Abstract
Variation in anthropometric measurements due to sexual dimorphism can be the result of genotype by sex interactions (G×S). The purpose of this study was to examine the sex-specific genetic architecture in anthropometric measurements in Alaskan Eskimos from the Genetics of Coronary Artery Disease in Alaska Natives (GOCADAN) study. Maximum likelihood based variance components decomposition methods, implemented in SOLAR, were used for GxS analyses. Anthropometric measurements included BMI, waist circumference (WC), waist/height ratio, percent body fat (%BF) and subscapular and triceps skinfolds. Except for WC, mean values of all phenotypes were significantly different in men and women (p < 0.05). All anthropometric measures were significantly heritable (p< 0.001). In a preliminary analysis not allowing for G×S interaction, evidence of linkage was detected between markers D19S414 and D19S220 on chromosome 19 for WC (LOD = 3.5), %BF (LOD = 1.7), BMI (LOD = 2.4), WHtR (LOD = 2.5), subscapular (LOD = 2.1) and triceps skinfolds (LOD = 1.9). In subsequent analyses which allowed for G×S interaction, linkage was again found between these traits and the same two markers on chromosome 19 with significantly improved LOD scores for: WC (LOD = 4.5), %BF (LOD = 3.8), BMI (LOD = 3.5), waist/height ratio (LOD = 3.2), subscapular (LOD = 3.0) and triceps skinfolds (LOD = 2.9). These results support evidence of a G×S interaction in the expression of genetic effects resulting in sexual dimorphism in anthropometric phenotypes and identify the chromosome 19q12-13 region as important for adiposity-related traits in Alaskan Eskimos.
Keywords: Abdominal obesity, Adiposity, Body composition, Linkage
Introduction
Alaskan Eskimos have been a genetically isolated population who until recent years followed a traditional lifestyle and diet, and had low rates of coronary artery disease. As late as 1965, mortality rates due to cardiovascular disease (CVD) were lower in Eskimos than in Whites in the United States (1). Since then, however, there have been many changes in diet and lifestyle of this population accompanied by a slowly increasing CVD mortality rate. This recent increase in CVD may, in large part, be attributed to the increasing availability of westernized diets along with a reduction in physical activity (2,3).
A key risk factor for CVD is abdominal obesity (4). Abdominal obesity is a major indicator of upper body fat accumulation. Increased waist circumference (WC), an important measure of abdominal obesity, is associated with several metabolic abnormalities (5). Greater visceral fat was associated with increased coronary lesions in two studies conducted in male adolescents and women < 50 years of age (6,7). In another study, WC and waist-hip ratio were independently associated with the risk of coronary heart disease in women (8). Increase in upper body fat is also associated with increased turnover of free fatty acids (9) which in turn contributes to defects in glucose metabolism leading to type 2 diabetes, dyslipidemia, hypertension, and gall bladder disease in addition to CVD.
The pattern of body fat distribution is greatly influenced by sexual dimorphism. Men tend to have more of an abdominal pattern of fat deposition, in contrast to women who tend to accumulate less fat in the abdominal region; even though the total amount of fat is greater in women than men (10). This disparity can be attributed, to a great extent, to the differences in reproductive biology (11). Given the differences in body fat distribution and the relative risk associated with related-metabolic disorders among the sexes (12,13), it is important to investigate the influence of sexual differences on genetic patterns of anthropometric measurements.
To understand the difference in sex-specific genetic architecture of anthropometric measurements we used a genotype by environment (GxE) interaction model. The GxE model can result in the same genotype giving rise to two different phenotypes in two different environments. The hormonal differences between men and women can be considered as two different environments. Genotype by sex (GxS) interaction can result in differential effects on the variation in the same trait in men and women. Thus our aim was to investigate the sex-specific genetic differences in anthropometric measurements in Alaskan Eskimos using a GxS interaction model.
Methods
Study design
The GOCADAN study recruited 1,214 individuals (over 18 years of age) from villages in the Norton Sound region of Alaska (14,15). The study population were members of multigenerational families, primarily Inupiat Eskimos. The average participation was 82.6 % in seven of the nine villages participating in the study. Of the total participants, 1,151 belong to the same extended pedigree. They are linked by marriage/matings within and between villages. All participants had a baseline examination. Diet, physical activity and medical history were recorded using a standardized interview protocol. Participants attended clinics for a blood draw following an overnight 12-hour fast. Blood was drawn by venipuncture and samples were stored in aliquots at −80 C for phenotypic analysis and DNA extraction. Physical examinations were performed along with electrocardiogram and carotid artery scans. Details of the study design, recruitment and methods have been reported previously by Howard et al (14) and Ebbesson et al (15). This study was approved by the Institutional Review Boards from all participating institutions and informed consent was obtained from all participants.
