Chronic renal failure patients have a higher risk for urogenic carcinoma (1,2). They need treatment with radical nephrectomy more frequently than a general population does. In addition, some non-dialysis-dependent patients may become dialysis-dependent after radical nephrectomy. Facing a choice of modality for renal replacement therapy (RRT) after nephrectomy, patients usually choose or change to hemodialysis.
Compared with the transperitoneal route, radical nephrectomy using the retroperitoneal approach can preserve peritoneal function. But the literature contains only a few reports of successful peritoneal dialysis (PD) after radical nephrectomy. Here, we report a case series of 5 patients who underwent radical nephrectomy by the retroperitoneal approach to preserve the peritoneum, allowing PD to be reinstated immediately after surgery without interruption or to be successfully started de novo.
METHODS
During 2004 – 2010 at our hospital, 3 end-stage renal disease (ESRD) patients receiving PD needed nephrectomy for a renal mass, and another 2 patients needed to start RRT after nephrectomy. The retroperitoneal approach was chosen to preserve peritoneal function, and all 5 patients maintained or started a PD program after their surgery. We retrospectively evaluated the records for these patients (Table 1).
TABLE 1.
Patient Data
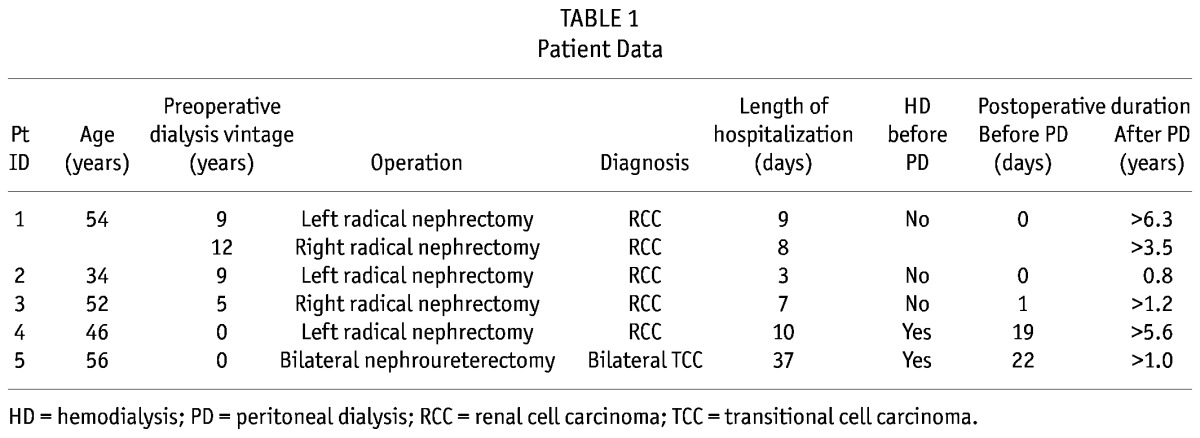
Patient 1, a 54-year-old man, had received PD for 15 years for ESRD secondary to hypertensive nephropathy. He underwent left and right radical nephrectomy procedures separated by an interval of 3 years. Both pathology reports showed renal cell carcinoma. This patient continued his PD program immediately after both surgeries without any complications. He was discharged 8 – 9 days after the operations.
Patient 2, a 34-year-old woman with ESRD secondary to chronic glomerulonephritis had received PD for 10 years. She underwent left radical nephrectomy and restarted PD immediately after surgery. The pathology report showed papillary renal cell carcinoma. This patient was discharged 3 days after her operation without any complications. She remained stable on her PD program until she died suddenly at home the next year. The possible cause of death was cardiac arrhythmia.
Patient 3, a 52-year-old man with ESRD secondary to hypertensive nephropathy had received PD for 6 years. He had right renal mass with presentation of painless hematuria. He underwent right radical nephrectomy with lymphadenectomy. The pathology reports showed renal cell carcinoma. This patient’s PD program was restarted the day after surgery. He was discharged 7 days after the operation without any complications.
Patient 4, a 46-year-old woman, was simultaneously diagnosed with a left renal mass and stage 5 chronic renal disease. After 5 days of hemodialysis, she underwent a left radical nephrectomy. The pathology report showed renal cell carcinoma. This patient underwent PD catheter insertion 3 days after the nephrectomy, and a PD program was started 18 days after catheter implantation.
Patient 5, a 56-year-old man, was diagnosed with a left renal pelvic tumor and right lower-ureter tumor, together with stage 3 chronic renal disease. He underwent a bilateral hand-assisted retroperitoneoscopic nephroureterectomy and hemodialysis on the day after surgery. The pathology report showed bilateral high-grade infiltrating urothelial carcinoma. A PD catheter was inserted 16 days after the nephrectomy. This patient started a PD program 6 days after catheter insertion without complications.
DISCUSSION
In 3 ESRD patients receiving PD, 4 open radical nephrectomies were performed by the retroperitoneal approach. All patients continued their PD program without interruption after surgery. During postoperative care, the dialysate volume was reduced to about one half or two thirds, and it titrated slowly upward according to patient’s clinical condition. No patient experienced peritoneal leakage, poor wound healing, or impaired ultrafiltration after surgery. For the patient who needed de novo RRT after nephrectomy, the retroperitoneal approach offered more freedom of choice with respect to RRT modality.
