Abstract
Background
Anemia is an important public health concern. Data from population-based surveys such as the National Health and Nutrition Examination Survey (NHANES) are the gold standard, but are obtained infrequently and include only small samples from certain minority groups.
Objectives
We assessed whether readily available databases of blood donor hemoglobin values could be used as a surrogate for population hemoglobin values from NHANES.
Design
Blood donor venous and fingerstick hemoglobin values were compared to 10,254 NHANES 2005-2008 venous hemoglobin values using demographically stratified analyses and ANOVA. Fingerstick hemoglobins or hematocrits were converted to venous hemoglobin estimates using regression analysis.
Results
Venous hemoglobin values from 1,609 first time donors correlated extremely well with NHANES data across different age, gender and demographic groups. Cigarette smoking increased hemoglobin by 0.26 to 0.59 g/dL depending on intensity. Converted fingerstick hemoglobin from 36,793 first time donors agreed well with NHANES hemoglobin (weighted mean hemoglobin of 15.53 g/dL for donors and 15.73 g/dL for NHANES) with similar variation in mean hemoglobin by age. However, compared to NHANES, the larger donor dataset showed reduced differences in mean hemoglobin between Blacks and other races/ethnicities.
Conclusions
Overall, first-time donor fingerstick hemoglobins approximate U.S. population data and represent a readily available public health resource for ongoing anemia surveillance.
Keywords: Erythrocyte count, Hematologic tests, Anemia, United States, blood donors, African Continental Ancestry Group
Introduction
Population-based measures of hemoglobin are important for surveillance of anemia, which has attracted increasing interest as a significant public health concern.[1] Anemia is associated with reduced cognitive function in women of child bearing age[2] and increased cardiovascular morbidity/mortality in those over 65 years old.[3, 4] Anemia disproportionately affects women and minorities.[5] In addition, the prevalence of anemia increases significantly with age and is present in 10% of those over 65 and 20% of those over 85.[6] The importance of anemia in the elderly will increase in the next 20 years as baby boomers age. In persons of all ages, amelioration of anemia has been shown to decrease the morbidity and mortality of associated disease.2
National estimates of hemoglobin and anemia are usually calculated using data from the National Health and Nutrition Examination Surveys (NHANES).[5, 7] This survey utilizes sophisticated samples designed to be representative of the United States population, but is limited by moderate sample size and periodic sampling in time. In contrast, blood donors are a very large population ranging in age from 16 to over 80 years old who are routinely tested for hemoglobin as part of the pre-donation qualification process. While most blood donors are white, there are also large numbers of donors from minority groups. In recent years, point of care measurement of quantitative fingerstick hematocrit or hemoglobin has replaced the qualitative copper sulfate density method, and the data are now being captured in large blood donor databases.
In 2006, 9.5 million individuals donated 15.7 million units of whole blood in the United States. Of these blood donors, 2.7 million (28.5%) were first time donors.[8] In addition to these successful donors, approximately 1 million individuals present to donate each year but are ineligible to do so because of hemoglobin below the 12.5 g/dL cut-off required for blood donation. Tracking demographic variation and secular trends in hemoglobin values provides an opportunity to readily identify changes in the prevalence of anemia in the United States. If the prevalence of anemia in the blood donor population reflects that of the overall population, the public health value of this readily available laboratory data is significant.
We therefore addressed, in a stepwise fashion, the research question of how closely hemoglobin data from four regional blood centers correlates with contemporaneous NHANES data. First, we compared venous hemoglobins in a subset of well-characterized donors to NHANES venous hemoglobins. Next, we performed a much larger comparison of donor fingerstick hemoglobin and hematocrit measurements using a correction factor to approximate venous hemoglobin. Our finding that blood donor data correlates well with NHANES data validates blood donors as a new and readily available source of hemoglobin measurement for public health surveillance of anemia among otherwise healthy, community-dwelling individuals in the United States.
Methods
Data was collected as part of the Retrovirus Epidemiology Donor Study-II (REDS-II) from four of six REDS-II blood centers: the American Red Cross, New England Region, Boston, MA (NEARC); the Blood Center of Wisconsin, Milwaukee, WI (BCW); the Hoxworth Blood Center, Cincinnati, OH (HOX); and the Institute for Transfusion Medicine, Pittsburgh, PA (ITxM). The other two REDS-II centers followed similar procedures but were excluded from this analysis because they did not perform complete blood count analyses on an automated hematology analyzer. Three sources of data were used for this analysis and are discussed in the following paragraphs: 1) Quantitative venous hemoglobin from blood donors enrolled in the REDS-II Donor Iron Status Evaluation (RISE) study; 2) Quantitative fingerstick hemoglobin/hematocrit from a larger set of REDS-II blood donors; and 3) Quantitative venous hemoglobin from participants in the 2005-2006 and 2007-2008 NHANES. All subjects in RISE and REDS-II gave informed consent, and protocols were consistent with ethical standards and approved by institutional review boards at each center.
Quantitative venous hemoglobin from blood donors in the RISE study
The RISE study enrolled 1624 subjects, 18 years of age or older, and who donated a unit of whole blood or double red cells at the four centers between January 1, 2008 and May 31, 2008. Thus, all enrolled donors had a fingerstick hemoglobin ≥12.5 g/dL. Of these subjects, 621 were first time donors or reactivated donors (no donations in the previous 24 months). The remaining 1003 subjects were regular whole blood donors, men with 3 or more donations in the previous 12 months and women with 2 or more donations in the previous 12 months. Further details regarding RISE including the enrollment procedures and donor demographics for the entire sample have been reported.[9, 10] Hemoglobin values were measured on venous blood on samples obtained at enrollment using ADVIA® 2120 or ADVIA® 120 Hematology Autoanalyzers (Siemens, Tarrytown, NY).
