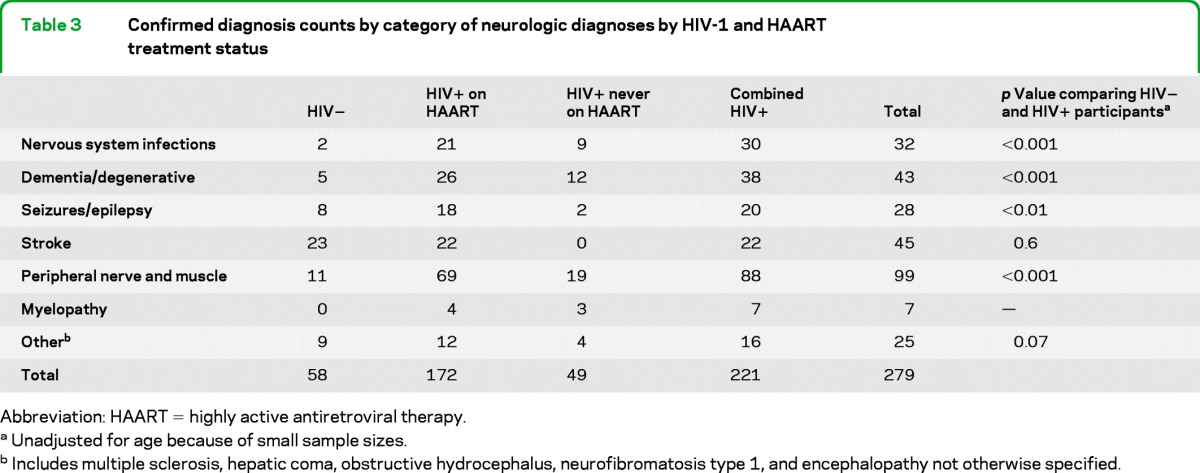
Description of neurologic diagnoses among men by disease category and HIV treatment status is given in table 3. The major excess burden of neurologic disorders among older HIV-positive participants occurred in the categories of infectious illnesses, dementia, seizures, and peripheral nervous system disorders. Peripheral neuropathy was the most frequently reported neurologic diagnosis among HIV-positive participants. Distinct entities included painful sensory peripheral neuropathy, toxic neuropathy, entrapment neuropathy, other HIV-related neuropathy, and demyelinating polyneuropathy.
Table 3.
Confirmed diagnosis counts by category of neurologic diagnoses by HIV-1 and HAART treatment status
Abbreviation: HAART = highly active antiretroviral therapy.
Unadjusted for age because of small sample sizes.
Includes multiple sclerosis, hepatic coma, obstructive hydrocephalus, neurofibromatosis type 1, and encephalopathy not otherwise specified.
There were 35 diagnoses of neurologic infections, excluding HIV-neurocognitive disorders and peripheral neuropathies, among HIV-positive participants, including 11 diagnoses in participants who were HIV positive but never took HAART. Diagnoses included acute hepatitis C with hepatic coma (n = 1), aspergillus infection of the CNS (n = 1), cryptococcal meningitis (n = 8), cytomegalovirus polyradiculitis (n = 2), herpes infection of the CNS (n = 1), HIV aseptic or unspecified viral meningitis (n = 7), HIV vacuolar myelopathy (n = 5), neurosyphilis (n = 5), PML (n = 3), and CNSL (n = 2).
An increased incidence of stroke among HIV-infected MSM was not found in this study when considering chart-confirmed cases of stroke. However, when considering both confirmed and unconfirmed self-reported stroke diagnoses together, the incidence of stroke was higher in HIV-infected vs HIV-uninfected MSM (57 vs 38 cases, p < 0.01).
In table 4, the mean incidence rates of selected HIV-associated neurologic diseases in men of all ages during the HAART era are compared with reports of incidence rates in this same cohort during earlier time periods. All cases in the most recent observation period were confirmed by review of the participants' medical records.
Table 4.