Nephrectomy can be performed by the transperitoneal or the retroperitoneal route (3). Transperitoneal procedures can be troublesome for patients requiring PD. It has traditionally been recommended that patients interrupt PD for at least 6 weeks after an open abdominal surgery to avoid complications (4), and removal of the PD catheter may be required. Insertion of a central venous catheter for maintenance hemodialysis exposes these patients to the risks of large-vessel access, hemodynamic changes, thrombosis, and greater dietary restrictions. The retroperitoneal approach can minimize damage to the peritoneum and preserve its integrity. Theoretically, a PD regimen can be restarted immediately after surgery, but there is little supporting evidence in the literature, except for 1 patient who returned to PD after pararectal retroperitoneal radical nephrectomy in a case report by Creagh et al. (5). Here, we report 4 successful experiences of PD continuing after peritoneum-preserving nephrectomy, with no negative effects on postoperative recovery.
Patients undergoing nephrectomy usually choose hemodialysis for subsequent RRT because of the potential risks of peritoneal fluid leakage, wound dehiscence, or abdominal hernia after surgery (6). Case reports have documented successful PD start simultaneously with retroperitoneal nephrectomy in children (7,8), but similar reports in adults are lacking.
In Taiwan, we usually initiate dialysate instillation about 1 – 2 weeks after the PD catheter is implanted. For that reason, patients 4 and 5 received temporary hemodialysis after nephrectomy and then switched successfully to PD.
Preserving the peritoneum so that patients can receive PD is especially valuable when patients are young and nondiabetic, given the mortality benefit often seen with PD (9,10).
CONCLUSIONS
For patients who need RRT after nephrectomy, the retroperitoneal approach to the surgery offers patient greater liberty to choose PD. Our successful experiences demonstrate that radical nephrectomy is absolutely not a contraindication for PD. We strongly recommend using the retroperitoneal route if there is any possibility of a subsequent need for RRT.
DISCLOSURES
MSW is a member of an advisory board and has received speaking honoraria and research funds from Baxter Healthcare Pty Ltd. He also received speaking honoraria from Novartis, Merck Sharp and Dohme, Roche, Astellas Pharma, AstraZeneca, and Pfizer. The remaining authors have no financial conflicts of interest to declare.
Acknowledgments
The authors are most grateful to the members of the PD unit at Chang Gung Memorial Hospital, Keelung, for excellent assistance with data collection.
REFERENCES
- 1. Savaj S, Liakopoulos V, Ghareeb S, Musso C, Sahu K, Bargman JM, et al. Renal cell carcinoma in peritoneal dialysis patients. Int Urol Nephrol 2003; 35:263–5 [DOI] [PubMed] [Google Scholar]
- 2. Ishikawa I. Renal cell carcinomas in patients on long-term hemodialysis. Contrib Nephrol 1999; 128:28–44 [DOI] [PubMed] [Google Scholar]
- 3. Wotkowicz C, Libertino JA. Renal cell cancer: radical nephrectomy. BJU Int 2007; 99(Pt B):1231–8 [DOI] [PubMed] [Google Scholar]
- 4. Teitelbaum I, Burkart J. Peritoneal dialysis. Am J Kidney Dis 2003; 42:1082–96 [DOI] [PubMed] [Google Scholar]
- 5. Creagh T, Grace P, Bouchier–Hayes D, McLean P. Peritoneum-preserving radical nephrectomy in a patient on continuous ambulatory peritoneal dialysis. Eur Urol 1992; 21:172–3 [DOI] [PubMed] [Google Scholar]
- 6. Del Peso G, Bajo MA, Costero O, Hevia C, Gil F, Díaz C, et al. Risk factors for abdominal wall complications in peritoneal dialysis patients. Perit Dial Int 2003; 23:249–54 [PubMed] [Google Scholar]
- 7. Szymanski KM, Bitzan M, Capolicchio JP. Is retroperitoneoscopy the gold standard for endoscopic nephrectomy in children on peritoneal dialysis? J Urol 2010; 184(Suppl 4):1631–7 [DOI] [PubMed] [Google Scholar]
- 8. Gundeti MS, Taghizaedh A, Mushtaq I. Bilateral synchronous posterior prone retroperitoneoscopic nephrectomy with simultaneous peritoneal dialysis: a new management for end-stage renal disease in children. BJU Int 2007; 99:904–6 [DOI] [PubMed] [Google Scholar]
- 9. Nelson CB, Port FK, Wolfe RA, Guire KE. Comparison of continuous ambulatory peritoneal dialysis and hemodialysis patient survival with evaluation of trends during the 1980s. J Am Soc Nephrol 1992; 3:1147–55 [DOI] [PubMed] [Google Scholar]
- 10. Collins AJ, Hao W, Xia H, Ebben JP, Everson SE, Constantini EG, et al. Mortality risks of peritoneal dialysis and hemo-dialysis. Am J Kidney Dis 1999; 34:1065–74 [DOI] [PubMed] [Google Scholar]