Demographic and phenotypic data
Standard demographic and genealogical data were collected during the surveys and included names, genders, dates, and places of birth, current home of the participant and his/her spouse and first degree relatives of all household members. Anthropometric measurements included height, weight, skinfolds and WC. Height was measured to the nearest quarter inch while the participant was standing, using a vertical mounted ruler; weight was determined to the nearest tenth of a pound, using a scale (Detecto, model 683-P, Cardinal Scale Mfg. Webb City, MO). Skinfolds (subscapular and triceps) were measured to the nearest millimeter with a Lange caliper. The subscapular measurement was taken 1 cm inferior to the angle of the right scapula while the participant was standing with shoulders relaxed and arms hanging loosely at his/her sides. The triceps measurement was taken directly over the right triceps muscle, halfway between the acromial and olecranon processes, with the arms hanging comfortably at the participant’s side. WC was measured at the level of the umbilicus with the subject in a supine position. Body mass index (BMI) was computed by dividing weight in kilograms by height (meters) squared. Waist-height ratio was calculated by dividing WC (cm) by height (cm).
Genotypic data
For each participant, 400 short tandem repeat (STR) markers (spaced at an average interval of 10cM throughout the genome) were amplified from genomic DNA in separate PCR reactions using fluorescently-labeled primer pairs (ABI PRISM Linkage Mapping Set MD 10 Version 2, Applied Biosystems, Foster City, CA). Pedigree and Mendelian errors were detected and corrected utilizing the software PREST (Pedigree Relationship Statistical Tests) and SIMWALK2(16). Multipoint identity-by-descent (IBD) matrices for genome-wide linkage analyses were calculated using the linkage analysis package (LOKI) (17). The chromosomal map (Haldane) used in these computations was based on marker locations reported by DeCode genetics(18).
Statistical analyses
Univariate genetic analysis
A variance components decomposition method was used to estimate heritability and linkage to chromosomal locations affecting variation in anthropometric measurements. This method is implemented in the software program SOLAR and has been described in detail elsewhere(19). All hypotheses were evaluated using standard likelihood ratio test procedures and the associated likelihood ratio test statistic (LRT). The LRT is computed as minus twice the difference in ln-likelihoods estimated under the null and alternative hypotheses, and is in standard cases distributed as a chi-square (χ2) with degrees of freedom (d.f.) given by the difference in the number of estimated or unconstrained parameters. However, as is often the case for variance components models, the non-standard case of testing a null hypothesis lying at a boundary of its acceptable parameter range yields a LRT distributed as a 50:50 mixture of a point-mass at 0 and a χ2 with 1 d.f. (20). Prior to conducting genetic analyses, distributional properties of all traits were evaluated. All values beyond four standard deviations of the mean were removed and the remaining traits were transformed by inverse normalization prior to analysis, to meet assumptions of normality.
Bivariate genetic analysis
Phenotypic, genetic and environmental correlations were calculated between plasma FAs and other adiposity-related traits as summarized by the following model:
where h12 andh22 are heritabilities of the two phenotypes being studied, and rhoG and rhoE are the additive genetic and environmental correlations between the traits, respectively(21).
To test whether the genetic correlation is significantly different from zero, a model in which all parameters were estimated was compared with a model in which the genetic correlation was constrained to zero. The LRT in this case is distributed as a χ2 with 1 d.f. To test for complete pleiotropy between the two traits, a model in which the genetic correlation was constrained to one was compared with a model in which all parameters were estimated. Since the null in this case lies at a boundary, the LRT is distributed as a 50:50 mixture of a point-mass at 0 and a χ2 with 1 d.f. Evidence of pleiotropy (a commonset of genes influencing more than one trait) was indicated bya genetic correlation significantly different from 0.
Genotype by sex interaction
To examine the sex-specific genetic architecture in anthropometric measurements, we first tested the GxS interaction in a basic polygenic model (not including linkage component). A variance components decomposition method was used to estimate the heritability. This method is implemented in the software package SOLAR, which has been described in detail previously (19). The approach for GxS interaction is an extension of the variance components decomposition approach and tests two hypotheses: 1) Whether variance due to genetic factors for men (σgM) and women (σgW) were significantly different from each other, and 2) whether the genetic correlation (ρG(GM, GW) between men and women was significantly different from one. Additive genetic variance was then modeled as the product of the genetic correlations between sexes and the sex-specific genetic standard deviations:
Where Ω is covariance between family members, Φ is kinship coefficient between the two individuals, ρG(GM, GW) is genetic correlation between the expression of the trait in men and women, σgM is genetic standard deviation of the trait in men, σgW is genetic standard deviation of the trait in women, I is identity matrix, and σe2 is environmental variance. For a GxS interaction to be significant, the genetic correlation between the two sexes should be significantly less than 1 and/or the genetic variance for men and women should not be equal. That is, rejection of either hypothesis by itself or both is taken as evidence of significant GxS interaction. For reasons discussed above, the LRT in the former case is distributed as a 50:50 mixture of a point-mass at 0 and a χ2 with 1 d.f., and in the latter case as a chi-square with 1 d.f. For future reference, we term this type of interaction polygenic GxS interaction.