Mean incidence rate of HIV-1–associated neurologic diseases during 4 time periods in the Multicenter AIDS Cohort Study, all ages (expanded from reference5)
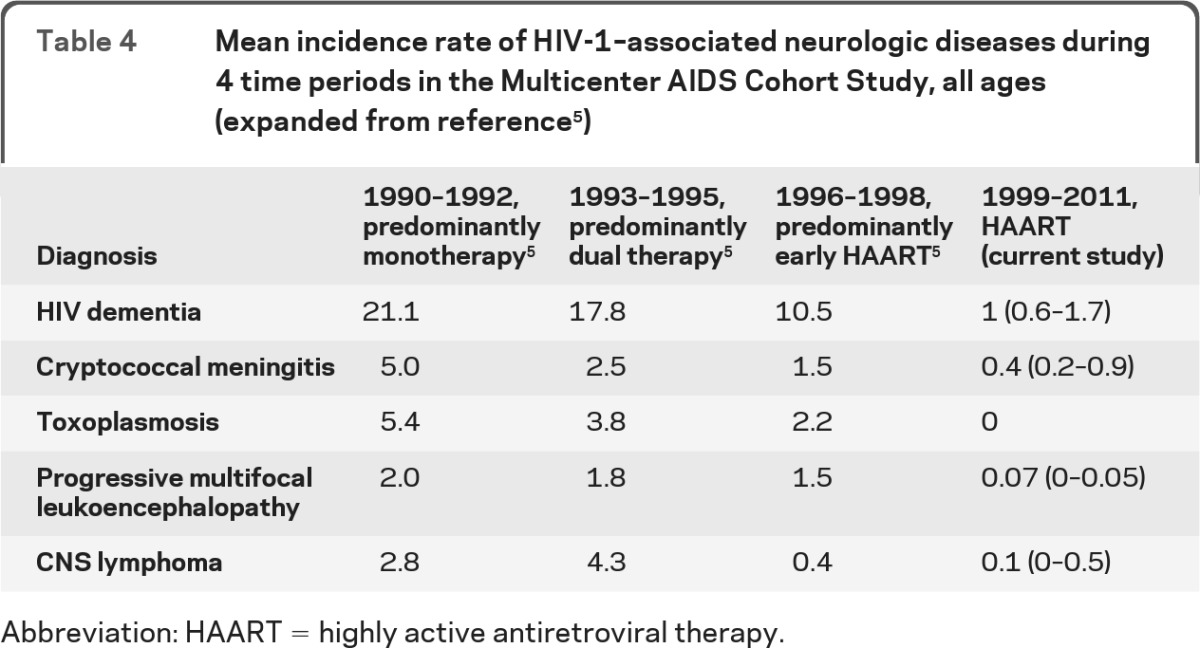
Abbreviation: HAART = highly active antiretroviral therapy.
DISCUSSION
MSM represent more than half of all cases of HIV among males in the United States10 and the incidence of HIV among MSM has risen since the early 1990s.11 In this large cohort study, we have found that HIV-positive MSM in the HAART era experience a higher incidence of neurologic disease compared with their age-matched HIV-negative counterparts. HIV-positive men also experience neurologic diagnoses at an earlier age. The majority of neurologic disorders involved the peripheral nervous system, but neuroinvasive infections and cognitive disorders were also important burdens of neurologic disease in HIV-positive MSM. The higher burden of neurologic disorders experienced in HIV-positive MSM represents potentially treatable and preventable neurologic disease.
By using data from the MACS, we extend the reporting of the incidence of key neurologic diseases that have been monitored since the beginning of the HIV epidemic. There is a low but persistent burden of HIV-related infectious neurologic diseases, including cryptococcal meningitis, PML, CNSL, and HIV dementia. In the HAART era, these diseases were found mostly among the diagnosed but untreated HIV-positive MSM. No cases of CNS toxoplasmosis were observed after 1998.
HIV-positive MSM, untreated with HAART, comprise a significant minority of HIV-positive participants with neurologic disorders in this study. In the HAART era, 21% of MACS participants with HIV and a first neurologic diagnosis were not on a HAART regimen. This has led to the continued observation of the untreated history of HIV infection and its sequelae. Approximately one-third of neurologic infectious diagnoses and HIV dementia cases in HIV-positive men were reported among HAART-naive individuals. This relatively large untreated group has important implications for continued efforts to improve access to care for people with HIV and to ensure availability of HIV testing and HAART treatment.
More than 85% of HIV-positive men in this study were older than 40 years, providing an initial look at neurologic diseases in a large middle-aged and elderly cohort of HAART-treated HIV-positive men. The MACS was not designed to study diseases of aging and indeed aimed to recruit participants younger than 60 years during initial enrollment.6 However, the MACS is now useful to examine broad estimations of incidence of neurologic disease in older HIV-positive individuals. The possibility that the nervous system ages differently in people with chronic HIV infection, even in the setting of long-term suppression of HIV viral load, suggests that aging with HIV may lead to a different pattern or earlier burden of neurologic disorders.
Previous clinical studies have found multiple explanations for this higher neurologic disease incidence. HIV testing among older persons is delayed compared with younger individuals,12 and older HIV-positive men are more likely to present with AIDS.13,14 Thus, the incidence in all HIV-positive MSM may be even higher than observed in this study.
