Abstract
♦ Introduction: Little is known regarding the causes and outcomes of peritoneal dialysis (PD) patients admitted to the intensive care unit (ICU). We explored the outcomes of technique failure and mortality in a cohort of PD patients admitted to the ICU.
♦ Methods: Using a provincial database of 990 incident PD patients followed from January 1997 to June 2009, we identified 90 (9%) who were admitted to the ICU. Parametric and nonparametric tests were used as appropriate to determine differences in baseline characteristics. The Cox proportional hazards and competing risk methods were used to investigate associations.
♦ Results: Compared with other patients, those admitted to the ICU had been on PD longer (p < 0.0001) and were more often on continuous ambulatory PD (74.2% vs 25.8%, p = 0.016). Cardiac problems were the most common admitting diagnosis (50%), followed by sepsis (23%), with peritonitis accounting for 69% of the sepsis admissions. The 1-year mortality was 53.3%, with 12% alive and converted to hemodialysis, and one third remaining alive on PD. In multivariate Cox modeling, age [hazard ratio (HR): 1.01; 95% confidence interval (CI): 0.99 to 1.03], white blood cell count (HR: 1.02; 95% CI: 1.00 to 1.04), temperature (HR: 0.75; 95% CI: 0.61 to 0.92), and peritonitis (1.64; 95% CI: 1.21 to 2.22) at admission to the ICU were associated with the composite outcome of technique failure or death. In a competing risk analysis, the risk for death was 30%, and for technique failure, 36% at 1 year.
♦ Conclusions: Patients on PD have high rates of death and technique failure after admission to the ICU.
Keywords: Outcomes, intensive care unit, critical care, mortality, technique failure, competing risk
As the steady worldwide rise in the peritoneal dialysis (PD) population continues, the requirements for and health resources consumed by this population rise in parallel. Today, PD patients are increasingly older, with lower functional status and a higher burden of comorbid illness (1–4). This situation has cumulated in a rise in critical illness and the need for admission to the intensive care unit (ICU) (5–7). Despite this escalation in ICU admission, little literature is available about the causes of critical illness, prognosis, or long-term outcomes to guide health care providers (8–18).
The existing data seem to suggest that, in the dialysis population, admission to the ICU portends a poor prognosis. However, the situation is seemingly more complicated, because after accounting for comorbid illnesses and illness acuity upon admission to the ICU, end-stage renal disease (ESRD) patients have outcomes similar to those for non-ESRD patients. This suggests that ESRD is not per se responsible for an increase in poor outcomes such as death and length of ICU stay (5,6).
To our knowledge, few studies have directly addressed the impact of dialysis modality on critical illness. One small study included 92 ESRD patients, 16 of whom were on PD (12). The PD patients experienced poor survival, having a 44% mortality compared with 25% in the hemodialysis (HD) cohort. Recent data collected by our group further strengthens the finding that dialysis modality may play a strong role in survival (19). Our group analyzed the long-term outcomes of 619 ESRD patients, 95 of whom were on PD; 334, on HD with a catheter; and 190, on HD with an arteriovenous fistula. Three different models accounting for case mix, comorbid illness, and physiologic variables found, as expected, that compared with HD patients having an arteriovenous fistula, HD patients with a catheter had an adjusted hazard ratio (HR) for death ranging from 1.50 to 1.58. What was unexpected and surprising was we also observed an increased association with long-term mortality for PD patients, with HRs of 1.63 to 1.75. Furthermore, the PD patients were younger, with significantly lower rates of vascular disease and in-hospital cardiac arrest, all factors associated with lower mortality. We felt this observation warranted further investigation to delineate potential mechanisms and causes.
The objectives of the present study were to describe the PD patients admitted to the ICU and to investigate the outcomes of technique failure (TF) and mortality and factors associated with those outcomes.
METHODS
STUDY POPULATION AND DESIGN
The study population consisted of all adults (≥18 years of age) on PD admitted to any of 11 ICUs in Manitoba, Canada, (catchment area 1.2 million) between January 2000 and December 2006, with follow-up to 30 June 2009.
DATA SOURCES
The study cohort was created by linking Manitoba’s PD database (PD-MAN) with the Manitoba ICU databases as described elsewhere (6,20). The Manitoba Renal Program (MRP) database captures prospective data on patient demographics, comorbid illness, date of dialysis initiation, modality, TF, peritonitis, and death. Of roughly 1200 prevalent dialysis patients in Manitoba, 20% are on PD. The Manitoba ICU database is a prospectively maintained database that captures information on demographics, physiologic and laboratory values, comorbidities, and outcomes for all patients admitted. Each patient may have up to 5 admission diagnoses on admission to the ICU (1 primary and 4 additional diagnoses).
