Abstract
♦ Aims: We investigated dialysis duration, dose of erythropoietin (EPO), and clinical manifestations in peritoneal dialysis (PD) patients with subclinical hypothyroidism.
♦ Methods: This cross-sectional study, performed in 3 centers, assessed 122 adult patients on PD for more than 6 months with regard to demographic data, dialysis duration, thyroid function, biochemical data, EPO dose, and clinical manifestations. Thyroid dysfunction was determined by serum thyroid-stimulating hormone, free thyroxine, total thyroxine, total triiodothyronine, antithyroid peroxidase antibodies, and auto-antibodies against thyroglobulin.
♦ Results: Of the 122 study patients, 98 (80.3%) were assessed as having euthyroidism; 19 (15.6%), subclinical hypothyroidism; and 5 (4.1%), subclinical hyperthyroidism. The proportion of women (74.2% vs. 57.1%, p = 0.038), the mean duration of PD (58.1 months vs. 37.9 months, p = 0.032), and the weighted mean monthly EPO dose (1.22 μg/kg vs. 1.64 μg/kg, p = 0.009) were significantly higher in the subclinical hypothyroidism group than in the euthyroidism group, but the prevalences of coronary artery disease and cerebrovascular disease were not. From the multivariate model, PD duration was more significant than sex as a risk factor for subclinical hypothyroidism (p = 0.0132).
♦ Conclusions: Subclinical hypothyroidism is frequent in PD patients, especially female patients and patients with a longer PD duration. Compared with euthyroid patients, patients with subclinical hyperthyroidism need a higher dose of EPO to maintain a stable hemoglobin level.
Keywords: Continuous ambulatory peritoneal dialysis, subclinical hypothyroidism, erythropoietin, cardiovascular disease, cerebrovascular disease
Thyroid dysfunction affects a significant portion of the general population (1–4) and of patients with renal failure (5–10). Thyroid function is reported to be abnormal in many patients once renal function declines below 50% (5,11). Subclinical hypothyroidism in dialysis patients has been reported to be causally related to the retention of excess iodide, to protein loss from dialysate, and to uremic toxins (5,12–17). Whether subclinical hypothyroidism is associated with the duration of peritoneal dialysis (PD) or with erythropoietin (EPO) dose is seldom investigated.
Subclinical hypothyroidism has been reported to be associated with cardiovascular disease (18–22) and cerebrovascular disease (23–24) in the general population. Coronary artery disease (CAD) and cerebrovascular disease are also associated with end-stage renal disease (25–29). Whether subclinical hypothyroidism is associated with these diseases in patients on PD is not well defined. Our study therefore investigated the associations of dialysis duration, EPO dose, and clinical manifestations such as CAD and cerebrovascular disease with subclinical hypothyroidism in PD patients.
METHODS
PARTICIPANTS
The study recruited 137 patients 18 years or older who had been treated with 3 or 4 daily 2-L exchanges of glucose-containing standard dialysate for more than 6 months at 3 in-hospital dialysis units in northern Taiwan starting from 1 January 2009. At the time of enrollment, patients also had to have been free of peritonitis for at least 6 months. Weekly Kt/V was at least 1.7.
To avoid misinterpretation of treatment response to EPO, patients with other causes of poor response to EPO therapy—such as malignancy, liver cirrhosis, thalassemia, iron deficiency, gastrointestinal bleeding, or a major operation within the 6 months preceding the thyroid function evaluation—were excluded. Patients with a pre-existing diagnosis of thyroid disease were not excluded from the study.
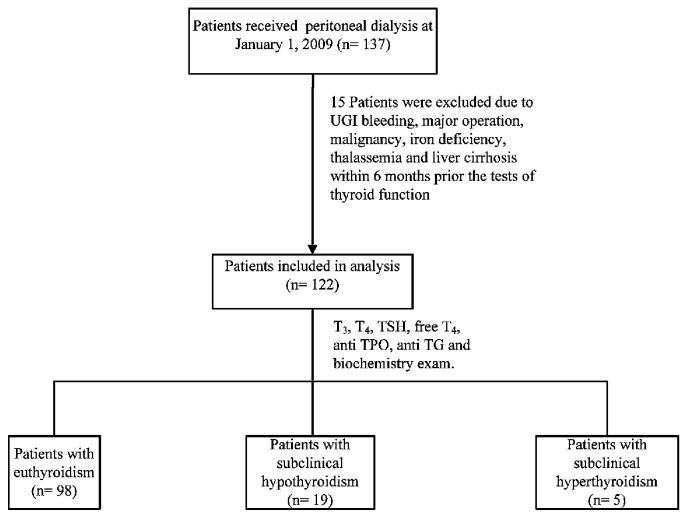
The 122 patients who met the inclusion criteria (Figure 1) all provided written informed consent. The study protocol was approved by the institutional review board of Taipei Veterans General Hospital and was performed in accordance with the ethical principles of the Declaration of Helsinki.
