Abstract
♦ Background: Cardiovascular (CV) disease is a major cause of morbidity and mortality in patients with end-stage renal disease. In recent years, arterial stiffness has taken on great importance in the pathophysiology of CV diseases. The independent predictive value of arterial stiffness for CV events and for all-cause and CV mortality has been demonstrated in the general population and in hemodialysis patients. Our aim in this study was to determine the relationship of arterial stiffness with mortality and fatal and nonfatal CV events in peritoneal dialysis (PD) patients.
♦ Methods: In this prospective observational cohort study with 2 years of follow-up, we studied a cohort of 156 PD patients with a mean follow-up of 19.2 ± 6.4 months. At baseline, echocardiography and standard clinical and biochemical analyses were performed in all patients and in 28 healthy subjects. Aortic stiffness index beta (ASIβ, a surrogate marker of arterial stiffness) was calculated as follows:
 |
♦ Results: During the follow-up period, 25 of the patients (16.0%) died, and 10 of those deaths had CV causes. Nonfatal CV events occurred in 15 patients. The median ASIβ was greater in PD patients than in control subjects (4.2 vs. 3.5; interquartile range: 3.2 – 5.5 vs. 2.5 – 4.8; p = 0.028]. In the fully adjusted multivariate Cox regression analysis (co-variates: age, sex, albumin, hemoglobin, diabetes mellitus, comorbid CV disease, left ventricular mass index, residual glomerular filtration rate, dialysate-to-plasma ratio of creatinine, Kt/V urea, left ventricular ejection fraction, duration of dialysis, smoking), ASIβ independently predicted fatal and nonfatal CV events (hazard ratio: 1.239; 95% confidence interval: 1.103 to 1.392), but not all-cause mortality.
♦ Conclusions: Our results provide the first direct evidence that arterial stiffness is an independent risk predictor of adverse CV outcome in PD patients.
Keywords: Arterial stiffness, cardiovascular disease outcome, mortality
Cardiovascular (CV) disease is a major cause of morbidity and mortality in patients with end-stage renal disease. In dialysis patients, CV mortality is 10 – 20 times that in a general population when stratified by age, sex, race, and the presence or absence of diabetes (1). In peritoneal dialysis (PD) patients, more than 50% of mortality can be attributed to CV disease (2). In addition to an increased occurrence of traditional risk factors, patients have additional uremia-related risk factors such as anemia, cardiovascular calcification, endothelial dysfunction, oxidative stress, volume overload, and uremic toxins that contribute to this increased burden of CV morbidity and mortality (3,4).
In recent years, arterial stiffness has taken on great importance in the pathophysiology of CV diseases. In the European Society of Hypertension/European Society of Cardiology guidelines, aortic pulse wave velocity (PWV), measurement of which is considered the “gold standard” method for determining aortic stiffness, was included among the risk factors influencing prognosis (5). The independent predictive value of arterial stiffness for CV events and for all-cause and CV mortality has been demonstrated in the general population and in hemodialysis patients (6–9). There is, however, no direct evidence showing an association between arterial stiffness and CV morbidity and mortality in PD patients.
Our aim in the present study was to determine the relationship of arterial stiffness with mortality and with fatal and nonfatal CV events in PD patients.
METHODS
PATIENTS AND STUDY DESIGN
This prospective observational cohort study with 2 years of longitudinal follow-up was started at the Erciyes University Medical Faculty PD unit in Kayseri in July 2007. The study cohort consisted of 156 PD patients and 28 healthy subjects who gave informed consent. Patients were eligible for entry into the study when they had been on PD for at least 3 months and if they had no malignant disease. Patients on automated PD were excluded. Biochemical analyses, echocardiographic examinations, assessment of dialysis indices, and determination of residual renal function were performed at study entry. Enrolment of a patient was postponed until at least 1 month after complete resolution of complications when the patient had infective, CV, or any other complications that required hospitalization.
ECHOCARDIOGRAPHY AND BLOOD PRESSURE MEASUREMENT
All subjects underwent a complete two-dimensional transthoracic echocardiographic and Doppler study in the left lateral decubitus position. The study was performed by a single experienced cardiologist using a GE–Vingmed Vivid 7 (GE–Vingmed Ultrasound AS, Horten, Norway) echocardiographic machine with a 2.5-MHz transducer operated from multiple windows. Measurements were made according to the guidelines of the American Society of Echocardiography (10). Systolic (SD) and diastolic ascending aortic diameter (DD) were measured in M-mode at a level 3 cm above the aortic valve from a parasternal long-axis view, according to a method previously described (11,12). The SD was recorded during ejection, and the DD, during pre-ejection. Simultaneously, blood pressure (BP) was measured by an oscillometric method using the MEC-1000 patient monitor (Mindray, Nanshan, Shenzhen, PR China). The average of 3 consecutive measurements was accepted as the BP.
Left ventricular mass (LVM) was calculated using the Devereux formula and was indexed by height (13).
MEASUREMENT OF AORTIC STIFFNESS
Noninvasive measurement of arterial stiffness involves measurement of surrogate parameters that are intrinsically related to stiffness. Three main techniques are used:
Pulse transit time
Analysis of the arterial pressure pulse and its wave contour
Direct stiffness estimation using measurements of diameter and distending pressure
A beta index model assesses the regional arterial stiffness based on change in pressure and diameter. Vascular diameters can be measured noninvasively with echocardiography, computed tomography, and magnetic resonance imaging. Simultaneously or within a few minutes, BP is measured at the brachial artery with an oscillometric device. The curvilinear relationship between BP and vascular diameter is approximated using a logarithmic transformation, resulting in the beta index reflecting stiffness (14).