COHORT DEFINITIONS
Peritoneal dialysis was defined as the insertion of a PD catheter: the date of PD initiation was the date of PD catheter insertion. More than 95% of patients would start PD within 30 days of insertion. Comorbidities were defined as follows: “Diabetes mellitus” was either type 1 or 2. “Coronary artery disease” was any of the presence of significant stenosis by angiography, a positive stress test, history of an acute coronary syndrome, or coronary artery bypass surgery; congestive heart failure by history of pulmonary edema by imaging; peripheral vascular disease by ankle brachial index less than 1.0 or stenosis on angiography; stroke by radiographic demonstration of an ischemic event, hemorrhage. or history of transient ischemic attack. The “distance to center” represents the direct linear distance from the patient’s postal code to the major PD hospital in Winnipeg, Manitoba, using ArcView (version 9.3: ESRI, Redlands, CA, USA) using the Vincenty formula (21). The peritoneal and renal Kt/V and peritoneal equilibration test values used for the analyses were those first recorded after patients initiated PD. The PD modality was classified as either continuous ambulatory PD (CAPD) or continuous cycling PD based on the modality that was prescribed the longest.
OUTCOME DEFINITIONS
The primary outcome was the composite of TF or death. Technique failure was the date of PD discontinuation.
DATA ANALYSES
Continuous variables of interest are summarized as mean or medians with standard deviations or interquartile ranges, as appropriate. Differences in baseline characteristics were determined using the Student t-test for continuous variables and the chi-square or Fischer exact test for dichotomous variables. Time-to-event analyses were used to determine survival curves and covariate associations. The Cox proportional hazards model was used to determine multivariate associations with the outcome of interest, with model selection based on univariate p values and clinical significance. Variables were removed from the model based on the significance of the change in the –2 log likelihood. A competing-risk survival analysis was performed to determine the earliest event of the outcomes of death or TF after ICU admission. Patients were censored at the end of the study period or after experiencing an outcome of either death or TF. The cumulative incidence was calculated as described by Pintilie (22). All analyses were performed using PASW (version 18.0: SPSS, Chicago, IL, USA).
RESULTS
During the study period, 990 patients started PD in Manitoba, among which 95 (9.6%) were admitted to the ICU. The crude rate of ICU admission in PD patients was 13.3 admissions per 100 patient–years on PD (95 admissions per 713 patient–years on PD). Full data were available for 90 patients (95% of those admitted), and those patients were included in the analyses.