Quantitative fingerstick hemoglobin/hematocrit from blood donors in the REDS-II database
Shared data on all blood donations and deferrals of REDS-II donors are compiled into a centralized research databases at the REDS-II coordinating center (Westat, Rockville, MD). In addition to information on the donation or deferral such as donation procedure, donation type or deferral codes, the database also contains donor demographic data, including gender, race/ethnicity, and date of birth. For two of the years of REDS-II data collection, quantitative fingerstick hemoglobin (two centers) or hematocrit (two centers) values for both donations and deferrals were reported by REDS-II centers on the majority of both their accepted donors and deferred donors. This analysis was limited to the donation and deferral records of first-time donor visits made between January 1, 2007 and December 31, 2008 at these centers. There were 51,091 first-time donor records of which 36,793 had a quantitative fingerstick measure. Donors were classified as first-time based on their response to the question “Is this the first time you've ever given blood” on a self-reported form.
Quantitative venous hemoglobin from participants in the 2005-2006 and 2007-2008 NHANES
The National Center for Health Statistics of the Centers for Disease Control conducts the National Health and Nutrition Examination Survey (NHANES) in two year cycles. NHANES participants are selected using a stratified, multistage probability design to represent the total civilian, non-institutionalized US population. The procedures for consent, data collection and analysis are published elsewhere.[11, 12] The sampling weights for the NHANES data include a post-stratification adjustment so that the NHANES distribution is standardized to the March Current Population Survey (CPS) estimate of the US population distribution (Sylvia Dohrmann (Westat, Rockville, MD) June 7th 2010, personal communication).
Data for NHANES is collected through household interviews and physical examinations at Mobile Examination Centers. Hemoglobin values are measured as part of a complete blood count using the Beckman Coulter MAXM hematology flow cytometer (Beckman Coulter Inc, Fullerton, CA).[13, 14]
We combined data from 2 cycles of NHANES (NHANES 2005–2006 and NHANES 2007–2008) to form our comparison population since the way that hemoglobin was measured did not differ for these two cycles of NHANES. This was done to increase the statistical power of this current analysis. From 2005-2008, the NHANES sample included 10,254 participants who were 18 or older, not pregnant, and had hemoglobin results (5,175 men and 5,079 non-pregnant women). Self-reported pregnancy status was used to exclude pregnant women.
Statistical analysis - comparison of RISE to NHANES
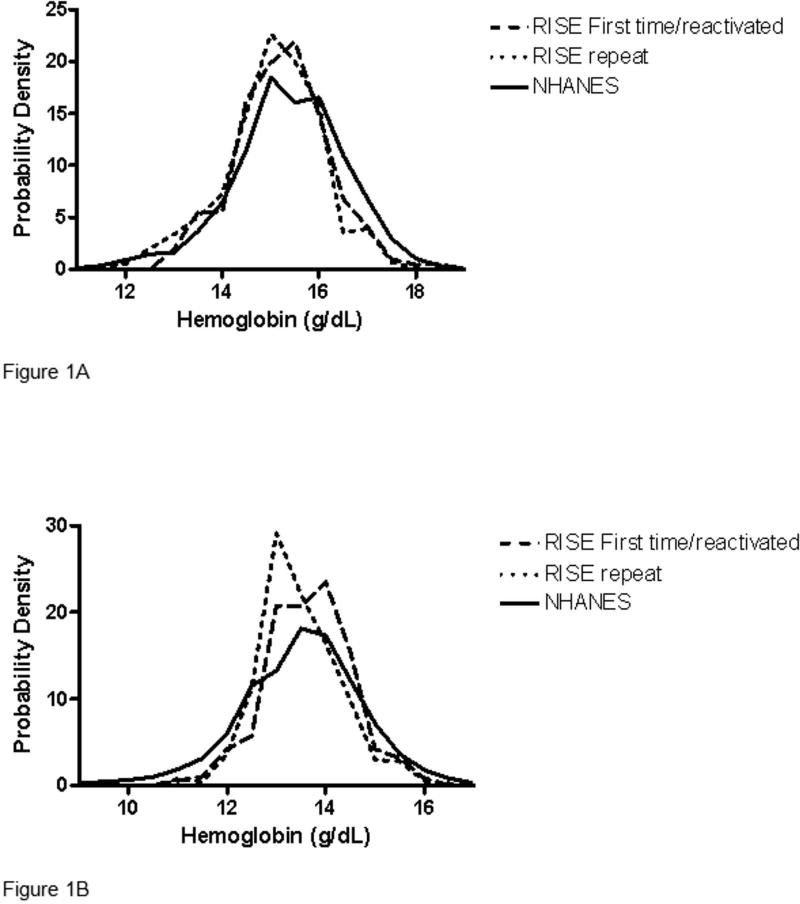
The RISE dataset was restricted to donors at enrollment at the 4 selected blood centers. In order to compare the RISE and NHANES hemoglobin distributions, it was necessary to adjust for the different characteristics of the two populations. A weight variable was developed for the RISE dataset such that these strata (i.e. gender, age, race, and smoking habit distributions) in RISE were standardized to the CPS estimate of the US population distribution. Hemoglobin probability density functions (Figure 1A and 1B) are based on the standardized population (i.e. the RISE first time/reactivated donor sample, the RISE repeat donor sample, and NHANES sample are each standardized to the US population).
Figure 1. Venous hemoglobin values in first-time blood donors and NHANES.
Distribution of venous hemoglobin values for RISE first-time/reactivated donors, RISE repeat donors and the 2005-2008 NHANES subjects. Results for (A) Males and (B) Females are standardized to adjust for differing demographics and smoking habits. Probability density reflects the proportion of each group with a certain value of hemoglobin. The sum of all values (area under the curve) equals 100%.