In the present study, we used aortic stiffness index beta (ASIβ) as a surrogate marker of arterial stiffness. The index was calculated as follows:
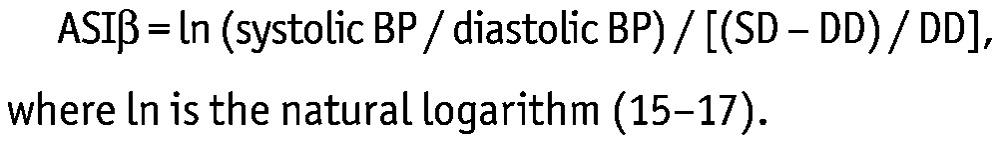 |
DIALYSIS INDICES AND RESIDUAL RENAL FUNCTION
A standard peritoneal equilibration test as described by Twardowski (18) was performed to determine peritoneal transport characteristics. Dialysis adequacy was calculated from 24-hour dialysate and urine collections by standard methods (19). Residual glomerular filtration rate was estimated as the average of urea and creatinine clearances in 24-hour urine (20).
OUTCOME MEASURES
Clinical outcomes included all-cause mortality, CV mortality, and first episode of a fatal or nonfatal CV event. Cardiovascular events included cerebrovascular disease (thromboembolic or hemorrhagic), transient ischemic attack, peripheral vascular disease, congestive heart failure, documented arrhythmia, myocardial ischemia, and myocardial infarction. Cardiovascular mortality was defined as a death whose cause was one of the CV events or sudden death. A witnessed death that occurred within 1 hour after the onset of acute symptoms and without any previous condition that would seem to be fatal was accepted as sudden death (21). In case of death out of hospital, family members were interviewed to discover the possible cause of death. When multiple CV events occurred, survival analysis was limited to the first episode. Deaths within 3 months after transfer to HD were accepted as PD-related mortalities.
STATISTICAL ANALYSIS