Table 1 presents the baseline characteristics of patients admitted to the ICU and those not requiring ICU admission. Significant baseline differences for the patients requiring ICU admission compared with those not admitted were longer PD vintage (1457 vs 756 days, p < 0.0001) and a greater likelihood of being on CAPD (74% vs. 26%, p = 0.016). The average age of patients admitted to the ICU was 61.2 years, and their most common cause of ESRD and comorbidity was diabetes mellitus. Most were high-average transporters with a total initial Kt/V above 2.4.
TABLE 1.
Baseline Demographics, Causes of End-Stage Renal Disease (ESRD), Comorbidities, and Peritoneal Dialysis (PD) Characteristics of Patients Requiring and Not Requiring Admission to the Intensive Care Unit (ICU)
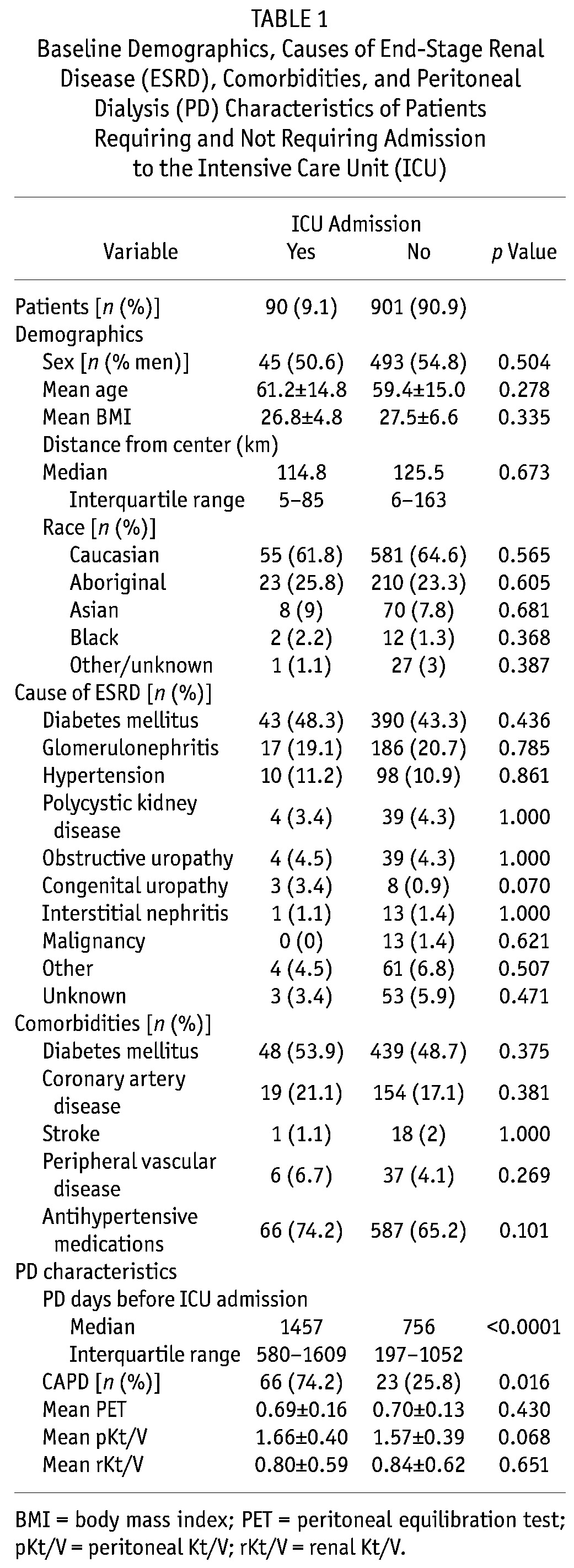
Figure 1 presents the most common ICU admission diagnoses. Owing to the medical complexity of patients admitted to the ICU, most had more than 1 diagnosis at admission; all causes of admission (n = 234) are presented. Of all admissions, 23% (54 of 234) were for sepsis or peritonitis, with 3 of 54 (5.6%) having peritonitis as the primary diagnosis and 37 of 54 (68.5%) having peritonitis among any of the 5 admission diagnoses. Cardiac disease (acute coronary syndrome, cardiogenic shock, arrhythmias, post–cardiac surgery, and congestive heart failure) accounted for 117 of all admissions (50%).
Figure 1.
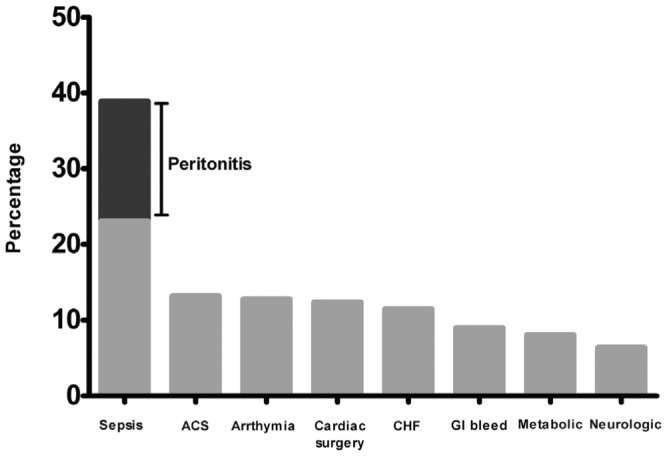
— Sepsis and cardiac disease were the most common diagnoses in peritoneal dialysis patients at admission to the intensive care unit. ACS = acute coronary syndrome; CHF = congestive heart failure; GI = gastrointestinal.
Figures 2 and 3 depict the evolution of mortality and TF. Death in the ICU occurred in a relatively modest 10% of the cohort; 18.9% were permanently converted to HD. At 6 months and 12 months post ICU admission, 57.6% and 46.7% of patients were alive, with 44% and 33% remaining on PD. A competing-risk survival analysis (Figure 3) shows that most death and TF occurred within the first 24 months post ICU discharge, with the initial risk being greater for TF than for death until 14 months post ICU discharge, after which the risk of death was greater. The 1-year risks for death and TF were 30% and 36% respectively.
Figure 2.
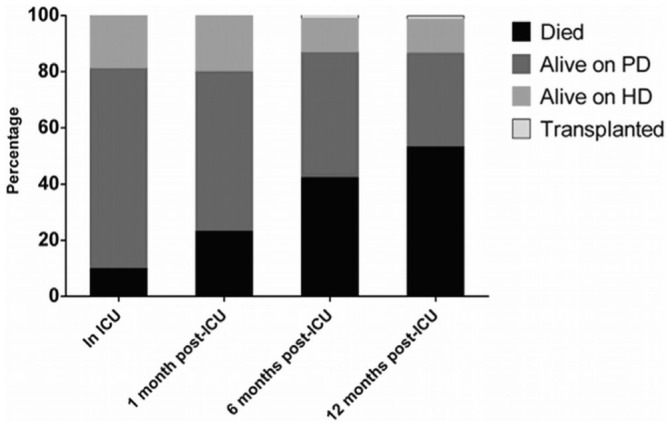
— Proportional outcomes among peritoneal dialysis (PD) patients admitted to the intensive care unit (ICU) and with up to 12 months’ follow-up. Few patients remained on peritoneal dialysis (PD) at 12 months. HD = hemodialysis.