To identify chromosomal locations that might be regulating the GxS interaction, we conducted a genome-linkage scan. An extension of the variance component model was used in which the phenotypic covariance among family members was modified to include GxS effects at a quantitative trait locus (QTL). In this case, the LRT is distributed as a χ2 with 1 d.f. We term this type of interaction QTL GxS interaction. All logarithm of odds (LOD) scores estimated under this model were corrected for increased degrees of freedom relative to the standard model. To verify our linkage results, we determined empirical LOD scores which are computed by multiplying the observed LOD score by a correction constant. In SOLAR, a correction constant was estimated by regressing the expected LOD scores on the observed simulated LOD scores (22,23)
RESULTS
Descriptive statistics
A total of 1,214 individuals (men = 537, women = 677) were included in these analyses. Women had higher BMI, waist-height ratio, percent body fat and skinfold measurements as compared to men (p < 0.001). WC was not significantly different between the sexes (Table 1).
Table 1.
Descriptive statistics of the participants
| Trait | Men (SEM) | Women (SEM) | p value |
|---|---|---|---|
| N | 537 | 677 | |
| Age (years) | 42 (0.68) | 42.9 (0.62) | NS |
| BMI (kg/m2) | 26.57 (0.22) | 28.55 (0.24) | < 0.001 |
| Waist circumference (inches) | 34.4 (0.21) | 34.8 (0.22) | NS |
| Waist/height ratio | 0.51 (0.003) | 0.56 (0.003) | < 0.001 |
| Body fat (%) | 31.5 (0.28) | 43.2 (0.22) | < 0.001 |
| Subscapular skinfold (cm) | 14.61 (0.33) | 20.41 (0.35) | < 0.001 |
| Triceps skinfold (cm) | 12.88 (0.28) | 21.30 (0.30) | < 0.001 |
- SEM = Standard error of mean
- NS – Not significant
Heritabilities of anthropometric measurements
The pedigrees included in the analyses for these anthropometric traits ranged between 315 and 325. All anthropometric measurements were significantly heritable and their heritabilities ranged from 0.48 to 0.58. The highest heritability was obtained for subscapular skinfold (0.58) (Table 2). Sex-specific heritabilities showed higher estimates for these measurements in women than men (Table 2).
Table 2.
Heritabilities of anthropometric traits
| All | Men | Women | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Trait | h2 (SE) | p value | h2 (SE) | p value | h2 (SE) | p value |
| BMI (kg/m2) | 0.55 (0.07) | 1.9 × 10−17 | 0.49 (0.15) | 3.7 × 10−4 | 0.69 (0.12) | 1.1 × 10−9 |
| Waist circumference (inches) | 0.55 (0.07) | 2.3 × 10−16 | 0.67 (0.16) | 3.9 × 10−5 | 0.56 (0.12) | 1.0 × 10−7 |
| Waist/height ratio | 0.53 (0.08) | 4.2 × 10−15 | 0.62 (0.16) | 1.1 × 10−4 | 0.56 (0.12) | 5.0 × 10−7 |
| Body fat (%) | 0.56 (0.07) | 3.4 × 10−17 | 0.54 (0.16) | 2.6 × 10−4 | 0.66 (0.12) | 2.4 × 10−9 |
| Subscapular skinfold | 0.58 (0.07) | 1.7 × 10−19 | 0.51 (0.17) | 1.5 × 10−3 | 0.70 (0.11) | 1.4 × 10−12 |
| Triceps skinfold | 0.48 (0.07) | 3.7 × 10−14 | 0.36 (0.16) | 0.010 | 0.50 (0.11) | 2.0 × 10−7 |
- h2 = heritability
- SE = standard error of variance
Genetic and phenotypic correlations
All anthropometric measurements were highly correlated with each other, with genetic and phenotypic correlations ranging between 0.78 and 0.96 (p < 10−10), and 0.68 and 0.92 (p < 10−125), respectively.
Genotype by sex (GxS) interaction
In evaluating GxS interaction, we tested a model with a null hypothesis of no interaction against a model where there is interaction. There are two types of interaction models. The first one tests the hypothesis that the variances due to the genetic effects in the two sexes are significantly different from each other. In our study we found genetic variances of BMI, and skinfold measures to be significantly different between the sexes (Table 4). The second hypothesis that we tested was that the genetic correlation between the sexes is significantly different from one. In our present study, we did not find genetic correlations between sexes to be different from one for any of the analyzed traits (Table 4).
Table 4.