In separate analyses, mean hemoglobin values were calculated for RISE first time/reactivated donors, RISE repeat donors and NHANES subjects at the strata level. The mean and standard errors for the NHANES strata were calculated using Proc Surveymeans (SAS version 9.1) in order to properly account for the complex survey design (i.e. NHANES data was weighted). RISE donors were treated as self-representing (i.e. RISE data was not weighted). Mean differences were calculated for each stratum and aggregated for combinations of gender and donor status (e.g. first time/reactivated or repeat males compared to NHANES males), using the RISE sample to weight the calculations. Standard errors for the mean differences were calculated based on the sum of standard errors from the two mean analyses and assumed to be independent.
Finally, RISE FT/RA donors and NHANES data were combined into a single dataset and analyzed using analysis of variance (ANOVA) as shown in Appendix One. For this analysis no weight variable was used (each record was self-representing). The motivation for this analysis was to test for a center effect relative to NHANES after controlling for gender, age, race/ethnicity, and smoking intensity. NHANES is used as a reference group for analyzing the center effect.
Statistical Analysis - Comparison of REDS-II to NHANES
The REDS-II dataset was restricted to first time donors who donated in 2007-2008 at the 4 selected blood centers (n=51,202). Values from fingerstick hemoglobin and hematocrit were converted to estimates of venous hemoglobin using a regression model that estimated venous hemoglobin from fingerstick values from the RISE data. [15] For each center a separate regression model was developed for conversion of fingerstick measurement to venous hemoglobin using the fingerstick measurement and gender as predictors.
The REDS-II quantitative fingerstick values were missing or not recorded in the database in 28 percent of the records, thus the analysis was performed on 36,793 donors. Since blood centers vary in the order of operations in qualifying blood donors (i.e. the order of donor history questionnaire or fingerstick hemoglobin), some donors were deferred for behavioral characteristics before their hemoglobin could be determined. To compensate for missing values a weight variable was calculated for each record with non-missing fingerstick values in order to mitigate bias in the analysis. A separate weighting procedure was used based on the order of qualifying operations at blood centers. A weight class was defined by gender, age, and race/ethnicity; factors known to affect hemoglobin. The smoking habits of the 36,793 REDS-II donors were not recorded and, therefore, no adjustments for smoking could be made. Anemia prevalence was calculated for both data sets using the same hemoglobin cutoffs of <13.5 g/dL for men and <12.0 g/dL for women.
Separate ANOVAs were applied to the combined NHANES data (2005-2008) and the REDS-II data. Both analyses used their respective weights and the same model parameterization except that the REDS-II model included a blood center effect.
Results
Study populations
The study included a total of 1609 blood donors participating in RISE; 36,793 first time blood donors with fingerstick hemoglobin or hematocrit values participating in the parent REDS-II study (some of whom may have also been enrolled in the RISE study); and 10,254 NHANES 2005-08 subjects with venous hemoglobin values. Demographic characteristics of the three study populations are given in Table I. First-time REDS-II donors were younger and slightly more likely to be female than RISE or NHANES subjects. Minority representation was greatest in NHANES, followed by REDS-II and then RISE.
Table I.
Demographic characteristics of the three study populations, showing the percentage of each population within each demographic category.
| RISE* 2008 (%) | NHANES 2005-2008 (%) | REDS-II# 2008 (%) | |
|---|---|---|---|
| Overall | N= 1,609 | N=10,254 | N=36,793 |
| Male | 48.7 | 48.1 | 41.4 |
| Female | 51.3 | 51.9 | 58.6 |
| Ages | |||
| 18-19 | 5.2 | 3.4 | 31.9 |
| 20-29 | 12.2 | 17.6 | 26.8 |
| 30-39 | 13.2 | 18.0 | 14.3 |
| 40-49 | 21.3 | 20.5 | 13.8 |
| 50-59 | 27.3 | 17.7 | 9.3 |
| 60-69 | 15.9 | 11.2 | 3.2 |
| 70+ | 4.9 | 11.6 | 0.7 |
| White | 94.7 | 70.9 | 84.6 |
| Black | 2.2 | 11.0 | 4.9 |
| Hispanic | 1.8 | 12.5 | 5.2 |
| Other | 1.3 | 5.7 | 5.2 |
| Blood Center | |||
| A | 26.4 | N/A | 18.1 |
| B | 24.3 | N/A | 13.1 |
| C | 25.2 | N/A | 16.0 |
| D | 24.1 | N/A | 52.8 |
Abbreviations: NHANES: National Health and Nutrition Examination Survey
First-time/Reactivated and Repeat Donors combined
0.2% of the race data for multicenter study is missing
Comparison of RISE blood donor venous hemoglobins to NHANES 2005-08
In order to determine the validity of comparing blood donor hemoglobin values to those of the general population, we first compared the venous hemoglobins measured by auto analyzers in the RISE study to similar data from NHANES 2005-08. Cigarette smoking was found to be an important factor (ANOVA analysis below) in explaining hemoglobin. Since the smoking rate is generally lower in blood donors than in the general population, smoking intensity was included in all three RISE analyses. Distributions of hemoglobin values from the subset of blood donors enrolled in the RISE study with a venous hemoglobin measured by autoanalyzer and those from NHANES 2005-08 are presented in Figures 1A and 1B for men and women, respectively. For males, values from RISE first-time and repeat blood donors closely approximate those from NHANES 2005-08, although there are proportionally fewer RISE than NHANES subjects with high hemoglobin values in the right “tails” and more RISE than NHANES subjects with lower hemoglobins in the left “tails” of the curves. For females, again the RISE data approximate the NHANES data although there are proportionally more RISE than NHANES females near mean hemoglobin values, as indicated by higher peaks and narrower tails of the RISE distributions. Distribution curves for female RISE repeat blood donors are shifted slightly to the left compared to female RISE first time donors, indicating lower hemoglobin values likely due to previous blood donation.