Figure 3.
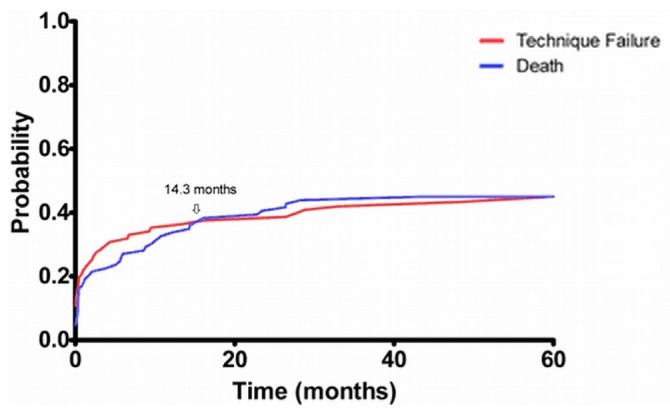
— Cumulative incidence curves for death and technique failure for peritoneal dialysis patients after admission to the intensive care unit (ICU). The risk for death or technique failure was highest early after discharge from the ICU, with the risk for technique failure initially being greater than the risk for death.
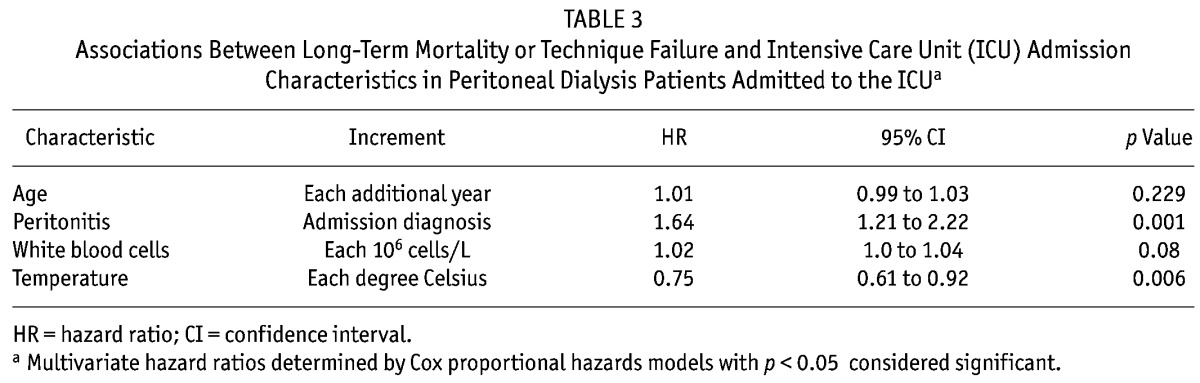
Tables 2 and 3 present the variables associated with the composite outcome of TF or mortality. Univariate associations with the composite outcome were statistically significant for various ICU admission characteristics: mean arterial pressure, white blood cells (WBCs), and heart rate. In multivariate Cox modeling, patient age [HR: 1.01; 95% confidence interval (CI): 0.99 to 1.03], white blood cell count (HR: 1.02; 95% CI: 1.00 to 1.04), temperature (HR: 0.75; 95% CI: 0.61 to 0.92), and peritonitis (HR: 1.64; 95% CI: 1.21 to 2.22) at admission to the ICU were associated with the composite outcome of TF or death.
TABLE 2.