Figure 1.
— Study design and classification of thyroid dysfunction.
BLOOD SAMPLES AND LABORATORY DATA
For the sake of reliability, venous blood samples were drawn at each participating site and were then sent to the central laboratory at Taipei Veterans General Hospital for measurement of serum thyroid-stimulating hormone (TSH), antithyroid peroxidase antibodies (anti-TPO), auto-antibodies against thyroglobulin (anti-TG), free thyroxine (FT4), total thyroxine (T4), and total triiodothyronine (T3). Levels of TSH, anti-TPO, and anti-TG were measured by chemiluminescent immunometric assay (DPC Immulite 2000: Seracon Diagnostics, Brownsville, TX, USA). Levels of FT4, T4, and T3 were measured by a chemiluminescent competitive analog immunoassay (DPC Immulite 2000). In Taiwan, each PD patient undergoes a routine monthly biochemistry profile that includes blood urea nitrogen; serum levels of creatinine, sodium, potassium, bicarbonate, albumin, cholesterol, triglycerides, uric acid, calcium, phosphate, glucose, intact parathyroid hormone, and hemoglobin; and efficacy of dialysis (demonstrated by Kt/V). Data for the foregoing variables were therefore available for a 6-month period preceding the thyroid function test. For each patient, the study analysis used the mean of those monthly values.
PERSONAL DATA
Demographic data for the patients (age, sex, body weight, height, date of PD) were collected from each participating site. Any history of diabetes mellitus, thyroid diseases, upper gastrointestinal bleeding, major operation, malignancy, liver cirrhosis, and treatment with EPO over the 6-month period preceding the thyroid function test was also recorded at each participating site and was sent to Taipei Veterans General Hospital. Comorbid conditions, including underlying CAD and cerebrovascular disease, were also recorded. We defined CAD and cerebrovascular disease as any hospital admission with a diagnosis of coronary disease or cerebrovascular accident.
DEFINITIONS OF EUTHYROIDISM AND SUBCLINICAL HYPOTHYROIDISM
Reference ranges for the biochemical variables of interest are these: TSH, 0.4 – 4 μIU/mL; FT4, 0.8 – 1.9 ng/dL; T3, 72 – 170 ng/dL; T4, 4.5 – 12.5 μg/dL; anti-TPO, <35 IU/mL; and anti-TG, <40 IU/mL. Hypothyroidism was defined as TSH > 4 μIU/mL and FT4 < 0.8 ng/dL. Subclinical hypothyroidism was defined as TSH > 4 μIU/mL and normal FT4. Hyperthyroidism was defined as TSH < 0.4 μIU/mL and T3 > 170 ng/dL or T4 > 12.5 μg/dL. Subclinical hyperthyroidism was defined as TSH < 0.4 μIU/mL and normal FT4. Patients with normal levels of TSH and FT4, but receiving treatment with thyroxine were categorized as having euthyroidism.
STATISTICAL ANALYSIS
Data were analyzed using the SPSS software application (release 13.0: SPSS, Chicago, IL, USA) for Windows (Microsoft, Redmond, WA, USA). The prevalences of thyroid disease are shown as frequencies. Because of the small number of cases of subclinical hyperthyroidism (only 5), those cases were not analyzed any further in the study. Chi-square and two-sample t-tests were conducted to test the differences between the euthyroidism and subclinical hypothyroidism groups. A p value less than 0.05 indicated statistical significance. Stepwise logistic regression models were used to assess associations of risk factors in the subclinical hypothyroidism group.