Summary of the GxS interaction for anthropometric traits
| GxS Interaction | QTL specific effects | ||
|---|---|---|---|
|
| |||
| Trait | σgM = σgW p value | ρG(M,W) = 1 p value | σqM = σqW p value |
| BMI | 0.004 | 0.26 | 0.000175 |
| Waist circumference | 0.30 | 0.29 | 0.000273 |
| Waist/height ratio | 0.076 | 0.37 | 0.000257 |
| Percent fat | 0.30 | 0.39 | 0.008207 |
| Subscapular skinfold | 0.01 | 0.12 | 0.d000567 |
| Triceps | 0.035 | 0.5 | 0.000883 |
Genome wide scan for anthropometric measurements and QTL effects
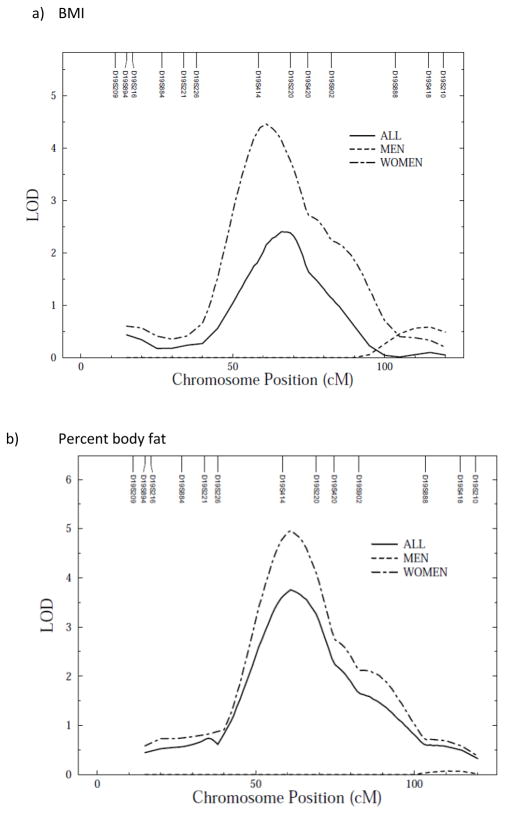
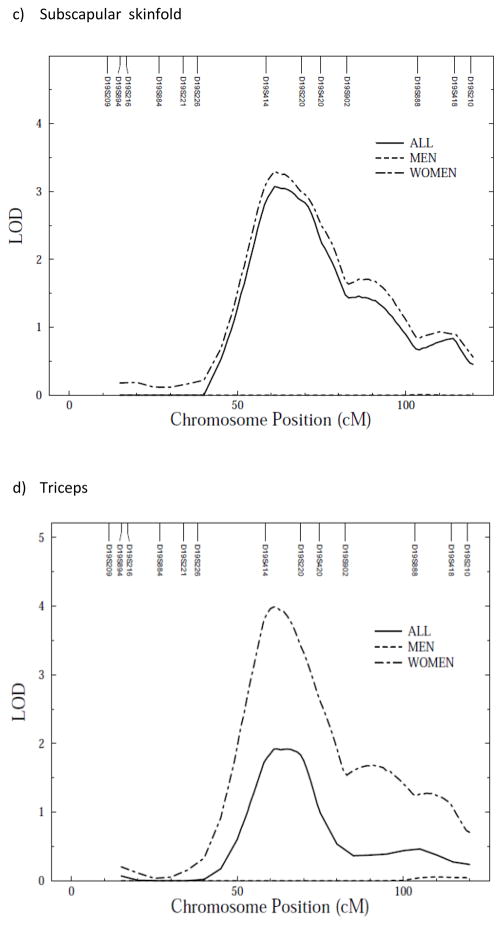
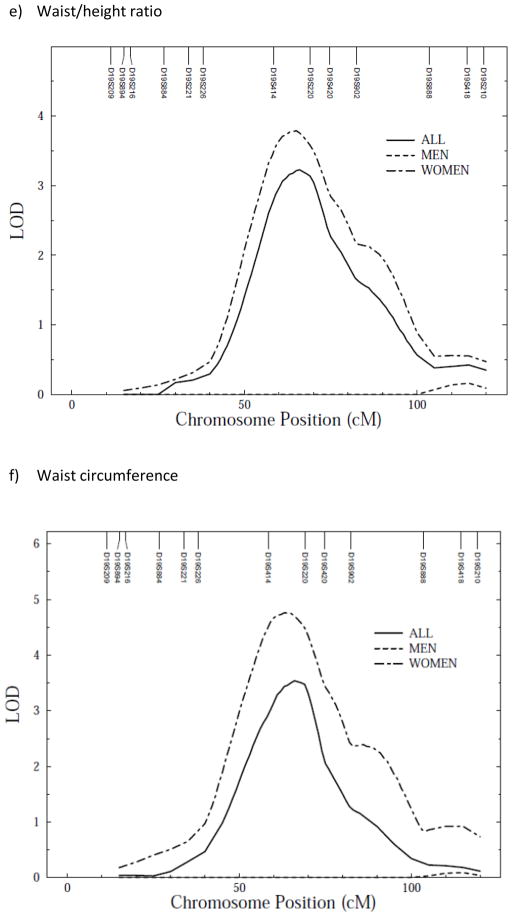
We conducted a genome-wide scan for anthropometric measurements with age, sex, age2, age*sex and age2*sex as covariates. Linkage scan plots for the anthropometric traits for autosomes are given in Supplementary figure 1 a–f online. In a preliminary analysis not allowing for sex-specific effects, evidence of linkage was detected between markers D19S414 and D19S220 on chromosome 19 for waist circumference (LOD = 3.5, empirical LOD score = 3.6), with suggestive evidence of linkage for percent body fat (LOD = 1.7, empirical LOD = 1.7), BMI (LOD = 2.4, empirical LOD = 2.4), waist/height ratio (LOD = 2.5, empirical LOD = 2.5), subscapular (LOD = 2.1, empirical LOD = 1.7) and triceps skinfolds (LOD = 1.9, empirical LOD = 1.6) (Figure 1a–f). All LOD scores shown are empirical LOD scores computed based on observed LOD scores and correction constant.
Figure 1.