Associations Between Long-Term Mortality or Technique Failure and Intensive Care Unit (ICU) Admission Characteristics in Peritoneal Dialysis (PD) Patients Admitted to the ICUa
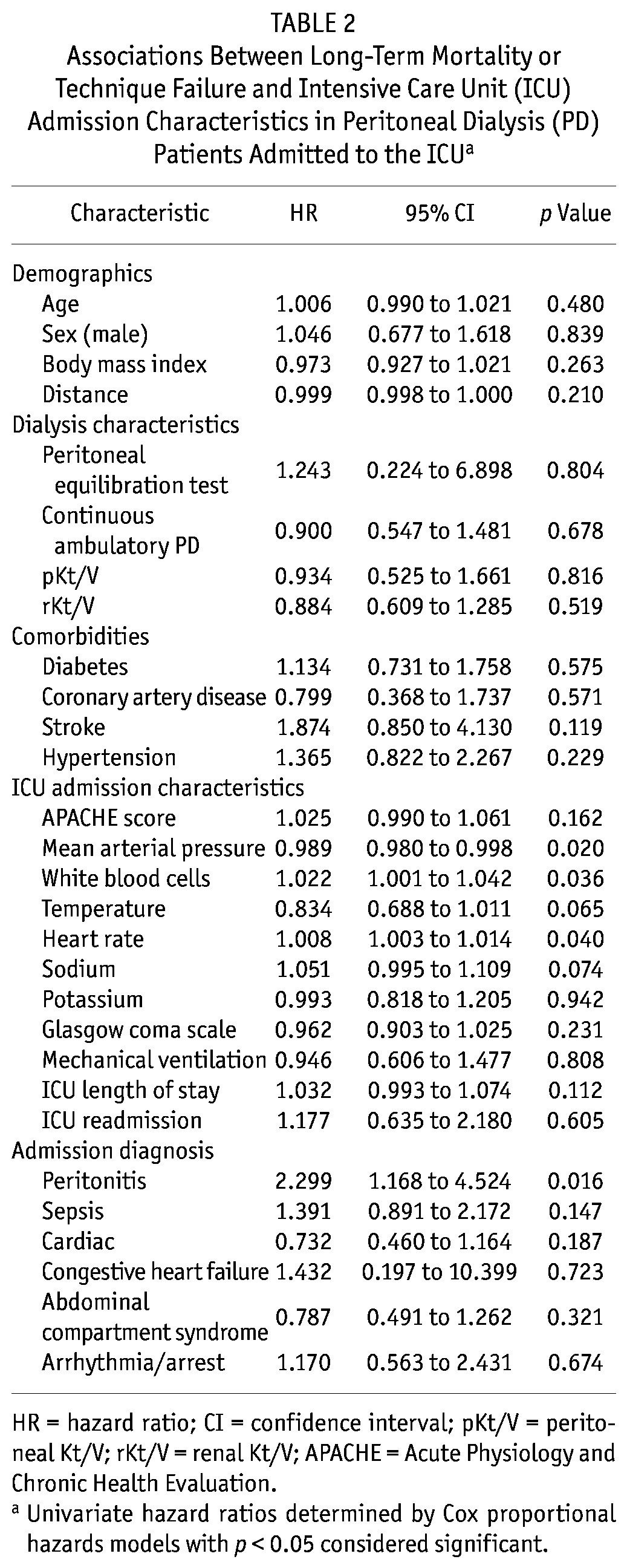
TABLE 3.
Associations Between Long-Term Mortality or Technique Failure and Intensive Care Unit (ICU) Admission Characteristics in Peritoneal Dialysis Patients Admitted to the ICUa
DISCUSSION
There is a paucity of data regarding PD patients admitted to the ICU. We found that, compared with PD patients not requiring ICU admission, PD patients with critical illnesses were more likely to be on CAPD and to have been on dialysis longer. The most frequent admission diagnoses were infection or sepsis, and cardiac disease. Furthermore, long-term outcomes after ICU discharge were poor, with high rates of TF and mortality. Physiologic factors (high WBCs and low temperature) upon admission to the ICU and admission for peritonitis were predictive of these poor outcomes.
Death and TF were common in our cohort, with the greatest risk occurring within the first 2 years after ICU discharge. Mortality in the ICU itself was relatively low at 10%; however, that percentage climbed rapidly to 42% at 6 months post ICU admission, meaning that one third of patients who survive their ICU admission will die within 6 – 12 months. Those findings were confirmed in a competing-risk survival analysis that accounted for death or TF as individual outcomes (23–26).
The risk of death starts to plateau at between 12 and 24 months (Figure 3), approaching the pre–ICU admission mortality. This high risk of death in the immediate post-ICU care period has been demonstrated in other cohorts and implies a high level of fragility in the ESRD population (11,19,27,28).
Roughly one fifth of PD patients admitted to the ICU are converted to HD. Critical illness appears to be a nidus for TF, and that risk persists steadily over time, increasing to 36% at 1 year (Figure 3). Comparing the risks of TF and mortality, TF is highest in the first 14 months, after which the risk of mortality is greater. At the end of 1 year, only 12% of the initial cohort remained alive on HD, suggesting that most who experienced TF died. Thus, TF is potentially a poor prognostic sign. The reasons for TF were not captured; however, it is plausible that the cause of the ICU admission (peritonitis, gastrointestinal illness) or changes in the patient’s functional status after critical illness, or both, may significantly impair the ability to perform PD.