Data are presented as mean ± standard deviation unless otherwise specified. Comparisons between patients and healthy subjects were performed using the unpaired Student t-test or Mann–Whitney U-test, as appropriate. Multivariate linear regression was used to evaluate the correlations of various parameters with ASIβ. Because of its skewed distribution, ASIβ was log-transformed before entry into the regression model. Baseline variables were added into the model, and backward stepwise elimination was applied to remove insignificant variables. The Cox proportional hazards model was used to determine independent predictors of outcome. The covariates for the models were age, sex, duration of dialysis, diabetes mellitus, pre-existing CV disease, smoking, dialysate-to-plasma ratio (D/P) of creatinine, Kt/V urea, residual glomerular filtration rate, left ventricular ejection fraction, LVM index (LVMi), hemoglobin, and albumin. The cohort was stratified into quartiles according to ASIβ value. Survival rates for fatal or nonfatal CV events by quartile were analyzed using the Kaplan–Meier method. Differences in survival were compared using the log-rank test.
Statistical analyses were performed using the SPSS software package (version 13.0: SPSS, Chicago, IL, USA).
RESULTS
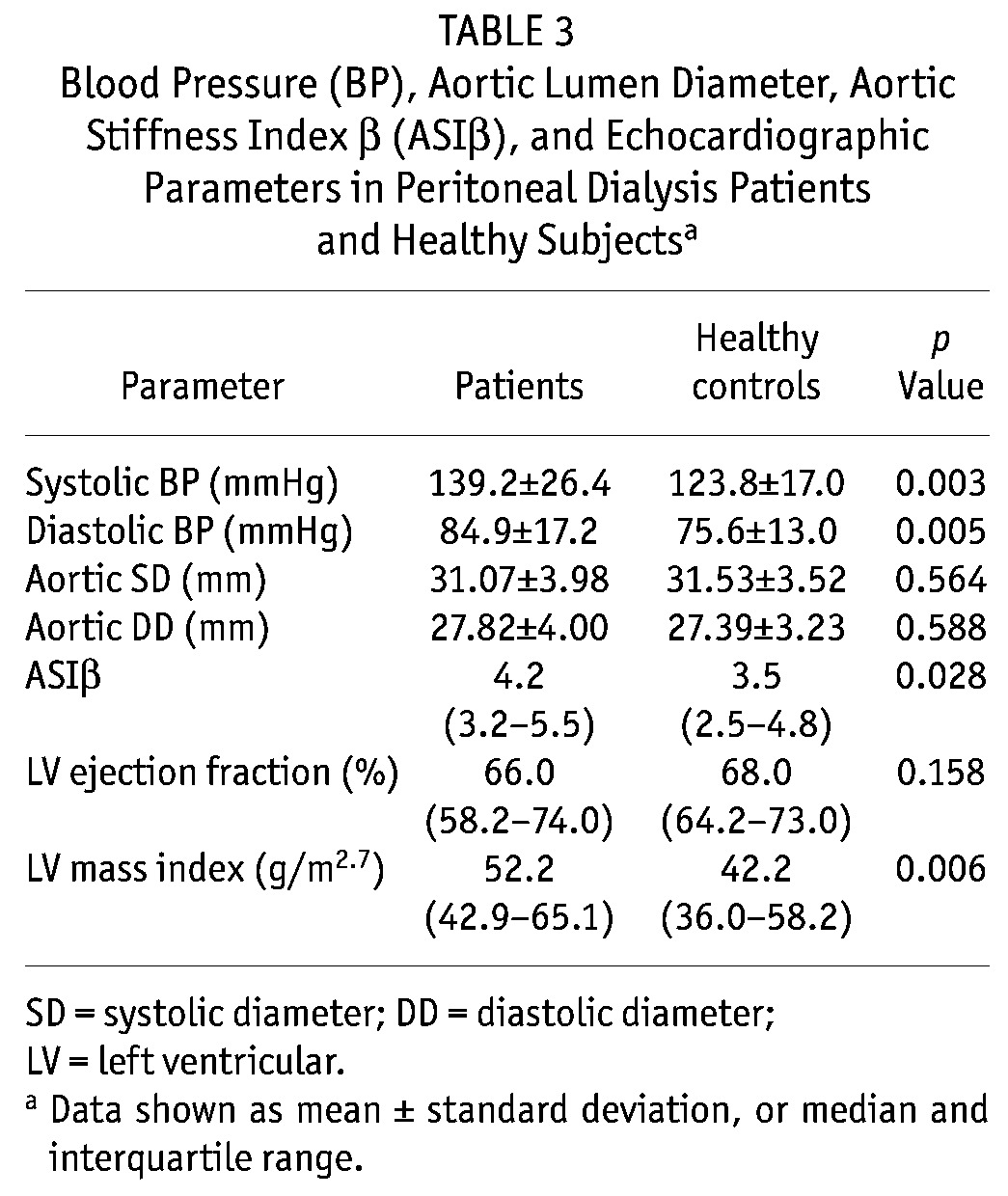
Table 1 shows the baseline demographic characteristics of the patients. Mean age was 48.2 ± 13.7 years, and mean dialysis follow-up was 19.3 ± 6.5 months. Table 2 shows the demographic and biochemical parameters of the PD patients and healthy subjects. Table 3 shows the values for BP, ASIβ, and echocardiographic parameters. The median ASIβ was significantly higher in patients than in healthy subjects (4.2 vs. 3.5).
TABLE 1.
Baseline Demographic and Clinical Characteristics of the Patients
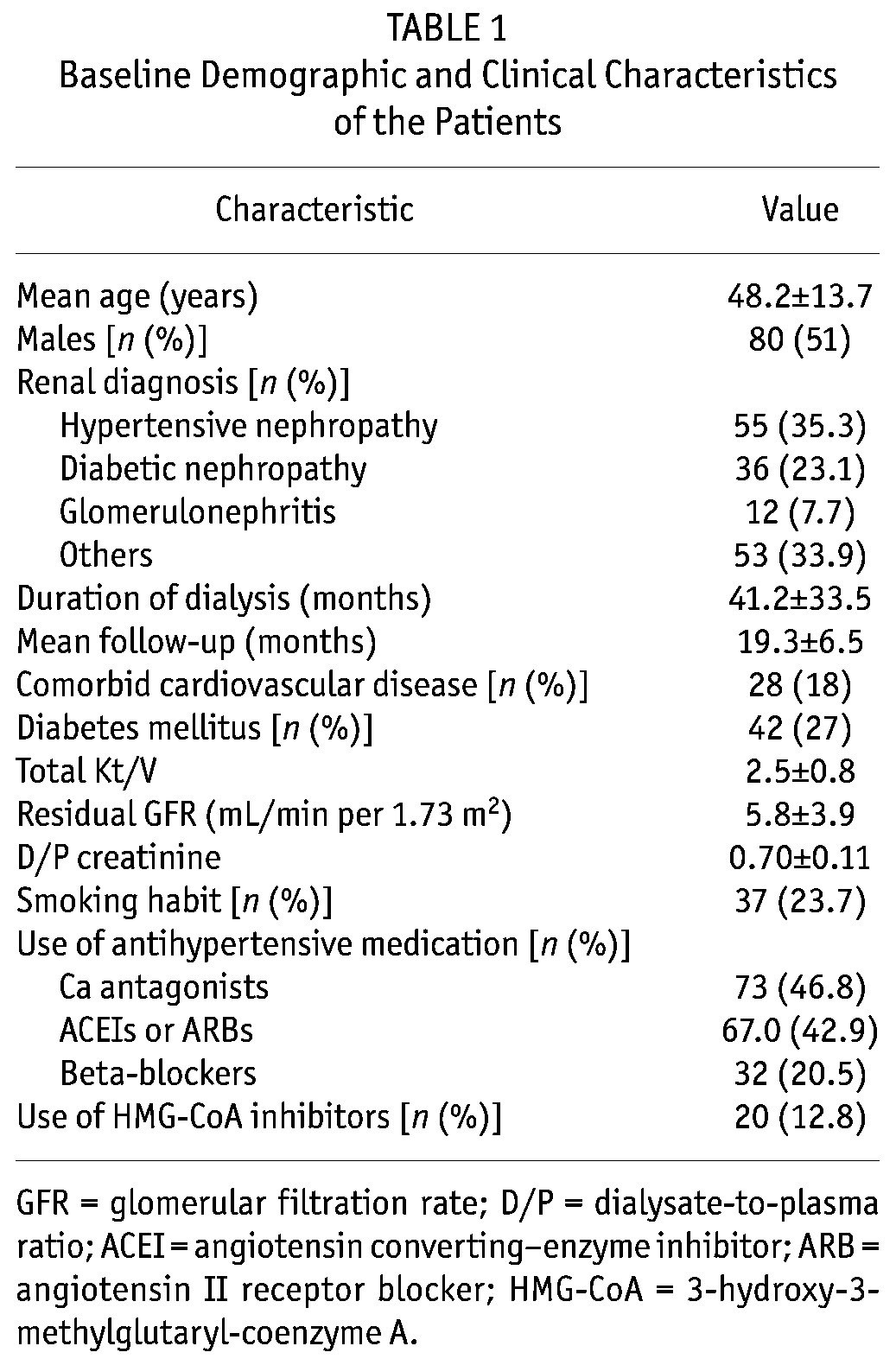
TABLE 2.