There are other explanations for an actual increase in incidence. HAART is an incomplete treatment for HIV infection and imperfectly prevents HIV-related nervous system manifestations, most notably peripheral neuropathy and cognitive disorders.15,16 HAART itself may be related to neurotoxicity. This may occur directly, as in the case of length-dependent toxic polyneuropathy, or indirectly, by increasing the tendency toward important risk factors, such as hyperglycemia. It remains uncertain whether long-term HAART has any adverse effects on the CNS, although a toxic effect has been suggested.17 Notably, HIV infection is often associated with other high-risk behaviors, nervous system diagnoses, and physiologic risk profiles that act synergistically or are comorbid with HIV infection. Some examples of this include IV injection drug use, head trauma, alcohol abuse, and hepatitis C and neurosyphilis coinfections. Given that the opportunity to study older HIV-positive individuals has heretofore been limited, the higher burden of neurologic diagnoses in older HIV-positive men may have multiple underlying causes, not all of which can be fully explained in an incidence study.
Our study has limitations. The number of HIV-positive individuals older than 60 years is not high and inferences to the eldest age groups should be made with caution. In the MACS, it is likely that neurologic diagnoses are incompletely captured rather than overcounted, contributing to a conservative estimation of incidence. To demonstrate the difficulties with chart confirmation of all neurologic diagnoses from multiple centers over 15 years, we have presented estimates based on diagnoses that could be confirmed with medical records review by the MACS team. To provide a comparative estimation, we also include all cases, including confirmed and unconfirmed diagnoses, as a supplementary table. These estimates are overall similar but demonstrate that some events can be difficult to confirm. Importantly, results based on self-report of participants may differ from medically diagnosed cases. Ascertainment of certain diagnoses, including stroke and epilepsy, were not initially recorded in the MACS and longitudinal estimations are therefore difficult. The number of HIV-related peripheral neuropathy and dementia diagnoses may be undercounted, particularly given the more recent introduction of newer forms of HIV-associated neurocognitive disorder, such as asymptomatic neurocognitive impairment and mild neurocognitive disorder. Dropout bias may have occurred. Participants experiencing or at risk for severe neurologic disease may have been lost to follow-up in the MACS. The generalizability of these findings among MSM to other high-risk groups for HIV infection with different health behaviors, such as IV drug users, or to women with chronic HIV infection, is not known.
However, this study, the largest US cohort study of HIV-positive MSM, now extending over a quarter of a century, has the advantage of utilizing a large HIV-negative control group of similar demographics and derived from the same population as the HIV-positive MSM. This cohort has been used to estimate important trends in the incidence of neurologic disorders in HIV infection since its inception in the mid-1980s. The current finding that older HIV-positive individuals have a higher burden of neurologic disease, mainly attributable to peripheral nervous system, cognitive, and infectious disorders, has important implications. The findings in the MACS are consistent with reports from Europe,18 Canada,19 Southeast Asia,20 Japan,21 Puerto Rico,22 and Nigeria23 that treated HIV-positive individuals have a relatively high incidence of various neurologic disorders.
Our findings are also consistent with a growing literature on a potentially increased burden of HIV-associated neurologic disorders in aging individuals.24–26 Middle-aged and elderly HIV-positive individuals require in-depth clinical studies to fully understand the various mechanisms that may contribute to their excess burden of neurologic disease.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the MACS administrative support staff including Mr. Jonathan Kerman, BA, Ms. Andrea Stronski, MA, and Ms. Colette Dominicus, BSc, all at The Johns Hopkins Bloomberg School of Public Health, for their assistance with data extraction and file preparation.
GLOSSARY
- CNSL
CNS lymphoma
- HAART
highly active antiretroviral therapy
- ICD-9
International Classification of Diseases, Ninth Revision
- MACS
Multicenter AIDS Cohort Study
- MSM
men who have sex with men
- PML
progressive multifocal leukoencephalopathy
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Mateen participated in the study design, data acquisition, data analysis, data interpretation, writing, and editing of the manuscript. Dr. Shinohara participated in the study design, data analysis, data interpretation, writing, and editing of the manuscript. Dr. Carone participated in the study design, data analysis, data interpretation, and editing of the manuscript. Dr. Miller participated in the data acquisition, data interpretation, and editing of the manuscript. Dr. McArthur participated in the data acquisition and editing of the manuscript. Dr. Jacobson participated in the data acquisition, data interpretation, and editing of the manuscript. Dr. Sacktor participated in the data acquisition, data interpretation, and editing of the manuscript.