The rate of ICU admission among PD patients appears relatively low at 13.3 admissions per 100 patient–years on PD (95 admissions per 713 patient–years on PD). The crude rate of ICU admission in the overall ESRD cohort (PD and HD combined) was greater at 19.5 per 100 patient–years, illustrating that HD patients require more ICU admissions and likely experience more critical illness (6). Taken in context, PD patients may experience poor outcomes after ICU admission, but they require far fewer ICU admissions and likely experience fewer critical illnesses than their HD counterparts.
For our composite outcome of TF or death, the predictive factors were largely physiologic variables (high WBCs and low temperature) obtained at the time of ICU admission. That finding is consistent with those from other ICU studies in the general population that have led to the development of numerous predictive and illness severity scores (for example, APACHE, the Acute Physiology and Chronic Health Evaluation) with an emphasis on admission physiology (29,30). In our univariate and multivariate models, the APACHE score was not associated with outcomes, calling into question whether ICU scoring systems developed in the general population are predictive in ESRD, and specifically PD, patients. Future investigations should look specifically at developing and validating tools tailored for the ESRD population.
Peritonitis is a frequent complication in PD, with a wide spectrum of illness severity from low acuity infection requiring outpatient therapy, to critical illness and sepsis (31–36). Over our 6-year study period, 3 admissions to the ICU were attributable to a primary diagnosis of peritonitis (0.4 admissions per 100 patient–years on PD). In 34 ICU admissions, peritonitis was listed as the primary or a concurrent diagnosis (4.8 episodes per 100 patient–years on PD). In our total PD cohort over the same period, the rate of peritonitis was 65.0 episodes per 100 patient–years, meaning that roughly 0.6% of all episodes of peritonitis required ICU admission for primary illness, a percentage that increases to 7% of all peritonitis episodes if primary and concurrent diagnoses are both considered. That finding is interesting, because it suggests that peritonitis per se is rarely identified as the primary cause for critical illness, and yet its complications—sequelae such as volume overload, respiratory failure, or metabolic complications—may be leading to ICU admission. Whether the episodes of peritonitis that required ICU admission represent true primary infections of the peritoneum, or secondary infections because of sepsis from another source or gastrointestinal illness, remains unclear. Of concern is the significant mortality associated with a primary or concurrent diagnosis of peritonitis in both the univariate and the multivariate models. Emphasis should be placed on early detection, a high index of suspicion, and aggressive goal-directed therapy for peritonitis.
Among the several studied risk factors for admission to the ICU, 2 were found to be significantly different between the groups. Compared with PD patients who were not admitted to the ICU, those who were admitted had been on dialysis longer (756 days vs 1457 days respectively) and were more likely to be on CAPD (n = 66, 74.2%, p = 0.016). These observations may all be a result of survival bias, because patients with prolonged survival will have a higher chance of experiencing a critical illness. Furthermore, for adequate clearance, patients with higher peritoneal transport status are often converted early to continuous cycling PD, and higher peritoneal transport is associated with an increase in mortality (37,38). Alternatively, the suggestion that CAPD patients have higher rates of peritonitis and volume overload is controversial but, if true, could predispose them to critical cardiac disease or infection, an observation reported in other cohorts (39–42).
Our study has numerous limitations that need to be addressed. First, it used registry administrative datasets that might be limited in accuracy and validity. Patients classified in the ICU database as ESRD receiving PD were therefore cross-validated with our PD-MAN database. The decision to convert a patient to HD is often clinical and prone to subjectivity. Our study would have been strengthened if the indication for TF had been known. The lack of this information limited the interpretation of our results, which must be viewed as hypothesis-generating only. Because up to 5 admission diagnoses were recorded, it may have been difficult to discern primary from concurrent admission diagnoses because of the complexities of critically ill patients. Data about the HD modality used in the ICU (intermittent or continuous renal replacement therapy) were not available. Because of our small cohort size and few endpoints, the multivariate models could account only for a limited number of variables. Lastly, Manitoba has a unique population with a high number of aboriginal PD patients who are often younger, have high rates of diabetes, and experience higher rates of peritonitis and mortality (20). Thus, our findings have limited generalizability and require confirmation in other cohorts.
CONCLUSIONS
This study describes the admission diagnoses, outcomes, and factors predictive of outcome in a cohort of PD patients admitted to the ICU. Critical illness in the PD population leads to high rates of TF and death in long-term follow-up. Physiologic characteristics and a diagnosis of peritonitis at time of admission are predictive of subsequent outcomes. Further investigations to help tailor care and improve prognostication in this population is warranted.
DISCLOSURES
This research was funded by a grant from the Renal Research and Development Committee (RRDC).