Multipoint linkage analysis of anthropometric measurements (stratified by sex) on chromosome 19*. X axis represents chromosomal position in cM and Y axis represents strength of the signal via LOD score
Sex-specific linkage analysis
Because we detected polygenic GxS interaction effects, we tested whether there were any QTL GxS interaction effects on anthropometric traits. We found that QTL effects, all clustering around the same location on chromosome 19, were significantly different between the two sexes (variances due to QTLs) for all measured anthropometric traits (Table 4). Due to the powerful QTL GxSex interaction effects observed—across-sex QTL variance heterogeneity in particular—the sex-specific LOD score plots are quite divergent (Table 5; Figure 1 a–f). Indeed, sex-specific linkage results showed higher LOD scores for QTL on chromosome 19 in women than men (Table 5; Figure 1 a–f).
Table 5.
Sex-specific linkage results for anthropometric measurements used in this study
| Phenotype | Peak LOD score/Chromosome (location in cM) | ||
|---|---|---|---|
| Men | Women | All | |
| BMI | 1.5/9 (121) | 4.5/19 (61) | 2.4/19 (66) |
| Waist circumference | 1.9/8 (109) | 4.8/19 (63) | 3.5/19 (66) |
| Waist/height ratio | 1.9/8 (108) | 3.8/19 (65) | 2.5/19 (66) |
| Percent body fat | 1.8/7 (25) | 5.0/19 (61) | 1.7/19 (69) |
| Subscapular skinfold | 1.8/9 (101) | 3.3/19 (61) | 2.1/19 (69) |
| Triceps | 2.3/9 (99) | 4.0/19 (61) | 1.9/19 (62) |
Discussion
This study presents strong evidence for GxS interaction for anthropometric measurements and a QTL on chromosome 19q12-q13.3 that seems to be modulating this interaction. Although it is well known that significant differences in anthropometric and body fat measures exist between men and women, not many studies have looked at the interaction between genotypes and sexes as a plausible reason for these differences.
Significant differences in anthropometric measurements between sexes found in the present study were largely a confirmation of earlier studies conducted in various age groups and ethnicities. Lewis et al (10) in the HyperGen study found significant differences in percent fat between men and women, with women having higher percent fat than men. In a study conducted in Alaskan Eskimos but in a different cohort from ours, significant differences between men and women with respect to BMI, WCs, %BF and skinfold measures were observed. Women had higher BMI and %BF and larger waist and skinfold measures than men. (24). In addition, we were able to replicate and confirm the strong genetic influence on anthropometric traits with heritabilities ranging from 0.48 to 0.58. The heritabilities for anthropometric traits have been reported by several studies, with the estimates ranging from 0.3 to 0.8. These studies were conducted across several ages and ethnicities. For example, a study conducted in a Belgian population, obtained heritabilities as high as 0.7 and 0.74 for waist-hip ratio and sum of skinfolds, respectively (25). Findings in a similar range have been reported by researchers conducting studies on anthropometric traits such as BMI, WC, skinfold measures, waist-hip ratio etc in the United States (26,27), Canada (28), Mexico (29), Europe (21), and Asia (30,31).
Sexual dimorphism in anthropometric traits can be explained by the differences in reproductive biology between men and women. However, diet and social activities may also contribute to the observed differences in these traits. Since genotypes are known to express themselves differently in different environments, we hypothesized that genotypes for anthropometric traits may behave differently in men and women. This study provided a strong evidence of GxS interaction indicating that the phenotypic variation in these anthropometric measurements is a result of genetic architecture based on an individual’s sex. For the GxS interaction, genetic standard deviations were significantly different from each other for BMI and skinfold measurements. Different genetic standard deviations signify that the intensity of genetic effects varies by sex. Genetic correlation between sexes, on the other hand, if different from one, implies that distinct genetic effects are involved in the regulation of these traits in men and women. None of these traits showed genetic correlation to be different from one and we were not able to reject the hypothesis that the traits are being regulated by the same set of genes. Even though, no significant GxS interaction was obtained for WC and %BF, QTL specific effects were significant for all anthropometric traits, showing that the magnitude of the genetic effects due to a specific QTL was different between sexes. In a study in Mexican- Americans, Comuzzie et al (26) reported significant GxS interactions for all anthropometric traits. Similarly, significant sex-specific interactions were obtained for these traits in a study conducted in non-Hispanic Whites and African-Americans from the HyperGEN study (10). They also found significant GxS interaction as well as separate QTLs for adiposity measures in men and women. We replicated this in the sense that we found separate QTLs for men and women. The significant GxS interactions complement our sex-specific findings.
We conducted a genome-wide scan for detecting QTLs that regulate sex-specific architecture for these traits. We found a region on chromosome 19q12-p13 which harbored QTLs for all these anthropometric traits in both standard and QTL GxS interaction models. Important and relevant candidate genes in this region are transforming growth factor-beta 1 (TGFB1), glycogen synthase 1-muscle (GYS1), hormone sensitive lipase (LIPE), apolipoprotein E (APOE), gastric inhibitory polypeptide receptor (GIPR) and lipolysis-sensitive lipoprotein receptor (LSR). The peptides expressed by these genes are generally associated with growth and differentiation (32) or clearance of very low density lipoproteins and chylomicron remnants (33), insulin and glucocorticoid metabolism (34), mobilization of free fatty acids from adipose tissue (35) and lipid transporter activity and clearance of dietary triglycerides (36).