Baseline Demographic and Biochemical Parameters in Peritoneal Dialysis Patients and Healthy Subjectsa
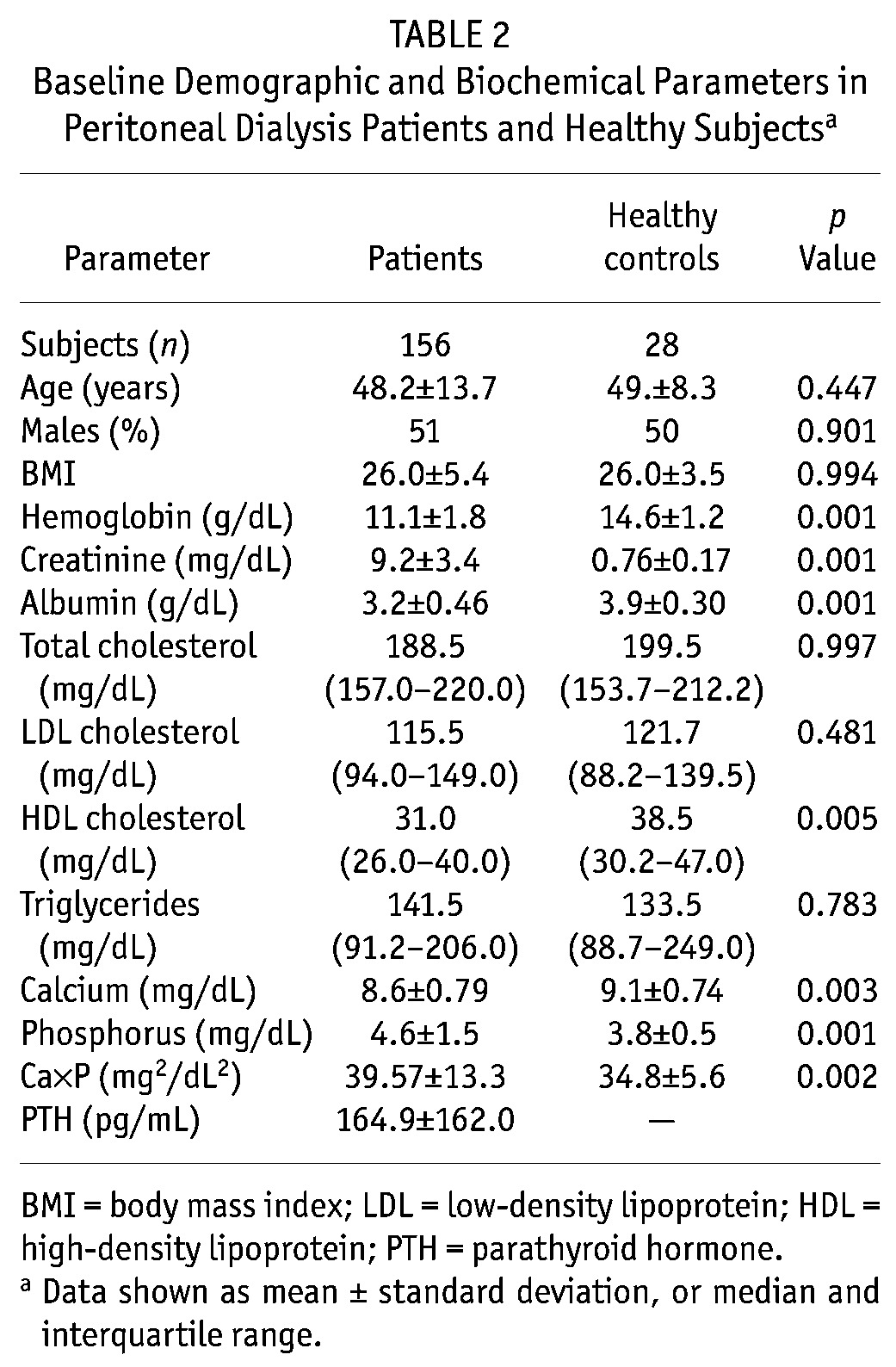
TABLE 3.
Blood Pressure (BP), Aortic Lumen Diameter, Aortic Stiffness Index β (ASIβ), and Echocardiographic Parameters in Peritoneal Dialysis Patients and Healthy Subjectsa
During the follow-up period, 25 patients (16.0%) died, 24 (15.4%) transferred to hemodialysis, 12 (7.7%) underwent renal transplantation, and 9 (5.8%) transferred to another units. Of the 25 deaths, 10 had CV causes (including ischemic heart disease in 5 patients, cerebrovascular disease in 2 patients, heart failure in 1 patient, arrhythmia in 1 patient, and sudden death in 1 patient). The causes of the 15 non-cardiac deaths included peritonitis in 4 patients, other infections in 7 patients, respiratory failure in 1 patient, pulmonary emboli in 1 patient, liver failure in 1 patient, and malignancy in 1 patient. Nonfatal CV events occurred in 15 patients (ischemic heart disease in 8, cerebrovascular event in 3, congestive heart failure in 3, and arrhythmia in 1).