RESULTS
PREVALENCE OF THYROID DYSFUNCTION
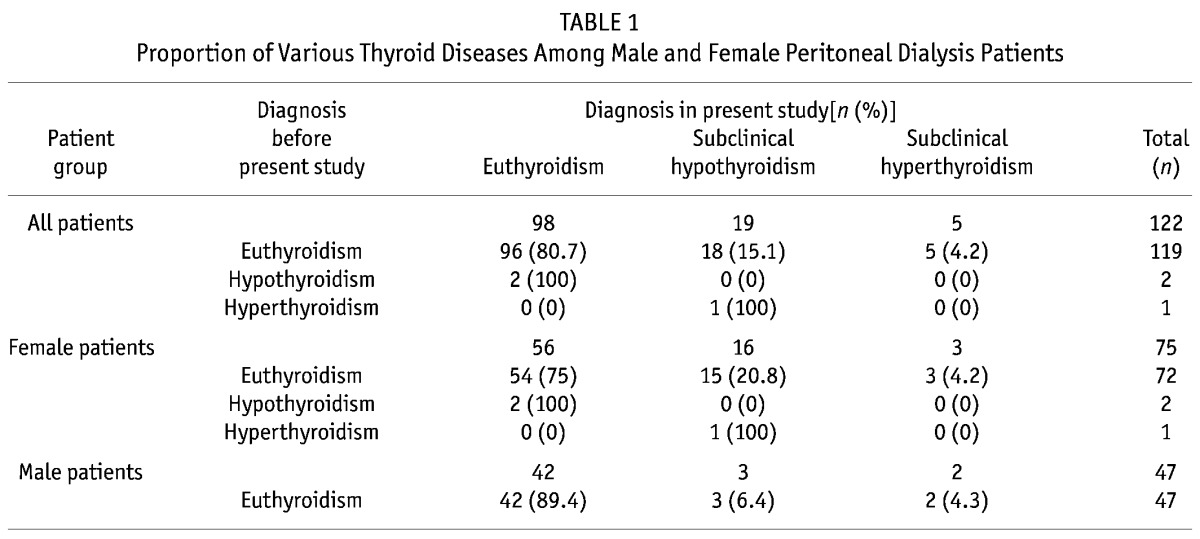
In this study, no patient was diagnosed with hypothyroidism or hyperthyroidism. After thyroid function testing, 98 of the 122 PD patients (80.3%) were defined as having euthyroidism; 19 (15.6%), subclinical hypothyroidism; and 5 (4.1%), subclinical hyperthyroidism (Figure 1). Of the 98 patients with euthyroidism, 2 (2.04%) had previously been diagnosed with hypothyroidism, and of the 19 patients with subclinical hypothyroidism, 1 (5.26%) had previously been diagnosed with hyperthyroidism (Table 1). The 2 patients with previous hypothyroidism were taking levothyroxine sodium (50 μg daily and 100 μg daily). The 1 patient with previous hyperthyroidism had undergone subtotal thyroidectomy and was not on medical treatment with levothyroxine sodium.
TABLE 1.
Proportion of Various Thyroid Diseases Among Male and Female Peritoneal Dialysis Patients
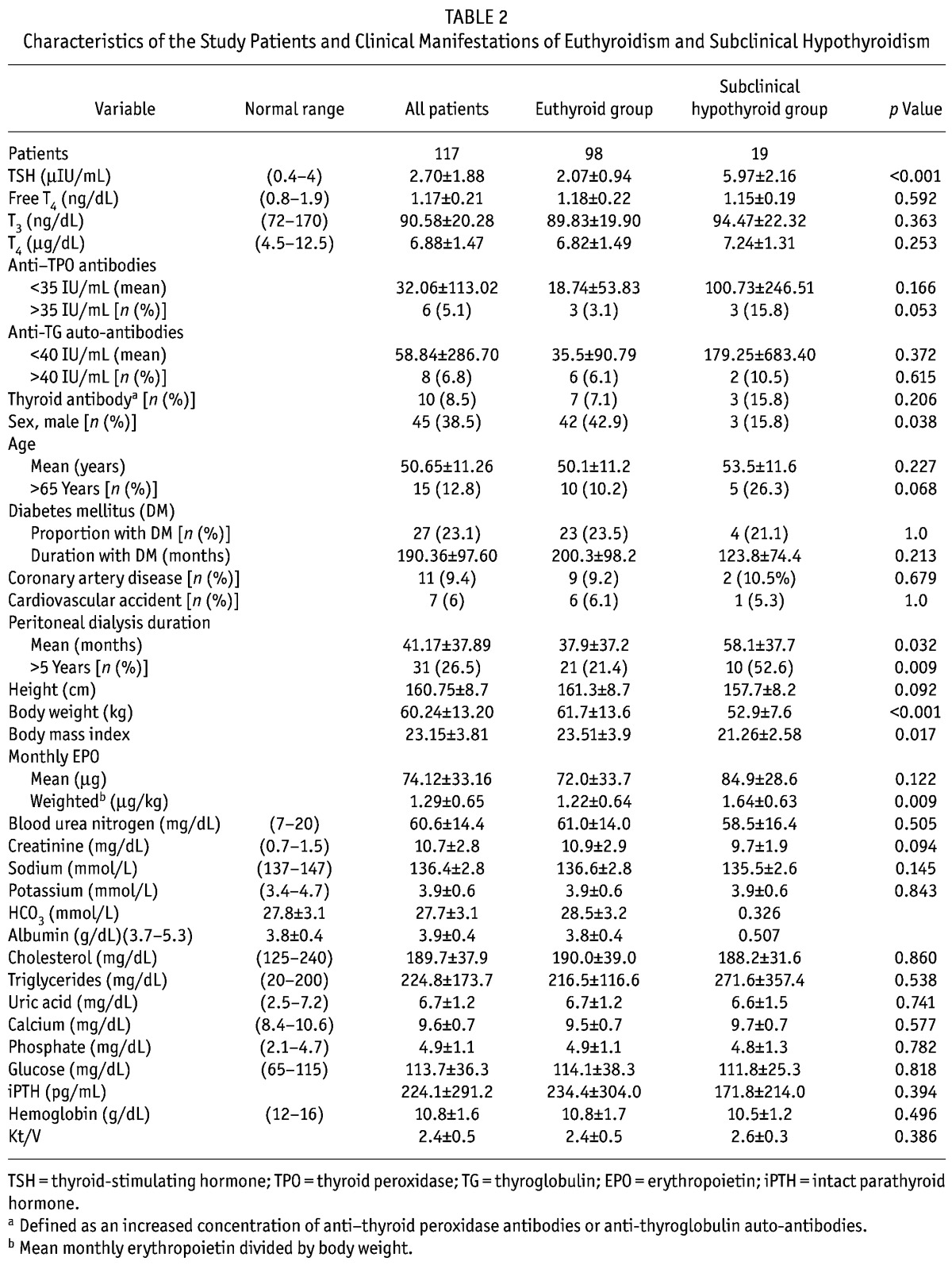
After exclusion of the 5 patients with subclinical hyperthyroidism, 117 patients [72 women (61.5%), 45 men (38.5%); 27 (23.1%) with diabetes mellitus; 15 (12.8%) over 65 years of age] remained for further analysis (Table 2).
TABLE 2.
Characteristics of the Study Patients and Clinical Manifestations of Euthyroidism and Subclinical Hypothyroidism
COMPARISON BETWEEN THE EUTHYROIDISM AND SUBCLINICAL HYPOTHYROIDISM GROUPS