Overall differences in mean hemoglobin were calculated after stratifying each study population according to sex, age, race and cigarette smoking intensity. Compared to NHANES 2005-08, RISE first-time blood donors had hemoglobin values that were overall 0.23 g/dL lower in men, and 0.01 g/dL higher in women. Again, compared to NHANES 2005-08, RISE repeat blood donors had mean hemoglobin values that were overall 0.40 g/dL lower in men and 0.25 g/dL lower in women, reflecting losses due to previous blood donation. Finally, center-to-center variability was observed between the four RISE centers after controlling for sex, age, race and cigarette smoking intensity, with relative differences compared to NHANES 2005-08 of +0.38 g/dL, -0.12 g/dL, - 0.10 g/dL and -0.39 g/dL. This was despite all four centers using the same methodology of obtaining venous hemoglobin results (see Appendix Table). This analysis also highlighted the importance of adjusting for cigarette smoking when comparing the two databases. In the combined RISE and NHANES ANOVA analysis, persons smoking <= 10 cigarettes per day had hemoglobin 0.26 g/dL higher and those smoking 11 or more cigarettes per day had hemoglobin 0.59 g/dL higher than non-smokers.
Comparison of REDS-II blood donor fingerstick hemoglobins to NHANES 2005-08
The overall agreement between RISE blood donor and NHANES 2005-08 hemoglobin values described above led us to extend the comparison more broadly to a much larger blood donor dataset of fingerstick hemoglobin measurements obtained using point of care devices or microcentrifuge spun hematocrit. For all comparisons, estimated venous hemoglobin values were derived from fingerstick hemoglobin values using a correction factor obtained from a regression analysis of RISE blood donors with simultaneous venous and fingerstick hemoglobin measurements.
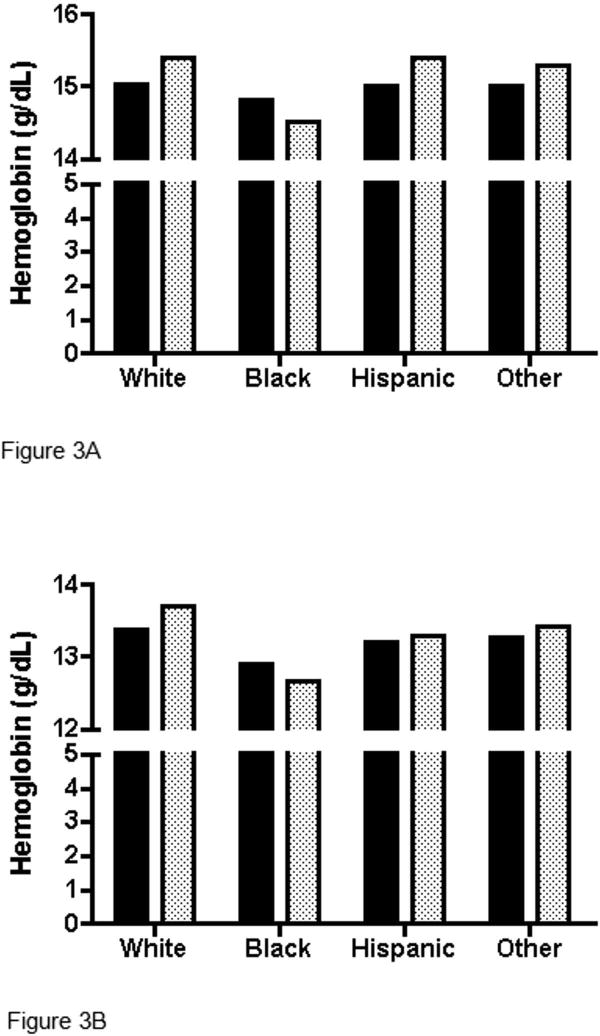
Hemoglobin values of demographic subgroups for both the REDS-II first-time donor and NHANES 2005-08 datasets were compared using separate ANOVA models (Table II and Figures 2, 3A and 3B). Comparison by smoking intensity was not possible because this variable is not available for the REDS-II dataset. Generally similar associations between hemoglobin and age and sex were observed for both datasets. For men, mean hemoglobin declined with age with small differences between NHANES and REDS-II data except for the oldest ages (Figure 2). For women, differences in mean hemoglobin were smaller between the two datasets and hemoglobin did not vary much with age, although a slight uptick was observed in postmenopausal women for both datasets (Figure 2). The main exception to similarity between the REDS-II and NHANES 2005-8 datasets was seen in reduced differences in hemoglobin between Blacks and other races. Specifically, Black men had hemoglobin values only 0.29 g/dL less than White men in the REDS-II dataset, while their hemoglobin was 0.89 g/dL less than White men in the NHANES dataset. For Black women, their hemoglobin was 0.48 g/dL less than White women in the REDS-II dataset, while hemoglobins were 1.03 g/dL less than White women in the NHANES 2005-08 dataset (Table II). In other words, the differences between REDS-II and NHANES were reduced or reversed in Blacks compared to other races/ethnicities (Figures 3A and 3B).
Table II.
Results of analysis of variance (ANOVA) models for the association of demographic characteristics with hemoglobin levels. Separate models were developed for the multicenter database and NHANES datasets; parameter estimates (standard errors) are given for each level of demographic characteristic relative to its reference category.