In the linear regression analysis, the log-transformed ASIβ showed the strongest correlation with age (standardized coefficient β = 0.373, p < 0.001), followed by total Kt/V (β = –0.180, p = 0.023) and diabetes (β = 0.159, p = 0.054) (for model: R2 = 0.182, p < 0.001).
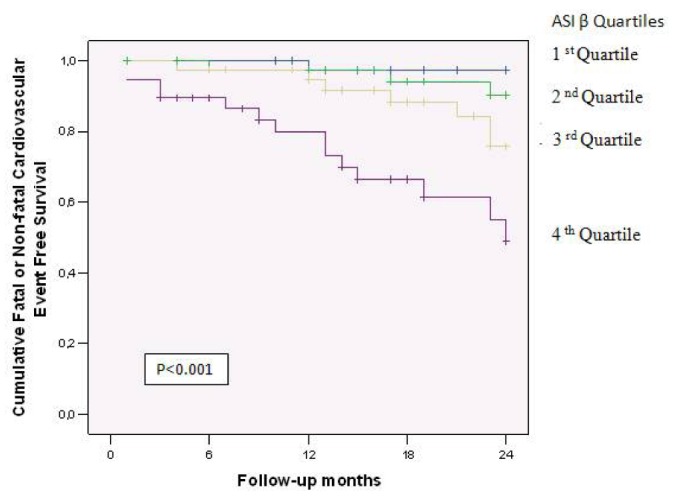
The number of CV deaths was small (only 10), and to avoid an underpowered analysis, multivariate Cox regression was not performed for CV deaths. In the fully adjusted multivariate Cox regression analysis, the ASIβ independently predicted fatal and nonfatal CV events (hazard ratio: 1.239; 95% confidence interval: 1.103 to 1.392), but not all-cause mortality. All-cause mortality was predicted by age, serum albumin, and LVMi. Increases in age and in LVMi increased the risk of all-case mortality, but increases in serum albumin reduced that risk. Table 4 sets out all the outcome results. Figure 1 shows cumulative event-free survivals for fatal and nonfatal CV events in relation to ASIβ quartiles. Comparisons between the survival curves were highly significant. Patients in the lower ASIβ quartiles had better event-free survival rates than did patients in the higher quartiles.
TABLE 4.
Cox Proportional Hazards Models for All-Cause Mortality and Fatal or Nonfatal Cardiovascular Events
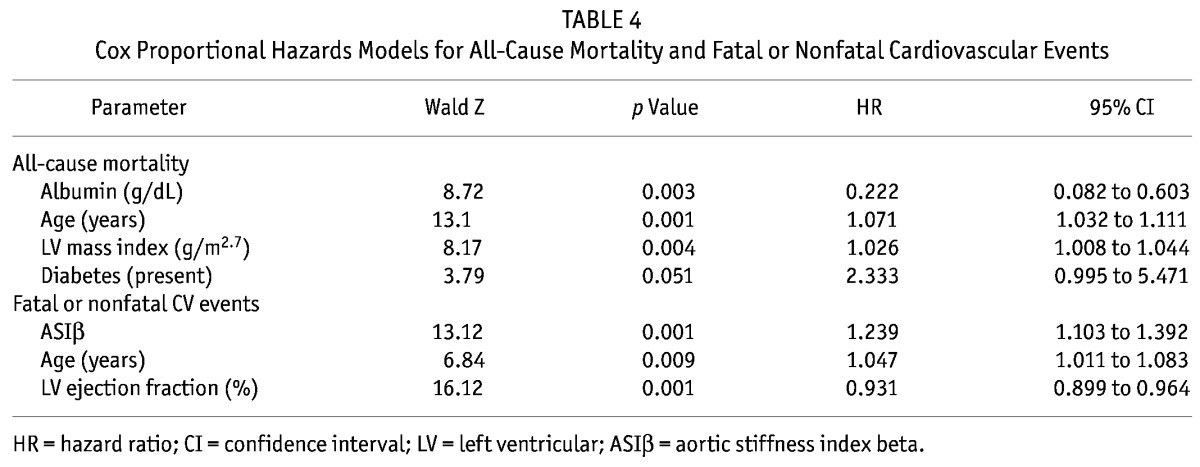
Figure 1.
— Comparison of cumulative event-free survival (Kaplan–Meier analysis) for fatal and nonfatal cardiovascular events in peritoneal dialysis patients stratified by quartiles of aortic stiffness index beta (ASIβ). A log-rank test showed a significant difference between the 1st and 3rd quartiles (p < 0.015), 1st and 4th quartiles (p < 0.001), 2nd and 4th quartiles (p < 0.001), and 3rd and 4th quartiles (p < 0.017).
DISCUSSION
Elasticity of the large arteries plays an important role in the transformation of pulsatile blood flow (a result of the intermittent nature of ventricular contraction) into steady blood flow. During the diastole, recoil of the central arteries pushes the blood forward and hence provides a continuous blood supply for organs and tissues. Reduction in arterial compliance, known as arterial stiffness, changes the BP profile: the systolic BP increases and the diastolic BP decreases, resulting in high pulse pressure. Increased pulse pressure raises the ventricular afterload, causes ventricular hypertrophy, and reduces coronary perfusion (22).