Compared with the euthyroidism group, the subclinical hypothyroidism group had a significantly higher TSH level (2.07 ± 0.94 μIU/mL vs. 5.97 ± 2.16 μIU/mL, p < 0.001), but the other parameters of thyroid function (FT4, T3, T4, anti-TPO, and anti-TG) were not significantly different between the groups. Other parameters—age; presence and duration of diabetes mellitus; body height; blood urea nitrogen; serum levels of creatinine, sodium, potassium, bicarbonate, albumin, cholesterol, triglycerides, uric acid, calcium, phosphate, glucose, intact parathyroid hormone, and hemoglobin; and efficacy of dialysis (Kt/V)—were also not significantly different between the euthyroidism and subclinical hypothyroidism groups. Subclinical hypothyroidism was significantly more prevalent among women than euthyroidism was (84.2% vs. 57.1%, p = 0.038). Mean body weight and body mass index were significantly lower in the subclinical hypothyroidism group than in the euthyroidism group (52.9 ± 7.6 kg vs. 61.7 ± 13.6 kg, p < 0.001). The mean duration of PD (58.1 ± 37.7 months vs. 37.9 ± 37.2 months, p = 0.032), the number of patients on PD for more than 5 years (52.6% vs. 21.4%, p = 0.009), and the weighted mean monthly EPO dose (1.22 μg/kg vs. 1.64 μg/kg, p = 0.009) were significantly higher in the subclinical hypothyroidism group than in the euthyroidism group (Table 2).
In the euthyroidism group (n = 98), 9 patients (9.2%) had CAD, and 6 (6.1%) had cerebrovascular disease. In the subclinical hypothyroidism group (n = 19), 2 patients had CAD (10.5%), and 1 (5.3%) had cerebrovascular disease. The prevalences of CAD (9.2% vs. 10.5%, p = 0.679) and cerebrovascular disease (6.1% vs. 5.3%, p = 0.999) were not significantly different between the groups (Table 2).
