Abstract
♦ Objective: An association of Streptococcus bovis bacteremia with carcinoma of colon has been reported, but data regarding peritoneal dialysis (PD) peritonitis caused by S. bovis is scarce. In this study, we examined the clinical characteristics, associations, and outcomes of this disease entity.
♦ Methods: The case records of patients with S. bovis PD peritonitis presenting to 2 renal centers between January 2000 and September 2010 were reviewed. Clinical features and outcomes were identified and analyzed.
♦ Results: Of cultures from 23 episodes of S. bovis peritonitis in 20 patients (1.28% of all peritonitis episodes at our center), 19 (82.6%) showed S. bovis alone, and 4 (17.4%) showed mixed growth. In 7 episodes, the S. bovis was moderately resistant to penicillin G. Rates of resistance to clindamycin and erythromycin were 43.5% and 47.8% respectively. In 18 episodes (78.3%), a primary response was achieved with a first-generation cephalosporin and an aminoglycoside. In 4 episodes, a secondary response was achieved after a switch from cephalosporin to vancomycin, and in 1 episode with mixed growth, the Tenckhoff catheter had to be removed. Repeat peritonitis occurred in 3 patients at a mean of 50.0 months (range: 24.2 – 83.1 months). Of the 20 patients of S. bovis peritonitis, 10 (50%) underwent either a barium enema or a colonoscopy. One patient had history of colonic carcinoma 2 years before the peritonitis, and a subsequent work-up revealed no recurrence. Three patients had diverticulosis, and one had a concomitant sigmoid polyp. Findings in the other 6 patients were normal. No colorectal malignancy had developed in the remaining 10 patients after a mean follow-up of 76.6 months (range: 0.8 – 125.1 months).
♦ Conclusions: Outcomes in S. bovis PD peritonitis were favorable, and an association with colorectal cancer was not found in our patients. Routine colonoscopy in these patients remains controversial and should be individualized.
Keywords: Streptococcus bovis, peritonitis
Although peritoneal dialysis (PD)–related peritonitis is often caused by gram-positive cocci such as Staphylococcus, up to 10% of all PD-related peritonitis is caused by streptococcal species. Among streptococcal peritonitis episodes, most are caused by Streptococcus viridans; S. bovis accounted for only 1.9% of such episodes (1). S. bovis is a Lancefield group D gram-positive coccus that forms part of the normal gut flora in about 10% of healthy adults (2). Although rarely a pathogen, S. bovis occasionally leads to invasive disease with serious consequences.
In recent years, extensive taxonomic change has occurred in this group; strains formerly known as human S. bovis are now designated as different species. The or ganism has now been renamed S. gallolyticus (S. bovis biotype I), S. infantarius (S. bovis biotype II/1), and S. pasteuranius (S. bovis biotype II/2) (3), changes that have furthered knowledge about the clinical behavior of this organism and the associated conditions. S. bovis I classically causes infective endocarditis and primary bacteremia, and its link with colorectal carcinoma is well established (4–7). S. bovis II is associated with chronic liver disease, and cases of spontaneous bacterial peritonitis have also been reported (7–10). However, data about PD peritonitis caused by S. bovis are lacking, and the association of this organism with colorectal malignancy and other medical diseases remains undefined. In that context, we aimed to outline the patient characteristics, microbiologic features, clinical outcomes, and medical conditions associated with this entity.
METHODS
This retrospective study was carried out in 2 renal centers, Tung Wah Hospital and Queen Mary Hospital, under the aegis of the University of Hong Kong. The case records of patients who experienced PD peritonitis between January 2000 and September 2010 were reviewed. Cases were included if the PD effluent culture was positive for S. bovis, and if the PD effluent had a total white cell count in excess of 100/μL with more than 50% neutrophils, and if signs of peritoneal inflammation were present. Data for age at the time of peritonitis, sex, medical comorbidities, duration of PD before peritonitis, symptomatology, exit-site condition, and the sensitivities and resistance patterns were obtained from the case records.