STUDY FUNDING
Dr. Mateen is supported by the 2010–2012 American Brain Foundation Practice Research Fellowship Grant and the Canadian Institutes of Health Research. Dr. Shinohara is supported by NIH Epidemiology and Biostatistics of Aging Training Grant T32 AG000247. Dr. McArthur and Dr. Sacktor are supported by the NIMH Center for novel therapeutics 5P30MH075673 (principal investigator, J.C.M.). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute: UO1-AI-35042, UL1-RR025005 (GCRC), UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, and UO1-AI-35041. The MACS Web site is located at http://www.statepi.jhsph.edu/macs/macs.html.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. UNAIDS UNAIDS report on the global AIDS epidemic 2010. Available at: http://www.unaids.org/en/dataanalysis/ Accessed April 28, 2012
- 2.Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med 2011;155:209–216 [DOI] [PubMed] [Google Scholar]
- 3.Wada N, Jacobson LP, Cohen M, et al. Cause-specific life expectancies after age 35 for HIV-infected and HIV-negative individuals followed simultaneously in long-term cohort studies: 1984–2008. Am J Epidemiol (in press 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacellar H, Munoz A, Miller EN, et al. Temporal trends in the incidence of HIV-1-related neurologic diseases: Multicenter AIDS Cohort Study, 1985–1992. Neurology 1994;44:1892–1900 [DOI] [PubMed] [Google Scholar]
- 5.Sacktor N, Lyles RH, Skolasky R, et al. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology 2001;56:257–260 [DOI] [PubMed] [Google Scholar]
- 6.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987;126:310–318 [DOI] [PubMed] [Google Scholar]
- 7.Detels R, Jacobson L, Margolick J, et al. The Multicenter AIDS Cohort Study, 1983 to …. Public Health 2012;126:196–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Academy of Neurology AIDS Task Force Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection: report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology 1991;41:778–785 [DOI] [PubMed] [Google Scholar]
- 9.Jacobson LP, Li R, Phair J, et al. Evaluation of the effectiveness of highly active antiretroviral therapy in persons with human immunodeficiency virus using biomarker-based equivalence of disease progression. Am J Epidemiol 2002;155:760–770 [DOI] [PubMed] [Google Scholar]
- 10.Liang KY, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13–22 [Google Scholar]
- 11.Smith RD, Delpech VC, Brown AE, Rice BD. HIV transmission and high rates of late diagnoses among adults aged 50 years and over. AIDS 2010;24:2109–2115 [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention (CDC). HIV testing among men who have sex with men: 21 cities, United States, 2008. MMWR Morb Mort Wkly Rep 2011;60:694–699 [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention (CDC) HIV surveillance: United States, 1981–2008. MMWR Morb Mort Wkly Rep 2011;60:21:689–693 [PubMed] [Google Scholar]
- 14.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA 2008;300:520–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann Neurol 2010;67:699–714 [DOI] [PubMed] [Google Scholar]
- 17.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007;21:1915–1921 [DOI] [PubMed] [Google Scholar]
- 18.d'Arminio Monforte A, Cinque P, Mocroft A, et al. ; EuroSIDA Study Group Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol 2004;55:320–328 [DOI] [PubMed] [Google Scholar]
- 19.Vivithanaporn P, Heo G, Gamble J, et al. Neurologic disease burden in treated HIV/AIDS predicts survival. Neurology 2010; 75:1150–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright E, Brew BA, Arayawichanont A, et al. Neurologic disorders are prevalent in HIV-positive outpatients in the Asia-Pacific region. Neurology 2008;71:50–56 [DOI] [PubMed] [Google Scholar]
- 21.Yoritaka A, Ohta K, Kishida S. Prevalence of neurological complications in Japanese patients with AIDS after the introduction of HAART. Rinsho Shinkeigaku 2007;47:491–496 [PubMed] [Google Scholar]
- 22.Wojna V, Skolasky RL, Hechavarría R, et al. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol 2006;12:356–364 [DOI] [PubMed] [Google Scholar]
- 23.Oshinaike OO, Okubadejo NU, Ojini FI, Danesi MA. The clinical spectrum of neurological manifestations in HIV/AIDS patients on HAART at the Lagos University Teaching Hospital, Lagos, Nigeria. Nig Q J Hosp Med 2009;19:181–185 [DOI] [PubMed] [Google Scholar]
- 24.Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology 2004;63:822–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cherry CL, Affandi JS, Imran D, et al. Age and height predict neuropathy risk in patients with HIV prescribed stavudine. Neurology 2009;73:315–320 [DOI] [PubMed] [Google Scholar]
- 26.Becker JT, Maruca V, Kingsley LA, et al. ; Multicenter AIDS Cohort Study Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology 2012;54:113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.