| REDS-II 2007-2008 | NHANES 2005-2008 | |||
|---|---|---|---|---|
| Estimate (Standard Error) in g/dL | Estimate (Standard Error) in g/dL | |||
| Intercept* | 15.53 (0.01) | 15.73 (0.08) | ||
| Center | ||||
| A | — | N/A | ||
| B | -0.02 (0.01) | N/A | ||
| C | -0.58 (0.02) | N/A | ||
| D | -0.34 (0.01) | N/A | ||
| Female 18-19 | -1.92 (0.01) | -2.19 (0.12) | ||
| Male 18-19 | — | — | ||
| Male | Female | Male | Female | |
| 18-19 | — | — | — | — |
| 20-29 | -0.02 (0.02) | 0.03 (0.01) | 0.07 (0.09) | 0.09 (0.10) |
| 30-39 | -0.11 (0.02) | -0.02 (0.02) | -0.08 (0.09) | 0.05 (0.10) |
| 40-49 | -0.18 (0.02) | -0.06 (0.02) | -0.19 (0.09) | 0.10 (0.10) |
| 50-59 | -0.32 (0.02) | 0.06 (0.02) | -0.35 (0.09) | 0.49 (0.10) |
| 60-69 | -0.52 (0.04 | 0.10 (0.03) | -0.59 (0.10) | 0.38 (0.10) |
| 70-99 | -0.69 (0.09) | -0.09 (0.06) | -1.16 (0.10) | 0.01 (0.10) |
| Male | Female | Male | Female | |
| White | — | — | — | — |
| Black | -0.29 (0.03) | -0.48 (0.02) | -0.89 (0.05) | -1.03 (0.05) |
| Hispanic | -0.14 (0.03) | -0.15 (0.02) | 0.01 (0.05) | -0.40 (0.05) |
| Other | -0.07 (0.03) | -0.10 (0.02) | -0.12 (0.07) | -0.29 (0.07) |
Intercept represents estimated mean hemoglobin for reference categorization (i.e. White male 18-19 years old from center 21).
Figure 2. Mean hemoglobin values (g/dL) in REDS-II donors versus NHANES subjects, by age and gender.
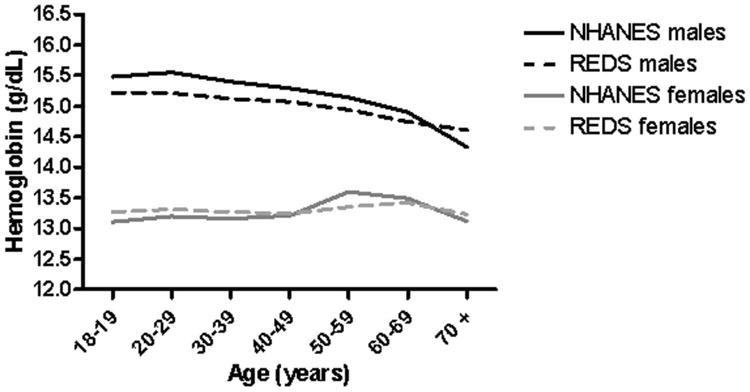
Figure 3. Mean hemoglobin values (g/dL) in REDS-II donors versus NHANES subjects among (A) Males, (B) Females.
Black bars are REDS-II; Stippled bars are NHANES.
Comparison of the prevalence of anemia in the REDS-II blood donor and NHANES 2005-08 datasets
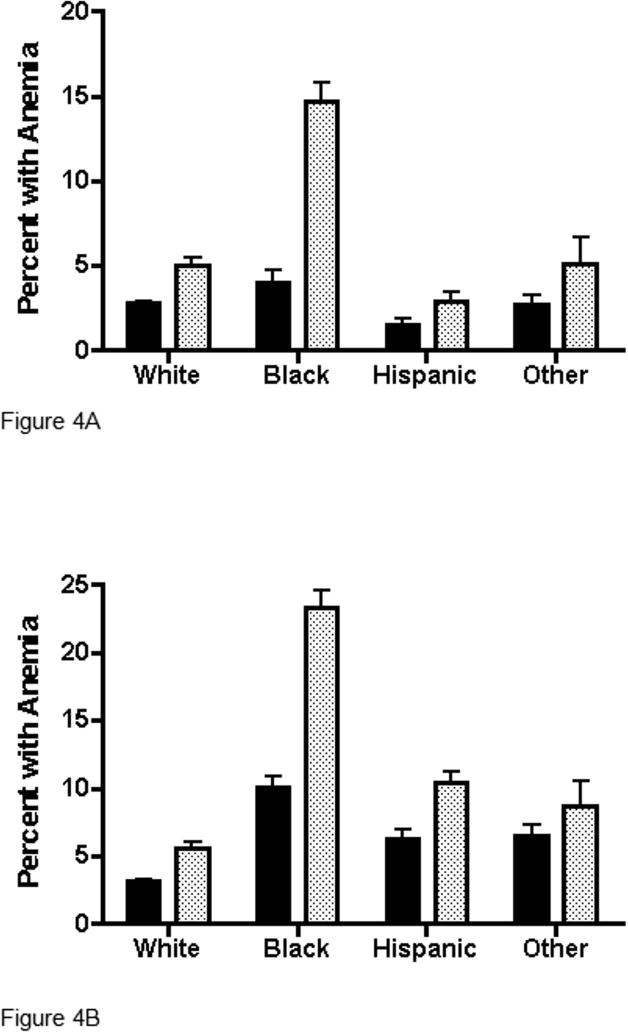
The prevalence of anemia for the two datasets was determined using cut-off values of 13.5 g/dL for men (Figure 4A) and 12.0 g/dL for women (Figure 4B). In men, there is an approximately 50 percent lower prevalence of anemia in REDS-II subjects than in NHANES subjects in most races/ethnicities (for example 2.8% vs. 5.1% in Whites and 1.5% vs. 2.9% in Hispanics) except that Black REDS-II men have a much lower prevalence of anemia than Black NHANES men (4.0% vs. 14.7%). A similar pattern is seen for women, namely a 50 percent lower prevalence in most races/ethnicities (for example 3.2% vs. 5.6% in Whites and 6.2% vs. 10.4% in Hispanics) but a much lower anemia prevalence among Black REDS-II women compared to Black NHANES women (10.0% vs. 23.3%).
Figure 4. Prevalence of anemia by race/ethnicity for first-time REDS-II blood donors versus NHANES among (A) Males and (B) Females.
Solid bars are REDS-II, Stippled bars are NHANES. Anemia was defined as hemoglobin < 13.5 g/dL for males and < 12.0 g/dL for females. Ninety-five percent upper confidence limits on prevalences are indicated by whiskers above each bar.