Outcome measures were primary response, secondary response, relapse peritonitis, repeat peritonitis, refractory peritonitis requiring Tenckhoff catheter removal, and death. “Primary response” was defined as clinical response with a PD effluent total cell count of less than 100/μL at day 3. “Secondary response” was defined as failure to reach a total cell count of less than 100/μL at day 3, but a response to second-line antibiotics. A peritonitis episode with the same organism within 4 weeks of treatment for the previous episode was regarded as a relapse; infection with the same organism after 4 weeks of treatment was considered repeat peritonitis. In both centers, the first-line treatment for PD peritonitis was intraperitoneal cefazolin, plus an aminoglycoside if the patient was not allergic to it. Patients who did not respond to this regimen were switched to second-line agents according to culture results and sensitivities. Patients who were willing to have a further gastrointestinal investigation were offered colonoscopy or barium enema.
RESULTS
CLINICAL FEATURES AND OUTCOME
During the study period, 1285 patients had been undergoing PD therapy for a total of 48 711 patient–months at the 2 centers. The overall peritonitis rate was 1 episode in 27.1 patient–months. Of the 1797 episodes of peritonitis experienced by those patients, 23 were episodes of S. bovis peritonitis (1.28% of all episodes) in 20 patients.
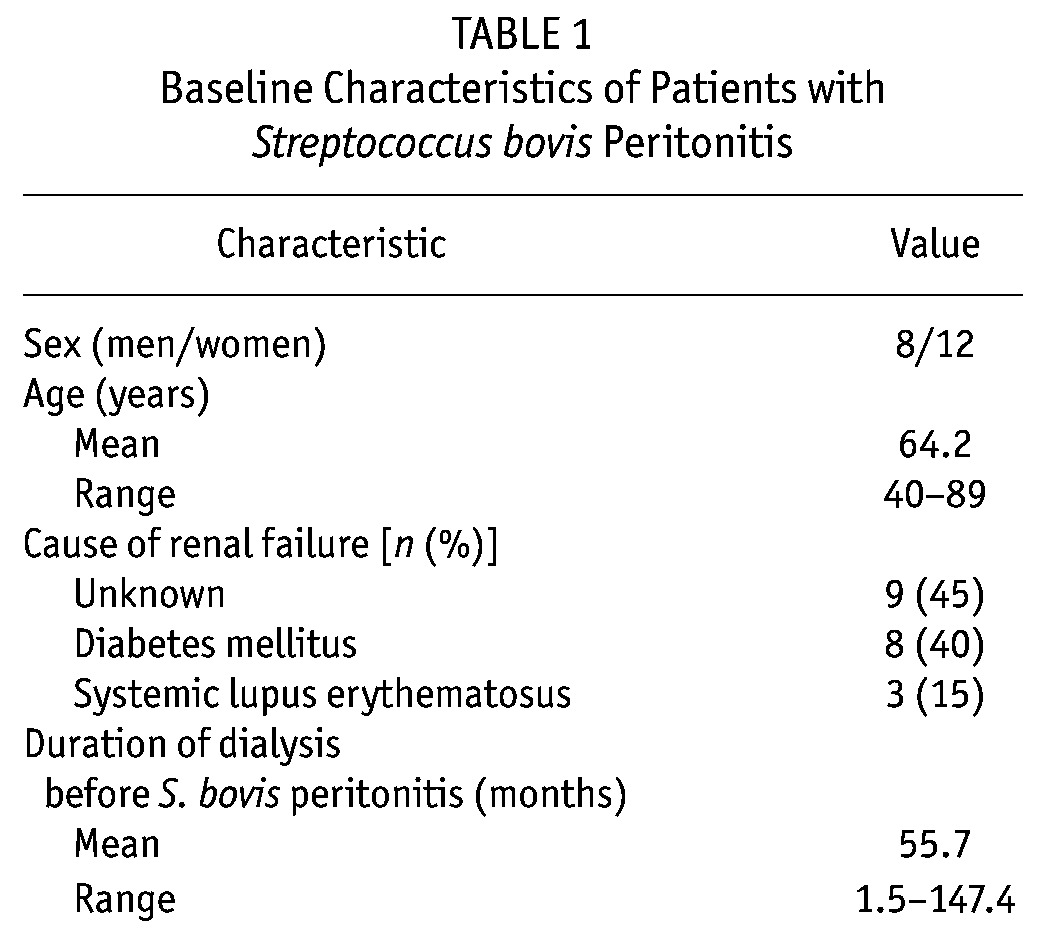
Table 1 presents the baseline characteristics of the patients [8 men, 12 women; mean age at onset: 64.2 years (range: 40 – 89 years)]. The primary causes of renal failure were unknown (n = 9, 45%), diabetes mellitus (n = 8, 40%), and systemic lupus erythematosus (n = 3, 15%). The mean duration of dialysis before peritonitis was 55.7 months (range: 1.5 – 147.4 months).