Arterial stiffness can be measured noninvasively by various methods. Measurement of the aortic PWV is generally considered the “gold standard” because it is simple, noninvasive, and reproducible (23). The local and noninvasive ASIβ is comparable, with a high degree of accuracy, to invasive methods (17).
Increased arterial stiffness commonly occurs in patients with chronic renal failure even in the early period of disease (24,25). Endothelial dysfunction, chronic inflammation, vascular calcification, advanced glycation end products (AGEs), and increased renin–angiotensin–aldosterone activity are the main causes of arterial stiffness associated with chronic renal failure (26–32). In the present study, age (positively), Kt/V urea (negatively), and diabetes were found to correlate with arterial stiffness. It is well known that vessels stiffen as age increases (33) because of an overproduction of abnormal collagen fibers and a relative loss of elastin in the extracellular matrix of arteries. The change in the collagen/elastin ratio causes an increase in arterial stiffness (34). Uremic toxins such as asymmetric dimethylarginine (ADMA) and AGEs also play a role in the development of arterial stiffness (35,36). A decrease in dialysis adequacy may increase the level of uremic toxins, including ADMA and AGEs, and thus may contribute to the development of arterial stiffness. Furthermore, uremic toxins may cause arterial stiffness through vascular calcification. The transformation of vascular smooth muscle cells to osteoblast-like cells and the resulting vascular calcification are induced by uremia (37).
Left ventricular hypertrophy is an expected result of vascular stiffness; we nevertheless found no relation between those parameters, which might be explained by the fact that the LVM can be also strongly affected by other parameters such as hypervolemia and hypertension rather than arterial stiffness.
Arterial stiffness is prevalent in hemodialysis and PD patients alike (38–40). The impact of dialysis modality on arterial function is not clear. Although some cross-sectional studies reported that PD patients have stiffer arteries (41,42), no longitudinal study has been undertaken in this field. As in the literature, the PD patients in our study had higher ASIβ values than did the healthy subjects. Zapolski et al. (43) assessed a mean ASIβ of 5.34 in 60 PD patients. The ASIβ in that cohort was higher than that in ours (5.34 vs. 4.2). The difference in age between the two cohorts (51.7 years vs. 48.2 years) may partly explain the difference in arterial stiffness. Because most studies of arterial stiffness in PD patients have used the aortic PWV method of measurement, it is difficult to compare our results with those of other studies.
Some studies in the literature support the notion that renal transplantation may lead to improvement in arterial stiffness in dialysis patients. Stompor et al. reported amelioration in the progression of arterial stiffness in PD patients after renal transplantation (44). In a cross-sectional study, Covic et al. (41) found that renal transplantation patients had lower PWV values than did HD and PD patients. However, the level of kidney function after transplantation is the main factor determining arterial stiffness.
A number of studies showed that arterial stiffness can predict the risk of the future fatal and nonfatal CV events and of all-cause mortality (7,45–48). In a recent meta-analysis that included 17 longitudinal studies evaluating arterial stiffness by aortic PWV in 15 877 subjects, Vlachopoulos et al. (49) reported that “an increase in aortic PWV by 1 m/s corresponded to an age–sex and risk factor adjusted risk increase of 14, 15, and 15% in total CV event, CV mortality, and all-cause mortality, respectively.”
The first study to clearly indicate that aortic stiffness is an independent risk factor for CV and all-cause mortality in HD patients was reported by Blacher et al. (8). In their study, which followed 241 HD patients for an average of 72 months, each aortic PWV increase of 1 m/s was related to a 39% adjusted risk increase in CV outcomes. In another study in 265 HD patients, Shoji et al. showed that PWV is a significant predictor for CV and overall mortality (9). However, in the study reported by Covic et al. (50), arterial stiffness measured as augmentation index did not correlate with mortality in HD patients. Those authors concluded that arterial stiffness might, in fact, depend on patient age and concurrent comorbidity.
Most of the studies that examined the association between arterial stiffness and outcomes in dialysis populations were conducted in HD patients. To date, only one study has involved PD patients. Gao et al. (51) studied 100 PD patients and used aortic PWV as a surrogate of arterial stiffness. They did not find any effect of arterial stiffness on mortality; however, they showed that PWV independently predicted the number of hospitalizations. Short follow-up duration (9.4 ± 4.6 months) was a possible reason for the absence of a relation between aortic stiffness and mortality in their study.
To the best of our knowledge, our study is the first to date to demonstrate that aortic stiffness can predict adverse CV outcomes in PD patients. Aortic stiffness independently predicted composite fatal and nonfatal CV events regardless of other factors known to affect outcome in PD patients—namely, age, pre-existing CV disease, overall duration of PD, diabetes mellitus, smoking, degree of left ventricular hypertrophy, ejection fraction, serum albumin, hemoglobin, and lipid levels. A 1-unit increase in ASIβ corresponded to an age–, sex–, and risk factor–adjusted 23% risk increase for fatal and nonfatal CV events.
Our study has several limitations. First, the number of endpoints for the outcome analysis was small, which might have reduced the power of the study. Second, all of the parameters were measured on a single occasion at study entry; changes over time were not included in the analyses. This omission may have decreased the predictive power of the parameters.
CONCLUSIONS
Our results provide the first direct evidence that arterial stiffness is an independent predictor of risk for adverse CV outcome in PD patients. By incorporating arterial stiffness measurement into regular CV assessments, PD patients who are at increased CV risk can be pinpointed earlier, and active preventive therapy can be started.