STEPWISE LOGISTIC REGRESSION MODEL ANALYSIS FOR SUBCLINICAL HYPOTHYROIDISM
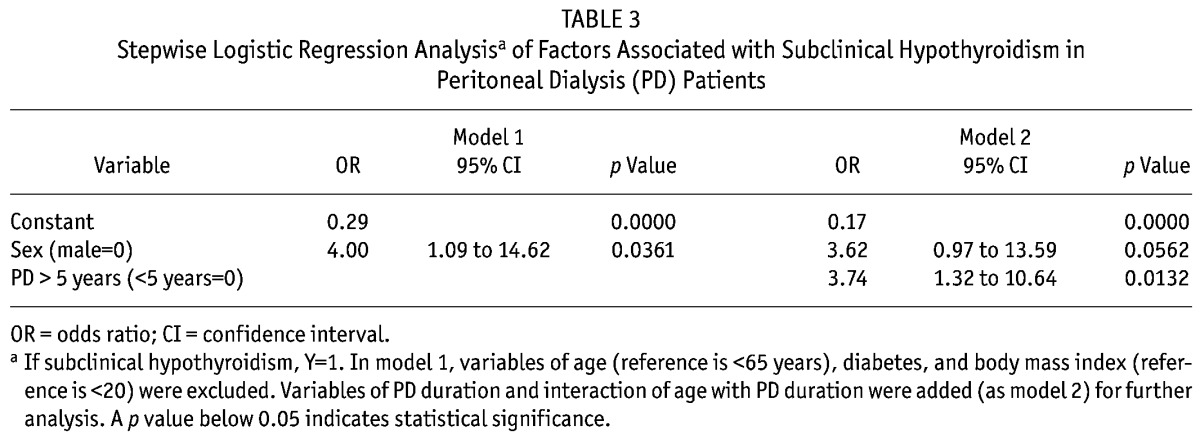
In Table 3, model 1 showed that subclinical hypothyroidism was associated with sex, but not with age, diabetes, or body mass index. Subclinical hypothyroidism occurred in women at a rate 4 times the rate in the overall group [odds ratio (OR) = 4, p = 0.0361]. When PD duration and the interaction of age with PD duration were added into model 1 (as model 2 for further analysis), a dialysis duration longer than 5 years was more significantly associated with subclinical hypothyroidism (OR = 3.74, p = 0.0132) than sex was (OR = 3.62, p = 0.0562).
TABLE 3.
Stepwise Logistic Regression Analysisa of Factors Associated with Subclinical Hypothyroidism in Peritoneal Dialysis (PD) Patients
DISCUSSION
The fact that no study patient was diagnosed with clinical hypothyroidism might be attributable to the low prevalence of this condition in renal failure patients (7) or to the relatively small study population. The substantial proportion of PD patients with subclinical hypothyroidism (Table 1) in this (15.6%) and a previous study [27.5% (30)] demonstrates that subclinical hypothyroidism is more common in PD patients than in the general population [4% – 10% (3,4,21,31)]. The higher prevalence of subclinical hypothyroidism observed in women in our study accords with findings in previous studies (2,32). Because women tend to have a lower body weight compared with body weight in men, the higher proportion of women in the subclinical hypothyroidism group may also explain the overall lower body weight and body mass index in that group (Table 2).
In the general population, the presence of antithyroid antibodies has been the most frequent cause of subclinical hypothyroidism [54% – 67% (2)]; but in PD patients with subclinical hypothyroidism, the prevalences of anti-TPO and anti-TG antibodies are low: 15.8% and 10.5% respectively in the present study, similar to rates in previous reports (30,33). The inconsistency in the causes of subclinical hypothyroidism in the general population and in PD patients was not clearly understood in the past. Impaired immunity in end-stage renal disease patients is not improved by maintenance dialysis (34,35), and therefore antibody titers continue to be low, and the response rate to vaccinations for viral hepatitis, tetanus, and diphtheria, poor (36,37). We therefore hypothesized that the inconsistency in the prevalence of antithyroid antibodies between subclinical hypothyroidism in the general population and in PD patients may be partly attributable to the impaired immune function in dialysis patients. On the other hand, the low prevalence of antithyroid antibodies in dialysis patients also supports suggestions in previous reports that the substantial number of dialysis patients with subclinical hypothyroidism may be related to retention of excess iodide, to protein loss from dialysate, or to uremic toxins rather than to autoimmune mechanisms (5,12–17). The longer PD duration in the subclinical hypothyroidism group might also support a suggestion of iodide retention or an effect of uremic toxins (Table 2).