TABLE 1.
Baseline Characteristics of Patients with Streptococcus bovis Peritonitis
On presentation, all patients had turbid PD effluent, 75% had abdominal pain, and 70% had fever. In 19 episodes (82.6%), the dialysate culture isolated S. bovis; in 4 episodes (17.4%), mixed growth was observed (3 with Escherichia coli, 1 with Enterobacter). Table 2 shows the sensitivities and resistance patterns. The rates of resistance to clindamycin and erythromycin were 43.5% and 47.8% respectively. The S. bovis sensitivity to penicillin G was 60.9%; 30.4% showed moderate resistance. All patients received intraperitoneal antibiotics as first-line treatment: 56.5% with cefazolin–tobramycin, 17.4% with cefazolin–amikacin, and 17.4% with vancomycin–amikacin.
TABLE 2.
Sensitivity and Resistance Pattern of Streptococcus bovis in 23 Peritonitis Episodes
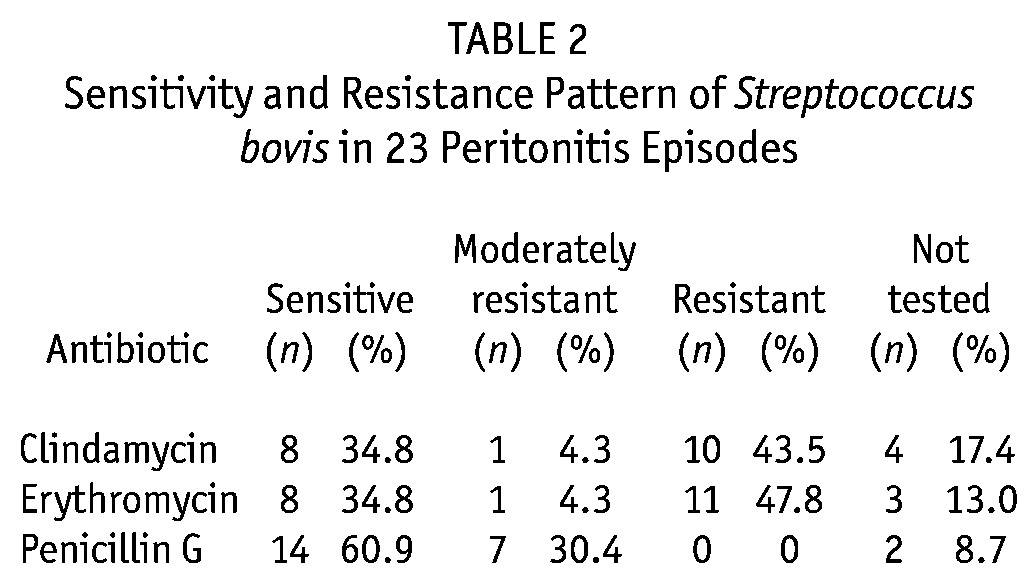
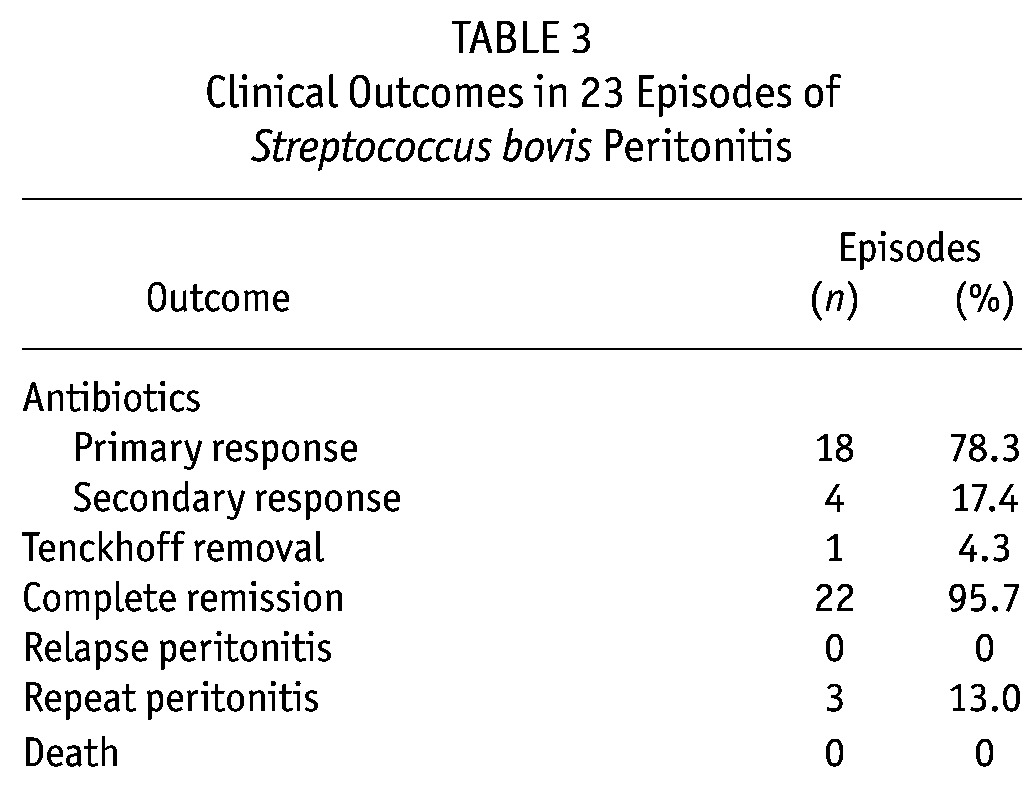
Table 3 shows clinical outcomes in the patients. A primary response was observed in 18 episodes (78.3%), and a secondary response, in 4 (17.4%). In 1 patient, the Tenckhoff catheter had to be removed. No relapses or mortality occurred. Patients who achieved a primary response were maintained on intraperitoneal cefazolin plus an aminoglycoside for 2 weeks. Of the 5 patients not responding to first-line antibiotics, 4 achieved a secondary response after a switch to intraperitoneal vancomycin. The remaining patient failed to respond after a switch to intraperitoneal meropenem, ceftazidime, and amikacin when concomitant E. coli were isolated, and the Tenckhoff catheter was removed.
TABLE 3.
Clinical Outcomes in 23 Episodes of Streptococcus bovis Peritonitis
Repeat peritonitis occurred in 3 patients at a mean of 50.0 months (range: 24.2 – 83.1 months) after the first episode; 1 had mixed growth (with E. coli). In 2 cases, sensitivities were unchanged; in 1 case, the organism had become resistant to erythromycin. In the 2 patients with single-organism S. bovis peritonitis, a primary response was achieved with intraperitoneal cefazolin plus tobramycin; in the 1 with mixed growth, a secondary response was achieved with intraperitoneal vancomycin plus ceftazidime and amikacin.