Discussion
This study has demonstrated that fingerstick hemoglobin and hematocrit values obtained when screening first time blood donors for anemia give a generally accurate representation of the US population hemoglobin values obtained from NHANES studies. As hemoglobin measurements obtained at blood centers are increasingly becoming available through electronic databases, these data represent a pre-existing and economical resource that is currently underutilized for public health assessment of hemoglobin and the prevalence of anemia in the US. Although the majority of blood donors are white individuals between 30 and 60 years old,[16] there are considerable numbers of minority as well as younger and older blood donors.[16] Thus it would be possible to statistically adjust for under representation of different demographic groups and obtain a nationally representative sample of community dwelling people in US.
In order to standardize for differences in demographic variables, it was necessary to adjust for imbalances in age, sex and race/ethnicity between blood donors and the general US population. It was also necessary to convert fingerstick hemoglobin measurement to that of venous blood.[17] We were able to use data from concurrent fingerstick and venous measurements obtained from blood donors enrolled in the RISE study to obtain adjustment factors.[15] Our analysis also revealed center-to-center variability in fingerstick hemoglobin measurement that was relatively modest in scale, but nevertheless will be important to quantify in future studies. The source of such variability is likely due to different point of care devices, differences in fingerstick blood sampling technique, training of operational staff and/or geographical variation in hemoglobin.[18] As quantitative hemoglobin measurement by fingerstick sampling becomes more widespread among blood centers, it is likely that operational research and quality control measures can be implemented to limit the effect of such variability.[19]
Despite demographic stratification and other statistical adjustment of the datasets, blood donor and NHANES data diverged among certain subgroups. First, the mean hemoglobin values for men were slightly lower in blood donors than in NHANES. This may have resulted from the lack of adjustment for smoking in the REDS-II donor database since male blood donors likely have lower smoking intensity than the male NHANES subjects. In our smaller RISE-NHANES comparison that did include donor smoking information, we found that cigarette smoking can raise hemoglobin levels by 0.26 to 0.59 g/dL depending on smoking intensity, values that are similar to those previously reported (0.4 g/dL).[20] In the future, it may be possible to impute smoking status from population surveys or to collect smoking status as a routine variable on blood donors, which would also be of use for cardiovascular risk screening that is often provided by blood centers to donors.[21, 22]
The hemoglobin differences between Blacks and other races/ethnicities were also smaller in blood donors than in their NHANES counterparts. Lower hemoglobin values in Blacks are well described and persist at various degrees of iron deficiency and following correction for disease status and behavioral risk factors.[23, 24] Since minorities are underrepresented among blood donors[16], positive selection for healthy individuals among Black first-time blood donors compared to the general population could explain why racial differences in hemoglobin are muted among blood donors. Further research to determine why this difference is smaller in blood donors than in the general population may help to further understand the genetic and physiological factors underlying the differences in hemoglobin values among races/ethnicities.[25]
Although there is good agreement of mean hemoglobin values between REDS-II blood donor and NHANES datasets among different demographic groups (Figures 2 and 3), there were more evident differences in the prevalence of anemia in Black men and women between the two datasets (Figure 4). This is because anemia, by definition, focuses on the leftward tail of the distributions shown in Figure 1 where differences between the REDS-II and NHANES curves are more accentuated. As noted above, possible reasons for these discrepancies include selection for healthier minority persons as blood donors, lack of adjustment for smoking and perhaps failure of our fingerstick-to-venous conversion algorithm to account for race/ethnicity. Future research can be directed at understanding these issues. Nevertheless, changes in anemia prevalence over time should still be evident in the blood donor sample, allowing the identification of trends relevant to public health.
Strengths of this study include its large size due to the inclusion of electronically recorded, routine hemoglobin and hematocrit values from four blood centers in the REDS-II blood donor database. Data were collected within a Food and Drug Administration regulated environment, with attention to quality control. Race and ethnicity information was available from all of the blood donors participating in the REDS-II research study. Although this may not be the case for all blood centers nationwide, increased collection of these data are likely to occur at blood centers in the future, particularly if new donor demographic based blood donation intervals are developed. Finally, the availability of pre-existing data allowed a low-cost analysis, important if such an approach is to be considered for routine public health monitoring.
Weaknesses of the study include the potential for selection bias inherent in the utilization of a blood donor population that are self-selected “healthy” individuals and screened for behavioral practices to remove those at high risk for HIV or hepatitis infection. This “healthy donor effect” is analogous to the “healthy worker effect” well-known in the occupational health literature. [26, 27] Sources of such bias may include deferral of the prospective blood donor by the blood center, self deferral of the donor for perceived ill health prior to presentation at the blood center, and socioeconomic and behavioral factors associated with the donation. Blood donors are also better educated than the general population, but educational status has little impact on blood donor hemoglobin values in the US.[23, 24, 28] We believe our statistical adjustments have minimized the effects of selection bias, but its effects need to be better understood. Another weakness is that the four centers where the study was conducted are in the Northeast and Midwest and, therefore, the study sample lacks populations of donors that may be of particular interest in the study of hemoglobin values, such as those that live at high altitude. Finally, although we developed a correction factor for fingerstick versus venous hemoglobin levels, measurement error attributable to the point of care devices and center-to-center variability needs to be better quantified.