When PD duration and the interaction of age with PD duration were added into the stepwise logistic regression model, PD duration was more significant than sex as a risk factor for subclinical hypothyroidism (Table 3). Patients with a longer PD duration also had longer retention of excess iodide and uremic toxins, possibly also explaining the presence of subclinical hypothyroidism.
Our study also showed a significantly higher weighted mean monthly EPO dose (1.22 μg/kg vs. 1.64 μg/kg, p = 0.009) in the subclinical hypothyroidism group (Table 2), supporting previous reports that a state of euthyroidism is essential for the action of EPO on bone marrow (38–40).
Previous reports showed an association between subclinical hypothyroidism and cerebrovascular diseases (25–27) and CAD (28,29) in dialysis patients, but in the present study, the prevalence of such diseases was not significantly different between the euthyroidism and subclinical hypothyroidism groups (Table 2). In addition, a cross-sectional analysis by Imaizumi et al. and a population study by Dorr et al. did not find evidence to support subclinical hypothyroidism as a potential risk factor for cerebrovascular disease (18,41). A study by Rodondi et al. (42) with a 4-year follow-up showed that subclinical hypothyroidism was not associated with an increased risk for CAD and stroke. In fact, other studies even showed associations of subclinical hypothyroidism with better functional outcomes in patients with ischemic stroke (43) and with good cardiorespiratory fitness (44), and a possibly protective effect of subclinical hypothyroidism on cardiovascular risk and functional mobility in the elderly (44,45). Taken together, findings in the present study and in previous reports suggest that the issue of cerebrovascular and cardiovascular disease risk remains controversial in PD patients with subclinical hypothyroidism. Further large-scale prospective multicenter studies are therefore needed to assess the association of subclinical hypothyroidism with cerebrovascular diseases and CAD in PD patients.
Several methodologic issues should be considered in the interpretation of our results:
First, ascertainment of cerebrovascular disease and CAD was not possible in all patients. Some patients with non-angina CAD (that is, patients with diabetes) or lacunar infarction, or those who were not willing to undergo confirmatory evaluation during hospital admission, could not be analyzed. Although we found no association between thyroid status and cardiovascular disease in the present study, we cannot draw a definitive conclusion. Being that this is only a cross-sectional study, there will be survivor effects, among others. There may be an association, but the present study was not designed to pick it up.
Second, the onset and duration of subclinical hypothyroidism in the patients could not be unequivocally defined in this study.
Finally, the sample size is not large enough to avoid the possibility of selection bias.
Despite those limitations, it should be noted that contrary to earlier assumptions, subclinical hypothyroidism may not be associated with cerebrovascular and CAD in PD patients.
CONCLUSIONS
A substantial number of PD patients unknowingly had laboratory evidence of thyroid disease. Subclinical hypothyroidism was common in female PD patients and was associated with longer PD duration. Subclinical hypothyroidism in PD patients contributes to the significantly higher dose of EPO needed to maintain hemoglobin. The relationships between subclinical hypothyroidism and cerebrovascular disease and CAD may need further large-scale prospective multicenter studies to delineate.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
Acknowledgments
This study was supported by a grant (NSC 96-2314-B-075-020-MY3,) from the National Science Council and another from the Taipei Veterans General Hospital (V98C1-106), Republic of China. We thank Ms. Lu, Jung Ai, Dr. Tsai-Hung Wu, Jinn-Yang Chen, Yao-Ping Lin, Chiao-Chuang Lin for their technical support. A preliminary report was selected for the poster session during the annual meeting of the Taiwan Society of Nephrology, 12 – 13 December 2009, Taipei, Taiwan.