Association of polymorphisms in the above mentioned genes with obesity-related anthropometric traits has been reported. Variants in TGFB1 have been associated with abdominal obesity and BMI (37). Similarly, variants in APOE gene have been associated with percent fat mass, BMI and waist circumference (37). To check whether the APOE gene variants in this region might be responsible for the linkage signal, we analyzed the phenotypes using APOE alleles, e2, e3, e4 as covariates. These alleles were not significant therefore could not be used in the final model, therefore indicating that the strength of the linkage signal is not explained by theses alleles. Body composition measures have been consistently associated with polymorphisms in the hormone sensitive lipase gene (LIPE) (38). Knockout animal studies for LIPE have shown reduced abdominal fat mass and resistance to diet-induced obesity (39). In addition, this region has been associated with obesity-related traits in genome-wide linkage studies (40). Bell et al (41) found a QTL in this region that was associated with severe obesity (BMI > 35) in French Caucasians.
Other QTLs that were found in this region are for triglycerides and adiposity (42), development of type 2 diabetes in Dutch population (43) and blood pressure in a Nigerian population (44).
In summary, this study provides strong evidence of GxS interaction on anthropometric measurements in Alaskan Eskimos. In addition to the formal demonstration of GxS interaction effects, we also found a QTL on chromosome 19q12-q13.3 that may have strong differential effects on the anthropometric measurements in men and women.
Supplementary Material
Table 3.
Genetic and phenotypic correlations between the anthropometric measurements
| Phenotype1 | Phenotype2 | rhog (SE) | p value | rhop (SE) | p value |
|---|---|---|---|---|---|
| BMI | Waist circumference | 0.95 (0.02) | 4.9 × 10−16 | 0.91 (0.06) | 1.0 × 10−30 |
| Waist/height ratio | 0.96 (0.01) | 1.6 × 10−15 | 0.92 (0.06) | 1.0 × 10−30 | |
| Percent body fat | 0.91 (0.02) | 2.2 × 10−15 | 0.89 (0.03) | 1.0 × 10−30 | |
| Subscapular skinfold | 0.87 (0.03) | 1.3 × 10−15 | 0.81 (0.04) | 7.4 × 10−21 | |
| Triceps | 0.80 (0.05) | 5.9 × 10−12 | 0.71 (0.05) | 1.8 × 10−14 | |
| Waist circumference | Waist/height ratio | 0.95 (0.10) | 3.3 × 10−14 | 0.96 (0.05) | 1.0 × 10−30 |
| Percent body fat | 0.95 (0.02) | 4.7 × 10−16 | 0.89 (0.03) | 1.0 × 10−30 | |
| Subscapular skinfold | 0.88 (0.04) | 1.9 × 10−15 | 0.80 (0.02) | 4.7 × 10−20 | |
| Triceps | 0.82 (0.06) | 6.4 × 10−12 | 0.69 (0.05) | 2.4 × 10−12 | |
| Waist/height ratio | Percent body fat | 0.88 (0.03) | 1.1 × 10−13 | 0.84 (0.03) | 2.2 × 10−23 |
| Subscapular skinfold | 0.84 (0.04) | 1.2 × 10−13 | 0.79 (0.02) | 4.8 × 10−19 | |
| Triceps | 0.78 (0.06) | 1.1 × 10−10 | 0.68 (0.04) | 1.4 × 10−12 | |
| Percent fat | Subscapular skinfold | 0.88 (0.03) | 2.8 × 10−16 | 0.78 (0.07) | 1.0 × 10−19 |
| Triceps | 0.78 (0.05) | 1.3 × 10−11 | 0.71 (0.03) | 1.4 × 10−13 | |
| Subscapular skinfold | Triceps | 0.90 (0.05) | 6.3 × 10−15 | 0.76 (0.04) | 5.7 × 10−17 |
- rhog = estimate for genetic correlation
- rhop = estimated for phenotypic correlation
- SE = Standard error of variance
Acknowledgments
We thank the participants of the GOCADAN study for their generous participation. This investigation was conducted in facilities constructed with support from Research Facilities Improvement Program Grants [Numbers C06 RR014578, C06 RR013556, C06 RR015456, C06 RR017515] from the National Center for Research Resources, National Institutes of Health (NIH), and with support from NIH grants U01 HL64244 and MH59490.
Footnotes
Author contributions:
VSV and AGC performed or supervised all aspects of the statistical analyses and were helped by HHHG, SL, VPD, JB and SAC. KH and SAC were responsible for the 10cM STR genotyping. JGU, SL, and CRW helped with the recruitment, data entry and preparation of the manuscript. SOE, RBD, RRF, JWM, BVH and AGC are responsible for the execution of the study and contributed to the preparation of the manuscript.