ASSOCIATION WITH GASTROINTESTINAL PATHOLOGY
Ten patients (50%) underwent gastrointestinal evaluation by barium enema or colonoscopy. One patient had a history of carcinoma of the colon 2 years before the peritonitis, but a subsequent work-up did not reveal tumor recurrence. Three patients had diverticulosis, and one had a sigmoid polyp. The other 6 patients had normal findings upon gastrointestinal investigation. After a mean follow up of 76.6 months (range: 0.8 – 125.1 months), no colorectal malignancy was detected in any of the remaining 10 patients who did not undergo gastrointestinal investigation.
CONCOMITANT RISK FACTORS AND USE OF IMMUNOSUPPRESSIVE AGENTS
Among the 20 patients who experienced S. bovis peritonitis, 8 had diabetes mellitus, and 2 had documented liver cirrhosis. Among 11 patients who had undergone liver ultrasonography, 3 had early cirrhotic changes. Chronic immunosuppressive agents were being administered to 2 patients: one had systemic lupus erythematosus and was taking maintenance corticosteroid and azathioprine; the other had bullous pemphigoid and was taking high-dose corticosteroid. One patient with a prior failed renal allograft had tapered off all immunosuppressants at the time of peritonitis.
DISCUSSION
Although S. bovis is seldom a virulent human pathogen, it may occur as an invasive infection with serious clinical consequences. Classically, it presents as primary bacteremia or infective endocarditis, but reports have also described cases of meningitis and spontaneous bacterial peritonitis in cirrhotic patients (7–11). Data regarding PD peritonitis caused by S. bovis are lacking. In a single-center study of streptococcal PD peritonitis over 10 years, only 2 cases of S. bovis, accounting for 1.9% of all episodes, were observed (1). A single case report on S. bovis PD peritonitis (12) has also been published.