REFERENCES
- 1. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160:526–34 [DOI] [PubMed] [Google Scholar]
- 2. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87:489–99 [DOI] [PubMed] [Google Scholar]
- 3. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (oxf) 1995; 43:55–68 [DOI] [PubMed] [Google Scholar]
- 4. Chuang CC, Wang ST, Wang PW, Yu ML. Prevalence study of thyroid dysfunction in the elderly of Taiwan. Gerontology 1998; 44:162–7 [DOI] [PubMed] [Google Scholar]
- 5. Lim VS. Thyroid function in patients with chronic renal failure. Am J Kidney Dis 2001; 38(Suppl 1):S80–4 [DOI] [PubMed] [Google Scholar]
- 6. Lin CC, Chen TW, Ng YY, Chou YH, Yang WC. Thyroid dysfunction and nodular goiter in hemodialysis and peritoneal dialysis patients. Perit Dial Int 1998; 18:516–21 [PubMed] [Google Scholar]
- 7. Kaptein EM. Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocr Rev 1996; 17:45–63 [DOI] [PubMed] [Google Scholar]
- 8. Rao MB, Bay WH, George JM, Hebert LA. Primary hypothyroidism in chronic renal failure. Clin Nephrol 1986; 25:11–14 [PubMed] [Google Scholar]
- 9. Diez JJ, Iglesias P, Selgas R. Pituitary dysfunctions in uremic patients undergoing peritoneal dialysis: a cross sectional descriptive study. Adv Perit Dial 1995; 11:218–24 [PubMed] [Google Scholar]
- 10. Xess A, Gupta A, Kumar U, Sharma HP, Prasad KM. Evaluation of thyroid hormones in chronic renal failure. Indian J Pathol Microbiol 1999; 42:129–33 [PubMed] [Google Scholar]
- 11. Maues MG, Santos SF, Filho HP, Lugon JR, Cruz VP, Sampaio JC, et al. Thyroid hormone losses in CAPD. Perit Dial Int 1995; 15:266–9 [PubMed] [Google Scholar]
- 12. Sanai T, Inoue T, Okamura K, Sato K, Yamamoto K, Abe T, et al. Reversible primary hypothyroidism in Japanese patients undergoing maintenance hemodialysis. Clin Nephrol 2008; 69:107–13 [DOI] [PubMed] [Google Scholar]
- 13. Wiederkehr MR, Kalogiros J, Krapf R. Correction of metabolic acidosis improves thyroid and growth hormone axes in haemodialysis patients. Nephrol Dial Transplant 2004; 19:1190–7 [DOI] [PubMed] [Google Scholar]
- 14. Bando Y, Ushiogi Y, Okafuji K, Toya D, Tanaka N, Miura S. Non-autoimmune primary hypothyroidism in diabetic and non-diabetic chronic renal dysfunction. Exp Clin Endocrinol Diabetes 2002; 110:408–15 [DOI] [PubMed] [Google Scholar]
- 15. Konno N, Makita H, Yuri K, Iizuka N, Kawasaki K. Association between dietary iodine intake and prevalence of subclinical hypothyroidism in the coastal regions of Japan. J Clin Endocrinol Metab 1994; 78:393–7 [DOI] [PubMed] [Google Scholar]
- 16. Robey C, Shreedhar K, Batuman V. Effects of chronic peritoneal dialysis on thyroid function tests. Am J Kidney Dis 1989; 13:99–103 [DOI] [PubMed] [Google Scholar]
- 17. Gardner DF, Mars DR, Thomas RG, Bumrungsup C, Misbin RI. Iodine retention and thyroid dysfunction in patients on hemodialysis and continuous ambulatory peritoneal dialysis. Am J Kidney Dis 1986; 7:471–6 [DOI] [PubMed] [Google Scholar]
- 18. Imaizumi M, Akahoshi M, Ichimaru S, Nakashima E, Hida A, Soda M, et al. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J Clin Endocrinol Metab 2004; 89:3365–70 [DOI] [PubMed] [Google Scholar]
- 19. Monzani F, Di Bello V, Caraccio N, Bertini A, Giorgi D, Giusti C, et al. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. J Clin Endocrinol Metab 2001; 86:1110–15 [DOI] [PubMed] [Google Scholar]
- 20. Walsh JP, Bremner AP, Bulsara MK, O’Leary P, Leedman PJ, Feddema P, et al. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med 2005; 165:2467–72 [DOI] [PubMed] [Google Scholar]
- 21. Ochs N, Auer R, Bauer DC, Nanchen D, Gussekloo J, Cornuz J, et al. Meta-analysis: subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med 2008; 148:832–45 [DOI] [PubMed] [Google Scholar]
- 22. Squizzato A, Gerdes VE, Brandjes DP, Buller HR, Stam J. Thyroid diseases and cerebrovascular disease. Stroke 2005; 36:2302–10 [DOI] [PubMed] [Google Scholar]