Disclosure statement:
Authors have no conflict of interest
References
- 1.Schumacher C, Davidson M, Ehrsam G. Cardiovascular disease among Alaska Natives: A review of the literature. Int J Circumpolar Health. 2003;62:343–362. doi: 10.3402/ijch.v62i4.17579. [DOI] [PubMed] [Google Scholar]
- 2.Ebbesson SOE, Adler AI, Risica PM, et al. Cardiovascular disease and risk factors in three Alaskan Eskimo populations: The Alaska –Siberia Project. Int J Circumpolar Health. 2005;64:378–399. doi: 10.3402/ijch.v64i4.18014. [DOI] [PubMed] [Google Scholar]
- 3.Eilat-Adar S, Mete M, Nobmann ED, et al. Dietary patterns are linked to cardiovascular risk factors but not to inflammatory markers in Alaska Eskimos. J Nutr. 2009;139:2322–2328. doi: 10.3945/jn.109.110387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Despres J-P, Lemieux S. Abdominal obesity and the metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 5.Aronne LJ. Classification of obesity and assessment of obesity-related health risks. Obes Res. 2002;10:105S–115S. doi: 10.1038/oby.2002.203. [DOI] [PubMed] [Google Scholar]
- 6.Kortelainen ML, Sarkioja T. Extent and composition of coronary lesions in relation to fat distribution in women younger than 50 years of age. Arterioscler Thromb Vasc Biol. 1999;19:695–699. doi: 10.1161/01.atv.19.3.695. [DOI] [PubMed] [Google Scholar]
- 7.Kortelainen ML, Sarkioja T. Visceral fat and coronary pathology in male adolescents. Int J Obes. 2001;25:228–232. doi: 10.1038/sj.ijo.0801466. [DOI] [PubMed] [Google Scholar]
- 8.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1847. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989;83:1168–1173. doi: 10.1172/JCI113997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis CE, North KE, Arnett D, et al. Sex-specific findings from a genome-wide linkage analysis of human fatness in non-Hispanic whites and African Americans: The HyperGEN Study. Int J Obes. 2005;29:639–649. doi: 10.1038/sj.ijo.0802916. [DOI] [PubMed] [Google Scholar]
- 11.Wells JCK. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. 2007;21:415–430. doi: 10.1016/j.beem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Mittendorfer B. Sexual dimorphism in human lipid metabolism. J Nutr. 2005;135:681–686. doi: 10.1093/jn/135.4.681. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy JJ. Gene by sex interaction in the etiology of coronary heart disease and the preceding metabolic syndrome. Nutr Metab Cardiovasc Dis. 2007;17:153–161. doi: 10.1016/j.numecd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Howard BV, Devereux RB, Cole SA, et al. A genetic and epidemiological study of cardiovascular disease in Alaska Natives (GOCADAN): Design and Methods. Int J Circumpolar Health. 2005;64:206–221. doi: 10.3402/ijch.v64i3.17985. [DOI] [PubMed] [Google Scholar]
- 15.Ebbesson SOE, Laston S, Wenger CR, et al. Recruitment and community interaction in the GOCADAN study. Int J Circumpolar Health. 2006;65:23–32. doi: 10.3402/ijch.v65i1.17882. [DOI] [PubMed] [Google Scholar]
- 16.Sobel E, Lange K. Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker sharing statistics. Am J Hum Genet. 1996;58:1323–1337. [PMC free article] [PubMed] [Google Scholar]
- 17.Heath SC. Markov chain Monte Carlo segregation and linkage analysis for oligogenic models. Am J Hum Genet. 1997;61:748–760. doi: 10.1086/515506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong A, Gudbjartsson DF, Sainz J, et al. A high-resolution recombination map of the human genome. Nat Genet. 2002;31:241–247. doi: 10.1038/ng917. [DOI] [PubMed] [Google Scholar]
- 19.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1121. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopper JL, Mathews JD. Extensions to multivariate normal models for pedigree analysis. Ann Hum Genet. 1982;46:373–383. doi: 10.1111/j.1469-1809.1982.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 21.Self S, Liang K. Asymptotic properties of maximum likelihood estimators and likelihood ratio tests under nonstandard conditions. J Am Stat Assoc. 1987;82:605–610. [Google Scholar]
- 22.Williams JT, Van Eerdewegh P, Almasy L, Blangero J. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits. I. Likelihood formulation and simulation results. Am J Hum Genet. 1999;65:1134–1147. doi: 10.1086/302570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blangero J, Williams JT, Almasy L. Variance component methods for detecting complex trail loci. Adv Genet. 2001;42:151–182. doi: 10.1016/s0065-2660(01)42021-9. [DOI] [PubMed] [Google Scholar]
- 24.Risica PM, Ebbesson SOE, Schraer CD, Nobmann ED, Caballero BH. Body fat distribution in Alaskan Eskimos of the Bering Straits region: the Alaskan Siberia Project. Int J Circumpolar Health Int J Obes. 2000;24:171–179. doi: 10.1038/sj.ijo.0801103. [DOI] [PubMed] [Google Scholar]