Despite these limitations, hemoglobin measurement in blood donors represents a large scale and continuous sampling of the US population. It will allow for the identification and tracking of population subgroups (age, sex, race/ethnicity groups) with increased risk for anemia. Ongoing as opposed to episodic measurement will allow for assessment of response to public health interventions such as changes in iron and folate supplements to food.[29] In addition, continuous tracking of blood donor hemoglobin data may be useful in monitoring the unexplained decline in anemia identified in women through study of NHANES data between 1988 and 2002.[5] Since anemia is an independent indicator of poor outcomes in the elderly, blood donor data also may prove valuable to track trends in the prevalence of anemia in the elderly as the baby boomer population becomes senior citizens.[4]
The availability of hemoglobin data as well as other health screening tests such as cholesterol and hemoglobin A1c is consistent with the concept of the blood center as a health screening resource for the community.[21] Conceivably, large-scale blood center data could be used to study the effects of altitude and other environmental influences on hemoglobin levels and other health parameters.[30] In addition, the robustness of the data may allow for improved assessment of current diagnostic values for anemia in specific demographic subgroups.[31]
The growing availability of large databases of fingerstick hemoglobin also has implications for the management of donors by blood centers. First, blood collection itself clearly has an impact on hemoglobin balance, as demonstrated in the shift towards lower mean hemoglobin among frequent female blood donors demonstrated in Figure 1B. Analyses of trends in hemoglobin data in repeat donors over time may allow better management for blood collection to prevent iron deficiency.[32, 33] The RISE cohort study from which our venous hemoglobin data was derived is attempting to correlate hemoglobin levels with iron status and the ability of donors to meet the current donation criteria of hemoglobin greater or equal to 12.5 g/dL. Once this relationship is understood by more intensive measurements on a subset of donors, broader scale monitoring of small changes in hemoglobin levels over time could allow assessment of minimum hemoglobin cutoffs or recommendation of blood donation intervals based on the hemoglobin patterns in different blood donor populations.
In conclusion, we have demonstrated the feasibility of utilizing fingerstick hemoglobin and hematocrit measurements on first-time blood donors to approximate hemoglobin levels in the general United States population. We have identified specific areas of potential bias and measurement error that are amenable to further research and correction by better quality control of the fingerstick hemoglobin measurement within the blood center. Nevertheless, we believe these currently existing data that are available at very low cost will be useful for public health surveillance of anemia, within specific demographic sub-groups of the population. Blood centers may also be able to use similar hemoglobin analyses on a real-time basis for better prevention of iron deficiency anemia among frequent blood donors and for incorporation into donor health screening strategies while serving as an incentive to retain blood donor participation.
Acknowledgments
The authors thank the staff at all six participating blood centers. Without their help, this study would not have been possible.
This work was supported by NHLBI contracts N01-HB-47168, -47169, -47171, -47172, -47174 and -47175.
Appendix.
The Retrovirus Epidemiology Donor Study - II (REDS-II Study Group) is the responsibility of the following persons:
Blood Centers
American Red Cross Blood Services, New England Region
R. Cable, J. Rios and R. Benjamin
American Red Cross Blood Services, Southern Region/Department of Pathology and Laboratory Medicine, Emory University School of Medicine
J.D. Roback
Hoxworth Blood Center, University of Cincinnati Academic Health Center
R.A. Sacher, S.L. Wilkinson and P.M. Carey
Blood Centers of the Pacific, University of California San Francisco, Blood Systems Research Institute
E.L. Murphy, B. Custer and N. Hirschler
The Institute for Transfusion Medicine
D. Triulzi, R. Kakaiya and J. Kiss
Blood Center of Wisconsin
J. Gottschall and A. E. Mast
Coordinating Center: Westat, Inc
J. Schulman and M. King
National Heart, Lung, and Blood Institute, NIH
G.J. Nemo
Central Laboratory: Blood Systems Research Institute
M.P. Busch and P. Norris.
Footnotes
Disclosures: AEM provides educational talks about anemia for Siemens. The other authors declare that they have no conflicts of interest relevant to this manuscript.
Authorship Contributions: AEM, RGC, PC, JLG, JEK, TLS, and ELM designed research; RGC, PC, JLG, and JEK conducted research; AEM, WRS, BJ, DJW, and ELM analyzed data; AEM, WRS, BJ, DJW, and ELM wrote the paper; WRS, BJ, and DJW devised the analysis strategy; RGC, PC, JLG, JEK, and TLS reviewed the manuscript. All authors read and approved the final manuscript.
References
- 1.U.S. Department of Health and Human Services. With Understanding and Improving Health and Objectives for Improving Health. 2nd. Vol. 2. Washington, DC: U.S.: Government Printing Office; Nov, 2000. Healthy People 2010. [Google Scholar]
- 2.Murray-Kolb LE, Beard JL. Iron treatment normalizes cognitive functioning in young women. Am J Clin Nutr. 2007;85:778–787. doi: 10.1093/ajcn/85.3.778. [DOI] [PubMed] [Google Scholar]
- 3.Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 4.Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107:3841–3846. doi: 10.1182/blood-2005-10-4308. [DOI] [PubMed] [Google Scholar]
- 5.Cusick SE, Mei Z, Freedman DS, Looker AC, Ogden CL, Gunter E, Cogswell ME. Unexplained decline in the prevalence of anemia among US children and women between 1988-1994 and 1999-2002. Am J Clin Nutr. 2008;88:1611–1617. doi: 10.3945/ajcn.2008.25926. [DOI] [PubMed] [Google Scholar]