- 23. Karakurum Goksel B, Karatas M, Nebioglu A, Sezgin N, Tan M, Seydaoglu G, et al. Subclinical hypothyroidism, hyperhomocysteinemia and dyslipidemia: investigating links with ischemic stroke in Turkish patients. Neurol Res 2007; 29:871–6 [DOI] [PubMed] [Google Scholar]
- 24. Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke 1990; 21:637–76 [DOI] [PubMed] [Google Scholar]
- 25. Nakatani T, Naganuma T, Uchida J, Masuda C, Wada S, Sugimura T, et al. Silent cerebral infarction in hemodialysis patients. Am J Nephrol 2003; 23:86–90 [DOI] [PubMed] [Google Scholar]
- 26. Koren–Morag N, Goldbourt U, Tanne D. Renal dysfunction and risk of ischemic stroke or TIA in patients with cardiovascular disease. Neurology 2006; 67:224–8 [DOI] [PubMed] [Google Scholar]
- 27. Toyoda K, Fujii K, Ando T, Kumai Y, Ibayashi S, Iida M. Incidence, etiology, and outcome of stroke in patients on continuous ambulatory peritoneal dialysis. Cerebrovasc Dis 2004; 17:98–105 [DOI] [PubMed] [Google Scholar]
- 28. Patient mortality and survival. United States Renal Data System. Am J Kidney Dis 1998; 32(Suppl 1):S69–80 [DOI] [PubMed] [Google Scholar]
- 29. Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 1998; 9(Suppl):S16–23 [PubMed] [Google Scholar]
- 30. Kang EW, Nam JY, Yoo TH, Shin SK, Kang SW, Han DS, et al. Clinical implications of subclinical hypothyroidism in continuous ambulatory peritoneal dialysis patients. Am J Nephrol 2008; 28:908–13 [DOI] [PubMed] [Google Scholar]
- 31. Kasagi K, Takahashi N, Inoue G, Honda T, Kawachi Y, Izumi Y. Thyroid function in Japanese adults as assessed by a general health checkup system in relation with thyroid-related antibodies and other clinical parameters. Thyroid 2009; 19:937–44 [DOI] [PubMed] [Google Scholar]
- 32. Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 2004; 291:228–38 [DOI] [PubMed] [Google Scholar]
- 33. Lo JC, Chertow GM, Go AS, Hsu CY. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int 2005; 67:1047–52 [DOI] [PubMed] [Google Scholar]
- 34. Descamps–Latscha B, Herbelin A, Nguyen AT, Zingraff J, Jungers P, Chatenoud L. Immune system dysregulation in uremia. Semin Nephrol 1994; 14:253–60 [PubMed] [Google Scholar]
- 35. Girndt M. Humoral immune responses in uremia and the role of IL-10. Blood Purif 2002; 20:485–8 [DOI] [PubMed] [Google Scholar]
- 36. Sezer S, Ozdemir FN, Guz G, Arat Z, Colak T, Sengul S, et al. Factors influencing response to hepatitis B virus vaccination in hemodialysis patients. Transplant Proc 2000; 32:607–8 [DOI] [PubMed] [Google Scholar]
- 37. Girndt M, Sester M, Sester U, Kaul H, Kohler H. Molecular aspects of T- and B-cell function in uremia. Kidney Int Suppl 2001; 78:S206–11 [DOI] [PubMed] [Google Scholar]
- 38. Chang PM, Ng YY. Amiodarone-induced hypothyroidism with EPO-resistant anemia in a patient with chronic renal failure. J Chin Med Assoc 2008; 71:576–8 [DOI] [PubMed] [Google Scholar]
- 39. Dilek M, Akpolat T, Cengiz K. Hypothyroidism as a cause of resistance to erythropoietin. Nephron 2002; 92:248 [DOI] [PubMed] [Google Scholar]
- 40. Sungur C, Erbas T, Akpolat T, Arik N, Yasavul U, Turgan C, et al. Resistance to human recombinant erythropoietin in hypothyroidism. Acta Haematol 1992; 88:162 [DOI] [PubMed] [Google Scholar]
- 41. Dorr M, Empen K, Robinson DM, Wallaschofski H, Felix SB, Volzke H. The association of thyroid function with carotid artery plaque burden and strokes in a population-based sample from a previously iodine-deficient area. Eur J Endocrinol 2008; 159:145–52 [DOI] [PubMed] [Google Scholar]
- 42. Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 2005; 165:2460–6 [DOI] [PubMed] [Google Scholar]
- 43. Baek JH, Chung PW, Kim YB, Moon HS, Suh BC, Jin DK, et al. Favorable influence of subclinical hypothyroidism on the functional outcomes in stroke patients. Endocr J 2010; 57:23–9 [DOI] [PubMed] [Google Scholar]
- 44. Simonsick EM, Newman AB, Ferrucci L, Satterfield S, Harris TB, Rodondi N, et al. Subclinical hypothyroidism and functional mobility in older adults. Arch Intern Med 2009; 169:2011–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29:76–131 [DOI] [PubMed] [Google Scholar]