Acknowledgments
The authors thank Dolores Friesen, Amanda Eng, Loretta Eng, and the Manitoba Renal Program.
REFERENCES
- 1. Canadian Institute for Health Information (CIHI). Renal replacement therapy for end-stage renal disease. In: Treatment of End-Stage Organ Failure in Canada, 1998 to 2007: CORR 2009 Annual Report. Ottawa, ON: CIHI; 2009: 5–22 [Google Scholar]
- 2. Verger C, Ryckelynck JP, Duman M, Veniez G, Lobbedez T, Boulanger E, et al. French peritoneal dialysis registry (RDPLF): outline and main results. Kidney Int Suppl 2006; (103):S12–20 [DOI] [PubMed] [Google Scholar]
- 3. United States Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urologic, and Hematologic Diseases. USRDS 2008 Annual Data Report. Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: United States Renal Data System; 2007. [Google Scholar]
- 4. Jassal SV, Trpeski L, Zhu N, Fenton S, Hemmelgarn B. Changes in survival among elderly patients initiating dialysis from 1990 to 1999. CMAJ 2007; 177:1033–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hutchison CA, Crowe AV, Stevens PE, Harrison DA, Lipkin GW. Case mix, outcome and activity for patients admitted to intensive care units requiring chronic renal dialysis: a secondary analysis of the ICNARC Case Mix Programme Database. Crit Care 2007; 11:R50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strijack B, Mojica J, Sood M, Komenda P, Bueti J, Reslerova M, et al. Outcomes of chronic dialysis patients admitted to the intensive care unit. J Am Soc Nephrol 2009; 20:2441–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bagshaw SM, Mortis G, Doig CJ, Godinez–Luna T, Fick GH, Laupland KB. One-year mortality in critically ill patients by severity of kidney dysfunction: a population-based assessment. Am J Kidney Dis 2006; 48:402–9 [DOI] [PubMed] [Google Scholar]
- 8. Dara SI, Afessa B, Bajwa AA, Albright RC. Outcome of patients with end-stage renal disease admitted to the intensive care unit. Mayo Clin Proc 2004; 79:1385–90 [DOI] [PubMed] [Google Scholar]
- 9. Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP. Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int 2002; 62:986–96 [DOI] [PubMed] [Google Scholar]
- 10. Chapman RJ, Templeton M, Ashworth S, Broomhead R, McLean A, Brett SJ. Long-term survival of chronic dialysis patients following survival from an episode of multiple-organ failure. Crit Care 2009; 13:R65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bell M, Granath F, Schön S, Löfberg E, SWING, Ekbom A, et al. End-stage renal disease patients on renal replacement therapy in the intensive care unit: short- and long-term outcome. Crit Care Med 2008; 36:2773–8 [DOI] [PubMed] [Google Scholar]
- 12. Manhes G, Heng AE, Aublet–Cuvelier B, Gazuy N, Deteix P, Souweine B. Clinical features and outcome of chronic dialysis patients admitted to an intensive care unit. Nephrol Dial Transplant 2005; 20:1127–33 [DOI] [PubMed] [Google Scholar]
- 13. Rocha E, Soares M, Valente C, Nogueira L, Bonomo H, Jr, Godinho M, et al. Outcomes of critically ill patients with acute kidney injury and end-stage renal disease requiring renal replacement therapy: a case–control study. Nephrol Dial Transplant 2009; 24:1925–30 [DOI] [PubMed] [Google Scholar]
- 14. Uchino S, Morimatsu H, Bellomo R, Silvester W, Cole L. End-stage renal failure patients requiring renal replacement therapy in the intensive care unit: incidence, clinical features, and outcome. Blood Purif 2003; 21:170–5 [DOI] [PubMed] [Google Scholar]
- 15. Arulkumaran N, Eastwood JB, Banerjee D. Haemodialysis and peritoneal dialysis patients admitted to intensive care units. Crit Care 2007; 11:133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bagshaw SM, Uchino S. End-stage kidney disease patients in the intensive care unit. Nephrol Dial Transplant 2009; 24:1714–17 [DOI] [PubMed] [Google Scholar]
- 17. Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP. Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int 2002; 62:986–96 [DOI] [PubMed] [Google Scholar]