- 6.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 7.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–1750. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Department of Health and Human Services. The 2007 National Blood Collection and Utilization Survey Report. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- 9.Cable R, Glynn S, Kiss J, Mast A, Steele W, EL M, Wright D, Sacher R, Gottschall J, Vij V, Simon T. Analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011;51:511–522. doi: 10.1111/j.1537-2995.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cable RG, Glynn SA, Kiss JE, Mast AE, Steele WR, Murphy EL, Wright DJ, Sacher RA, Gottschall JL, Tobler LH, Simon TL the NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II) Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011 Oct 24; Epub ahead of print. [Google Scholar]
- 11.National Center for Health Statistics. 2005-2006 National Health and Nutrition Examination Survey (NHANES) Available from: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/nhanes05_06.htm (cited 7 June 2010)
- 12.National Center for Health Statistics. 2007-2008 National Health and Nutrition Examination Survey (NHANES) Available from: http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/nhanes07_08.htm (citer 7 June 2010)
- 13.National Center for Health Statistics. National Health and Nutrition Examination Survey. Questionnaires, Datasets and Related Documentation. NHANES 2005-2006. 2005-2006 Laboratory files Complete blood count. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/cbc_d_met.pdf(cited June 7, 2010)
- 14.National Center for Health Statistics. National Health and Nutrition Examination Survey. Questionnaires, Datasets and Related Documentation. NHANES 2007-2008. 2007-2008 Laboratory files Complete blood count. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/cbc_e_met.pdf (cited June 7,2010)
- 15.Cable RG, Steele WR, Melmed RS, Johnson B, Mast AE, Carey PM, Kiss JE, Kleinman SH, Wright DJ. The difference between fingerstick and venous hemoglobin and hematocrit varies by sex and iron stores. Transfusion. 2011 Oct 20; doi: 10.1111/j.1537-2995.2011.03389.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy EL, Shaz B, Hillyer CD, Carey P, Custer BS, Hirschler N, Fang J, Schreiber GB. Minority and foreign-born representation among US blood donors: demographics and donation frequency for 2006. Transfusion. 2009;49:2221–2228. doi: 10.1111/j.1537-2995.2009.02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross DG, Ross WB, Schreiner DE, Heaton WA. Rejection of prospective blood donors due to systematic errors in hematocrit measurement. Transfusion. 1983;23:75–77. doi: 10.1046/j.1537-2995.1983.23183147314.x. [DOI] [PubMed] [Google Scholar]
- 18.Bravo J, Hsueh Y, Gordeuk V, Querin J, Brittenham G, Keating L. Second fingerstick: a simple method to increase the blood supply. Transfusion. 1990;30:474–476. doi: 10.1046/j.1537-2995.1990.30590296384.x. [DOI] [PubMed] [Google Scholar]
- 19.Russell BL, Martin SM. Blood donor rejection rate: HemoCue hemoglobin analyzer vs microhematocrit. Clin Lab Sci. 1997;10:321–324. [PubMed] [Google Scholar]
- 20.Nordenberg D, Yip R, Binkin NJ. The effect of cigarette smoking on hemoglobin levels and anemia screening. JAMA. 1990;264:1556–1559. [PubMed] [Google Scholar]
- 21.Davey RJ. The blood centre as a community health resource. Vox Sang. 2006;91:206–213. doi: 10.1111/j.1423-0410.2006.00824.x. [DOI] [PubMed] [Google Scholar]
- 22.Pope CA, 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360:376–386. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson-Spear MA, Yip R. Hemoglobin difference between black and white women with comparable iron status: justification for race-specific anemia criteria. Am J Clin Nutr. 1994;60:117–121. doi: 10.1093/ajcn/60.1.117. [DOI] [PubMed] [Google Scholar]
- 24.Pan WH, Habicht JP. The non-iron-deficiency-related difference in hemoglobin concentration distribution between blacks and whites and between men and women. Am J Epidemiol. 1991;134:1410–1416. doi: 10.1093/oxfordjournals.aje.a116046. [DOI] [PubMed] [Google Scholar]
- 25.Mast AE, Lee TH, Schlumpf KS, Wright DJ, Johnson B, Carrick DM, Cable RG, Kiss JE, Glynn SA, Steele WR, Murphy EL, Sacher R, Busch MP. The impact of HFE mutations on haemoglobin and iron status in individuals experienceing repeated iron loss through blood donation. Br J Haematol. 2012;156:388–401. doi: 10.1111/j.1365-2141.2011.08952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atsma F, dV F, Verbeek A, Veldhuizen I, de Korti W. Healthy Donor Effect: Its Magnitude in Health Research Among Blood Donors (Abstract S73-0301) Transfusion. 2010;50(2-S):33A. doi: 10.1111/j.1537-2995.2010.03055.x. [DOI] [PubMed] [Google Scholar]
- 27.Pearce N, Checkoway H, Kriebel D. Bias in occupational epidemiology studies. Occupational Environmental Medicine. 2007;64:562–568. doi: 10.1136/oem.2006.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mast A, Schlumpf K, Wright D, Custer B, Spencer B, EL M, Simon T. Demographic correlates of low hemoglobin deferral among prospective whole blood donors. Transfusion. 2010;50:1794–1802. doi: 10.1111/j.1537-2995.2010.02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganji V, Kafai MR. Hemoglobin and hematocrit values are higher and prevalence of anemia is lower in the post-folic acid fortification period than in the pre-folic acid fortification period in US adults. Am J Clin Nutr. 2009;89:363–371. doi: 10.3945/ajcn.2008.26287. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Arguelles GJ, Sanchez-Medal L, Loria A, Piedras J, Cordova MS. Red cell indices in normal adults residing at altitude from sea level to 2670 meters. Am J Hematol. 1980;8:265–271. doi: 10.1002/ajh.2830080304. [DOI] [PubMed] [Google Scholar]
- 31.Beutler E, West C. Hematologic differences between African-Americans and whites: the roles of iron deficiency and alpha-thalassemia on hemoglobin levels and mean corpuscular volume. Blood. 2005;106:740–745. doi: 10.1182/blood-2005-02-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mast AE, Foster TM, Pinder HL, Beczkiewicz CA, Bellissimo DB, Murphy AT, Kovacevic S, Wroblewski VJ, Witcher DR. Behavioral, biochemical, and genetic analysis of iron metabolism in high-intensity blood donors. Transfusion. 2008;48:2197–2204. doi: 10.1111/j.1537-2995.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 33.Boulton F. Evidence-based criteria for the care and selection of blood donors, with some comments on the relationship to blood supply, and emphasis on the management of donation-induced iron depletion. Transfus Med. 2008;18:13–27. doi: 10.1111/j.1365-3148.2007.00818.x. [DOI] [PubMed] [Google Scholar]