- 25.Souren NY, Paulussen AD, Loos RJ, et al. Anthropometry, carbohydrate and lipid metabolism in the East Flanders Prospective Twin Survey: heritabilities. Diabetologia. 2007;50:2107–2116. doi: 10.1007/s00125-007-0784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comuzzie AG, Blangero J, Mahaney MC, Mitchell BD, Stern MP, MacCluer JW. Quantitative genetics of sexual dimorphism in body fat measurements. Am J Hum Biol. 1993;5:725–734. doi: 10.1002/ajhb.1310050616. [DOI] [PubMed] [Google Scholar]
- 27.Voruganti VS, Göring HH, Diego VP, et al. Genome-wide scan for serum ghrelin detects linkage on chromosome 1p36 in Hispanic children: results from the Viva La Familia study. Pediatr Res. 2007;62:445–450. doi: 10.1203/PDR.0b013e31813cbf02. [DOI] [PubMed] [Google Scholar]
- 28.Hunt MS, Katzmarzyk PT, Pérusse L, Rice T, Rao DC, Bouchard C. Familial resemblance of 7-year changes in body mass and adiposity. Obes Res. 2002;10:507–517. doi: 10.1038/oby.2002.69. [DOI] [PubMed] [Google Scholar]
- 29.Bastarrachea RA, Kent JW, Jr, Rozada G, et al. Heritability and genetic correlations of metabolic disease-related phenotypes in Mexico: preliminary report from the GEMM Family Study. Hum Biol. 2007;79:121–129. doi: 10.1353/hub.2007.0021. [DOI] [PubMed] [Google Scholar]
- 30.Arya R, Duggirala R, Comuzzie AG, et al. Heritability of anthropometric phenotypes in caste populations of Visakhapatnam, India. Hum Biol. 2002;74:325–344. doi: 10.1353/hub.2002.0026. [DOI] [PubMed] [Google Scholar]
- 31.Bayoumi RA, Al-Yahyaee SA, Albarwani SA, et al. Heritability of determinants of the metabolic syndrome among healthy Arabs of the Oman family study. Obesity (Silver Spring) 2007;15:551–556. doi: 10.1038/oby.2007.555. [DOI] [PubMed] [Google Scholar]
- 32.Dickinson ME, Kobrin MS, Silan CM, et al. Chromosomal localization of seven members of the murine TGF-beta superfamily suggests close linkage to several morphogenetic mutant loci. Genomics. 1990;6:505–520. doi: 10.1016/0888-7543(90)90480-i. [DOI] [PubMed] [Google Scholar]
- 33.Mooijaart SP, Berbee JFP, van Heemst D, et al. ApoE plasma levels and risk of cardiovascular Mortality in old age. PLoS Med. 2006;3:e176. doi: 10.1371/journal.pmed.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993;133:2861–2870. doi: 10.1210/endo.133.6.8243312. [DOI] [PubMed] [Google Scholar]
- 35.Holm C, Kirchgessner TG, Svenson KL, et al. Hormone-sensitive lipase: sequence, expression, and chromosomal localization to 19cent-q13.3. Science. 1988;241:1503–1506. doi: 10.1126/science.3420405. [DOI] [PubMed] [Google Scholar]
- 36.Yen FT, Masson M, Clossais-Besnard N, et al. Molecular cloning of a lipolysis-stimulated remnant receptor expressed in the liver. J Biol Chem. 1999;274:13390–13398. doi: 10.1074/jbc.274.19.13390. [DOI] [PubMed] [Google Scholar]
- 37.Long JR, Liu PY, Liu YJ, et al. APOE and TGF-beta1 genes are associated with obesity phenotypes. J Med Genet. 2003;40:918–924. doi: 10.1136/jmg.40.12.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garbenc C, Perusse L, Chagnon YC, et al. The hormone-sensitive lipase gene and body composition: the HERITAGE family study. Int J Obes Relat Metab Disord. 2002;26:220–227. doi: 10.1038/sj.ijo.0801872. [DOI] [PubMed] [Google Scholar]
- 39.Mutch DM, Clement K. Genetics of human obesity. Best Pract Res Clin Endocrinol Metab. 2006;20:647–664. doi: 10.1016/j.beem.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Rankinen T, Zuberi A, Chagnon YC, et al. The Human Obesity Gene Map: The 2005 Update. Obesity. 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- 41.Bell C, Benzinou M, Siddiq A, et al. Genome-wide linkage analysis for severe obesity in French Caucasians finds significant susceptibility locus on chromosome 19q. Diabetes. 2004;53:1857–1865. doi: 10.2337/diabetes.53.7.1857. [DOI] [PubMed] [Google Scholar]
- 42.Feitosa MF, Rice T, North KE, et al. Pleiotropic QTL on chromosome 19q13 for triglycerides and adiposity: the HERITAGE Family Study. Atherosclerosis. 2006;185:426–432. doi: 10.1016/j.atherosclerosis.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 43.van Tillburg JH, Sandkuijl LA, Strengman E, et al. A genome-wide scan in type 2 diabetes mellitus provides independent replication of a susceptibility locus on 18p11 and suggests the existence of novel Loci on 2q12 and 19q13. J Clin Endocrinol Metab. 2003;88:2223–2230. doi: 10.1210/jc.2002-021252. [DOI] [PubMed] [Google Scholar]
- 44.Cooper RS, Luke A, Zhu X, et al. Genome scan among Nigerians linking blood pressure to chromosomes 2, 3, and 19. Hypertension. 2002;40:629–633. doi: 10.1161/01.hyp.0000035708.02789.39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.