- 18. Ostermann M, Chang R, on behalf of the Riyadh ICU Program Users Group. Renal failure in the intensive care unit: acute kidney injury compared to end-stage renal failure. Crit Care 2008; 12:432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sood MM, Miller L, Komenda P, Reslerova M, Bueti J, Santhianathan C, et al. Long-term outcomes of end-stage renal disease patients admitted to the ICU. Nephrol Dial Transplant 2011; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20. Sood MM, Komenda P, Sood AR, Reslerova M, Verrelli M, Sathianathan C, et al. Adverse outcomes among aboriginal patients receiving peritoneal dialysis. CMAJ 2010; 182:1433–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vincenty T. Direct and inverse solutions of geodesics on the ellipsoid with application of nested equations. Surv Rev XXIII [misprinted as XXII] 1975; 176:88–93 [Google Scholar]
- 22. Pintilie M. Analysing and interpreting competing risk data. Stat Med 2007; 26:1360–7 [DOI] [PubMed] [Google Scholar]
- 23. Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res 2007; 13:559–65 [DOI] [PubMed] [Google Scholar]
- 24. Satagopan JM, Ben–Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer 2004; 91:1229–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jager KJ, Stel VS, Zoccali C, Wanner C, Dekker FW. The issue of studying the effect of interventions in renal replacement therapy—to what extent may we be deceived by selection and competing risk? Nephrol Dial Transplant 2010; 25:3836–9 [DOI] [PubMed] [Google Scholar]
- 26. Verduijn M, Grootendorst DC, Dekker FW, Jager KJ, le Cessie S. The analysis of competing events like cause-specific mortality—beware of the Kaplan–Meier method. Nephrol Dial Transplant 2011; 26:56–61 [DOI] [PubMed] [Google Scholar]
- 27. de Rooij SE, Govers A, Korevaar JC, Abu-Hanna A, Levi M, de Jonge E. Short-term and long-term mortality in very elderly patients admitted to an intensive care unit. Intensive Care Med 2006; 32:1039–44 [DOI] [PubMed] [Google Scholar]
- 28. Udekwu P, Gurkin B, Oller D, Lapio L, Bourbina J. Quality of life and functional level in elderly patients surviving surgical intensive care. J Am Coll Surg 2001; 193:245–9 [DOI] [PubMed] [Google Scholar]
- 29. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–29 [PubMed] [Google Scholar]
- 30. Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 1991; 100:1619–36 [DOI] [PubMed] [Google Scholar]
- 31. Nessim SJ, Bargman JM, Austin PC, Story K, Jassal SV. Impact of age on peritonitis risk in peritoneal dialysis patients: an era effect. Clin J Am Soc Nephrol 2009; 4:135–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bunke CM, Brier ME, Golper TA. Outcomes of single organism peritonitis in peritoneal dialysis: gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int 1997; 52:524–9 [DOI] [PubMed] [Google Scholar]
- 33. Mujais S, Story K. Peritoneal dialysis in the US: evaluation of outcomes in contemporary cohorts. Kidney Int Suppl 2006; (103):S21–6 [DOI] [PubMed] [Google Scholar]
- 34. Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int Suppl 2006; (103):S55–62 [DOI] [PubMed] [Google Scholar]
- 35. Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV. Predictors of peritonitis in patients on peritoneal dialysis: results of a large, prospective Canadian database. Clin J Am Soc Nephrol 2009; 4:1195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim DK, Yoo TH, Ryu DR, Xu ZG, Kim HJ, Choi KH, et al. Changes in causative organisms and their antimicrobial susceptibilities in CAPD peritonitis: a single center’s experience over one decade. Perit Dial Int 2004; 24:424–32 [PubMed] [Google Scholar]
- 37. Rumpsfeld M, McDonald SP, Johnson DW. Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. J Am Soc Nephrol 2006; 17:271–8 [DOI] [PubMed] [Google Scholar]
- 38. Lo WK, Bargman JM, Burkart J, Krediet RT, Pollock C, Kawanishi H, et al. Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit Dial Int 2006; 26:520–2 [PubMed] [Google Scholar]
- 39. Rabindranath KS, Adams J, Ali TZ, Daly C, Vale L, Macleod AM. Automated vs continuous ambulatory peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2007; 22:2991–8 [DOI] [PubMed] [Google Scholar]
- 40. Piraino B, Sheth H. Peritonitis—does peritoneal dialysis modality make a difference? Blood Purif 2010; 29:145–9 [DOI] [PubMed] [Google Scholar]
- 41. Oo TN, Roberts TL, Collins AJ. A comparison of peritonitis rates from the United States Renal Data System database: CAPD versus continuous cycling peritoneal dialysis patients. Am J Kidney Dis 2005; 45:372–80 [DOI] [PubMed] [Google Scholar]
- 42. Huang JW, Hung KY, Yen CJ, Wu KD, Tsai TJ. Comparison of infectious complications in peritoneal dialysis patients using either a twin-bag system or automated peritoneal dialysis. Nephrol Dial Transplant 2001; 16:604–7 [DOI] [PubMed] [Google Scholar]