In the present study, we describe our experience of S. bovis PD peritonitis in 20 patients over a period of 10 years. These data might better reflect the patient characteristics, clinical outcomes, and conditions associated with this entity. The clinical manifestations are similar to those in PD peritonitis caused by other organisms, with turbid effluent, abdominal pain, and fever being the leading symptoms. With regard to sensitivities, moderate resistance to penicillin G was seen in one third of cases, and in close to half, the organisms were resistant to erythromycin (47.8%) and clindamycin (43.5%). That resistance rate was higher than the rate reported in otherwise healthy patients with primary S. bovis bacteremia and endocarditis, in whom the infecting organisms showed a 98% penicillin sensitivity (7). The much higher resistance rate in our dialysis patients might be explained by the greater chance of prior use of antibiotics in those patients. The resistance to erythromycin had little clinical relevance, because that agent is seldom used in peritonitis treatment, but the resistance to clindamycin is a concern because intraperitoneal clindamycin may be used in patients with allergies to the penicillin group of antibiotics. Despite the high resistance rate, primary treatment with intraperitoneal cefazolin plus an aminoglycoside was successful in 80% of episodes. Most of the nonresponders improved after a switch to intraperitoneal vancomycin. As with other streptococcal peritonitis, S. bovis peritonitis usually has a milder manifestation than that seen with its Staphylococcus counterparts. Infection with S. bovis is also associated with favorable outcomes, as illustrated by the almost 100% overall response rate in our cohort, with the exception of 1 patient whose polymicrobial episode included infection with E. coli.
Several medical comorbidities may also contribute to an increased risk of S. bovis peritonitis in PD patients. A previous study highlighted diabetes mellitus as a risk factor for streptococcal PD peritonitis (1), and the 40% incidence of diabetes in our cohort echoes that finding. The use of immunosuppressants may also have a contributing effect (1). In the present report, 1 lupus patient was taking maintenance corticosteroid and azathioprine, and another patient with bullous pemphigoid had received high-dose corticosteroid shortly before peritonitis developed.
One important clinical feature that distinguishes infection with S. bovis from infection with other streptococcal species is the strong association with bowel and hepatic pathologies. Patients with S. bovis bacteremia and endocarditis have classically been tied to colorectal carcinoma. In one study, 56% of patients underwent colonoscopy or barium enema, and colorectal carcinoma was detected in 14% – 18% (7). Other studies have also reported much higher rates of colorectal cancer (5,6). We did not find such an association in our study. Of our 20 patients, 10 (50%) underwent gastrointestinal evaluation, but only 4 were found to have bowel pathologies, including 3 cases of diverticulosis. None had colorectal carcinoma, including 1 patient who had experienced a carcinoma 2 years earlier and in whom no recurrence was detected. The remaining 10 patients are unlikely to have colorectal cancer; they have been followed for a mean duration of 5 years without detection of any gastrointestinal malignancies. The incidence of diverticular disease among the patients that underwent gastrointestinal evaluation was similar to that in our previous report on colonic diverticulosis being a risk factor (24%) for enteric peritonitis (13).
Because of the association between S. bovis endocarditis and colorectal cancer, some authors have advocated routine colonoscopy screening (14). However, such a practice is not a casual decision, because colonoscopy in PD patients is not without complications (15).
Studies have also linked spontaneous bacterial peritonitis caused by S. bovis, especially biotype II, with chronic liver disease (7–10). One limitation of our study is the lack of biotyping data for the various S. bovis strains. In our locality, a significant proportion of S. bovis bacteremia is related to the biotype II strain, and the patients had underlying hepatobiliary pathology (16). In the present cohort, 2 patients had documented liver cirrhosis. Given that the proportion of the biotype I strain in our patients is unknown, we cannot totally exclude the possibility of an association between colorectal cancer and S. bovis biotype I peritonitis. The low incidence of an association with colorectal carcinoma in our cohort does not support the case for routine colonoscopy in PD patients with S. bovis peritonitis, but with more stereotyping, it may be possible to better establish any underlying association so that a policy of routine colonoscopy may have firmer grounds in the future.