DISCLOSURES
CU is a member of advisory boards for the Eczacibasi–Baxter Company. The other authors have no financial conflicts of interest to declare.
REFERENCES
- 1. Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 1998; 9(Suppl):S16–23 [PubMed] [Google Scholar]
- 2. Wang AY. Cardiovascular risk factors in peritoneal dialysis patients revisited. Perit Dial Int 2007; 27(Suppl 2):S223–7 [PubMed] [Google Scholar]
- 3. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003; 108:2154–69 [DOI] [PubMed] [Google Scholar]
- 4. Garcia–Lopez E, Carrero JJ, Suliman ME, Lindholm B, Stenvinkel P. Risk factors for cardiovascular disease in patients undergoing peritoneal dialysis. Perit Dial Int 2007; 27(Suppl 2):S205–9 [PubMed] [Google Scholar]
- 5. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25:1105–87 [DOI] [PubMed] [Google Scholar]
- 6. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121:505–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension 2010; 55:799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999; 99:2434–9 [DOI] [PubMed] [Google Scholar]
- 9. Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, et al. Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol 2001; 12:2117–24 [DOI] [PubMed] [Google Scholar]
- 10. Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989; 2:358–67 [DOI] [PubMed] [Google Scholar]
- 11. Gorgulu S, Uslu N, Eren M, Celik S, Yildirim A, Dagdeviren B, et al. Aortic stiffness in patients with cardiac syndrome X. Acta Cardiol 2003; 58:507–11 [DOI] [PubMed] [Google Scholar]
- 12. Stefanadis C, Dernellis J, Toutouzas P. Mechanical properties of the aorta determined by the pressure-diameter relation. Pathol Biol (Paris) 1999; 47:696–704 [PubMed] [Google Scholar]
- 13. Foppa M, Duncan BB, Rohde LE. Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovasc Ultrasound 2005; 3:17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pannier BM, Avolio AP, Hoeks A, Mancia G, Takazawa K. Methods and devices for measuring arterial compliance in humans. Am J Hypertens 2002; 15:743–53 [DOI] [PubMed] [Google Scholar]
- 15. Itoh S, Umemoto S, Hiromoto M, Toma Y, Tomochika Y, Aoyagi S, et al. Importance of NAD(P)H oxidase–mediated oxidative stress and contractile type smooth muscle myosin heavy chain SM2 at the early stage of atherosclerosis. Circulation 2002; 105:2288–95 [DOI] [PubMed] [Google Scholar]
- 16. Sugioka K, Hozumi T, Sciacca RR, Miyake Y, Titova I, Gaspard G, et al. Impact of aortic stiffness on ischemic stroke in elderly patients. Stroke 2002; 33:2077–81 [DOI] [PubMed] [Google Scholar]
- 17. Stefanadis C, Stratos C, Boudoulas H, Kourouklis C, Toutouzas P. Distensibility of the ascending aorta: comparison of invasive and non-invasive techniques in healthy men and in men with coronary artery disease. Eur Heart J 1990; 11:990–6 [DOI] [PubMed] [Google Scholar]
- 18. Twardowski ZJ. Peritoneal equilibration test. Perit Dial Bull 1987; 7:138–47 [Google Scholar]
- 19. van Olden RW, Krediet RT, Struijk DG, Arisz L. Measurement of residual renal function in patients treated with continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 1996; 7:745–50 [DOI] [PubMed] [Google Scholar]
- 20. Nolph KD, Moore HL, Twardowski ZJ, Khanna R, Prowant B, Meyer M, et al. Cross-sectional assessment of weekly urea and creatinine clearances in patients on continuous ambulatory peritoneal dialysis. ASAIO J 1992; 38:M139–42 [DOI] [PubMed] [Google Scholar]
- 21. Engelstein ED, Zipes DP. Sudden cardiac death. In: Alexander RW, Schlant RC, Fuster V, eds. The Heart, Arteries and Veins. New York, NY: McGraw–Hill; 1998. [Google Scholar]
- 22. Covic A, Gusbeth–Tatomir P, Goldsmith DJ. Arterial stiffness in renal patients: an update. Am J Kidney Dis 2005; 45:965–77 [DOI] [PubMed] [Google Scholar]
- 23. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–605 [DOI] [PubMed] [Google Scholar]
- 24. Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis 2005; 45:494–501 [DOI] [PubMed] [Google Scholar]
- 25. Mourad JJ, Pannier B, Blacher J, Rudnichi A, Benetos A, London GM, et al. Creatinine clearance, pulse wave velocity, carotid compliance and essential hypertension. Kidney Int 2001; 59:1834–41 [DOI] [PubMed] [Google Scholar]
- 26. Demirci MS, Ozkahya M, Asci G, Sevinc E, Yilmaz M, Demirci C, et al. The influence of dialysate calcium on progression of arterial stiffness in peritoneal dialysis patients. Perit Dial Int 2009; 29(Suppl 2):S15–17 [PubMed] [Google Scholar]
- 27. van Guldener C, Janssen MJ, Lambert J, Steyn M, Donker AJ, Stehouwer CD. Endothelium-dependent vasodilatation is impaired in peritoneal dialysis patients. Nephrol Dial Transplant 1998; 13:1782–6 [DOI] [PubMed] [Google Scholar]