CONCLUSIONS
S. bovis PD peritonitis was associated with favorable outcomes. The link between colorectal cancers in patients experiencing S. bovis infection was not established in our cohort. The case for routine colonoscopy is not currently substantiated, and the decision for further gastrointestinal evaluation should be individualized.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1. Shukla A, Abreu Z, Bargman JM. Streptococcal PD peritonitis—a 10-year review of one centre’s experience. Nephrol Dial Transplant 2006; 21:3545–9 [DOI] [PubMed] [Google Scholar]
- 2. Potter MA, Cunliffe NA, Smith M, Miles RS, Flapan AD, Dunlop MG. A prospective controlled study of the association of Streptococcus bovis with colorectal carcinoma. J Clin Pathol 1998; 51:473–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev 2002; 15:613–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klein RS, Recco RA, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Association of Streptococcus bovis with carcinoma of the colon. N Engl J Med 1977; 297:800–2 [DOI] [PubMed] [Google Scholar]
- 5. Ruoff KL, Miller SI, Garner CV, Ferraro MJ, Calderwood SB. Bacteremia with Streptococcus bovis and Streptococcus salivarius: clinical correlates of more accurate identification of isolates. J Clin Microbiol 1989; 27:305–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corredoira JC, Alonso MP, García JF, Casariego E, Coira A, Rodriguez A, et al. Clinical characteristics and significance of Streptococcus salivarius bacteremia and Streptococcus bovis bacteremia: a prospective 16-year study. Eur J Clin Microbiol Infect Dis 2005; 24:250–5 [DOI] [PubMed] [Google Scholar]
- 7. Fernández–Ruiz M, Villar–Silva J, Llenas–García J, Caurcel–Díaz L, Vila–Santos J, Sanz–Sanz F, et al. Streptococcus bovis bacteraemia revisited: clinical and microbiological correlates in a contemporary series of 59 patients. J Infect 2010; 61:307–13 [DOI] [PubMed] [Google Scholar]
- 8. Gonzlez–Quintela A, Martínez–Rey C, Castroagudín JF, Rajo–Iglesias MC, Domínguez–Santalla MJ. Prevalence of liver disease in patients with Streptococcus bovis bacteraemia. J Infect 2001; 42:116–19 [DOI] [PubMed] [Google Scholar]
- 9. Eledrisi MS, Zuckerman MJ, Ho H. Spontaneous bacterial peritonitis caused by Streptococcus bovis. Am J Gastroenterol 2000; 95:1110–11 [DOI] [PubMed] [Google Scholar]
- 10. Glória H, Ducla–Soares J, Serejo F, Póvoa P, Marques A, Ramalho F, et al. Streptococcus bovis spontaneous bacterial peritonitis in patients with alcoholic cirrhosis. Eur J Gastroenterol Hepatol 1996; 8:823–4 [PubMed] [Google Scholar]
- 11. Sturt AS, Yang L, Sandhu K, Pei Z, Cassai N, Blaser MJ. Streptococcus gallolyticus subspecies pasteurianus (biotype II/2), a newly reported cause of adult meningitis. J Clin Microbiol 2010; 48: 2247–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong SS, Woo PC, Ho PL, Wang TK. Continuous ambulatory peritoneal dialysis-related peritonitis caused by Streptococcus bovis. Eur J Clin Microbiol Infect Dis 2003; 22:424–6 [DOI] [PubMed] [Google Scholar]
- 13. Yip T, Tse KC, Lam MF, Cheng SW, Lui SL, Tang S, et al. Colonic diverticulosis as a risk factor for peritonitis in Chinese peritoneal dialysis patients. Perit Dial Int 2010; 30:187–91 [DOI] [PubMed] [Google Scholar]
- 14. Ferrari A, Botrugno I, Bombelli E, Dominioni T, Cavazzi E, Dionigi P. Colonoscopy is mandatory after Streptococcus bovis endocarditis: a lesson still not learned. Case report. World J Surg Oncol 2008; 6:49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yip T, Tse KC, Lam MF, Cheng SW, Lui SL, Tang S, et al. Risks and outcomes of peritonitis after flexible colonoscopy in CAPD patients. Perit Dial Int 2007; 27:560–4 [PubMed] [Google Scholar]
- 16. Lee RA, Woo PC, To AP, Lau SK, Wong SS, Yuen KY. Geographical difference of disease association in Streptococcus bovis bacteraemia. J Med Microbiol 2003; 52:903–8 [DOI] [PubMed] [Google Scholar]