- 28. London GM, Marchais SJ, Guerin AP, Metivier F, Adda H. Arterial structure and function in end-stage renal disease. Nephrol Dial Transplant 2002; 17:1713–24 [DOI] [PubMed] [Google Scholar]
- 29. Kobayashi S, Okamoto K, Maesato K, Moriya H, Ohtake T. Important role of blood rheology in atherosclerosis of patients with hemodialysis. Hemodial Int 2005; 9:268–74 [DOI] [PubMed] [Google Scholar]
- 30. Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant 2008; 23:586–93 [DOI] [PubMed] [Google Scholar]
- 31. Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol 2009; 54:505–12 [DOI] [PubMed] [Google Scholar]
- 32. Adragao T, Branco P, Birne R, Curto JD, de Almeida E, Prata MM, et al. Bone mineral density, vascular calcifications, and arterial stiffness in peritoneal dialysis patients. Perit Dial Int 2008; 28:668–72 [PubMed] [Google Scholar]
- 33. Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens 2002; 15:1101–8 [DOI] [PubMed] [Google Scholar]
- 34. Hopkins K, Bakris GL. Assessing blood pressure control in dialysis patients: finally a step forward. Hypertension 2009; 53:448–9 [DOI] [PubMed] [Google Scholar]
- 35. Ueno H, Koyama H, Tanaka S, Fukumoto S, Shinohara K, Shoji T, et al. Skin autofluorescence, a marker for advanced glycation end product accumulation, is associated with arterial stiffness in patients with end-stage renal disease. Metabolism 2008; 57:1452–7 [DOI] [PubMed] [Google Scholar]
- 36. Paiva H, Kahonen M, Lehtimaki T, Raitakari OT, Jula A, Viikari J, et al. Asymmetric dimethylarginine (ADMA) has a role in regulating systemic vascular tone in young healthy subjects: the Cardiovascular Risk in Young Finns Study. Am J Hypertens 2008; 21:873–8 [DOI] [PubMed] [Google Scholar]
- 37. Moe SM, Duan D, Doehle BP, O’Neill KD, Chen NX. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int 2003; 63:1003–11 [DOI] [PubMed] [Google Scholar]
- 38. Konings CJ, Hermans M, Kooman JP, Meinders JM, Hoeks AP, van der Sande FM, et al. Arterial stiffness and renal replacement therapy. Perit Dial Int 2004; 24:318–22 [PubMed] [Google Scholar]
- 39. Konings CJ, Dammers R, Rensma PL, Kooman JP, Hoeks AP, Kornet L, et al. Arterial wall properties in patients with renal failure. Am J Kidney Dis 2002; 39:1206–12 [DOI] [PubMed] [Google Scholar]
- 40. Gusbeth–Tatomir P, Covic A. Causes and consequences of increased arterial stiffness in chronic kidney disease patients. Kidney Blood Press Res 2007; 30:97–107 [DOI] [PubMed] [Google Scholar]
- 41. Covic A, Goldsmith DJ, Florea L, Gusbeth–Tatomir P, Covic M. The influence of dialytic modality on arterial stiffness, pulse wave reflections, and vasomotor function. Perit Dial Int 2004; 24:365–72 [PubMed] [Google Scholar]
- 42. Chung AW, Yang HH, Kim JM, Sigrist MK, Brin G, Chum E, et al. Arterial stiffness and functional properties in chronic kidney disease patients on different dialysis modalities: an exploratory study. Nephrol Dial Transplant 2010; 25:4031–41 [DOI] [PubMed] [Google Scholar]
- 43. Zapolski T, Wysokinski A, Janicka L, Grzebalska A, Ksiazek A. Aortic stiffness and valvular calcifications in patients with end-stage renal disease. Pol Arch Med Wewn 2008; 118:111–18 [PubMed] [Google Scholar]
- 44. Stompor T, Rajzer M, Kawecka–Jaszcz K, Dembinska–Kiec A, Janda K, Wojcik K, et al. Renal transplantation ameliorates the progression of arterial stiffness in patients treated with peritoneal dialysis. Perit Dial Int 2005; 25:492–6 [PubMed] [Google Scholar]
- 45. Anderson SG, Sanders TA, Cruickshank JK. Plasma fatty acid composition as a predictor of arterial stiffness and mortality. Hypertension 2009; 53:839–45 [DOI] [PubMed] [Google Scholar]
- 46. Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002; 106:2085–90 [DOI] [PubMed] [Google Scholar]
- 47. Mattace–Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam study. Circulation 2006; 113:657–63 [DOI] [PubMed] [Google Scholar]
- 48. Terai M, Ohishi M, Ito N, Takagi T, Tatara Y, Kaibe M, et al. Comparison of arterial functional evaluations as a predictor of cardiovascular events in hypertensive patients: the Non-Invasive Atherosclerotic Evaluation in Hypertension (NOAH) study. Hypertens Res 2008; 31:1135–45 [DOI] [PubMed] [Google Scholar]
- 49. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55:1318–27 [DOI] [PubMed] [Google Scholar]
- 50. Covic A, Mardare N, Gusbeth–Tatomir P, Prisada O, Sascau R, Goldsmith DJ. Arterial wave reflections and mortality in haemodialysis patients—only relevant in elderly, cardiovascularly compromised? Nephrol Dial Transplant 2006; 21:2859–66 [DOI] [PubMed] [Google Scholar]
- 51. Gao N, Kwan BC, Chow KM, Chung KY, Pang WF, Leung CB, et al. Arterial pulse wave velocity and peritoneal transport characteristics independently predict hospitalization in Chinese peritoneal dialysis patients. Perit Dial Int 2010; 30:80–5 [DOI] [PubMed] [Google Scholar]


