Mineral–bone disease (MBD), a constitutive component of chronic kidney disease (CKD) in any dialysis method, determines a patient’s clinical progress, complications, and survival (1). Several studies have confirmed that clinicians remain far from reaching and maintaining proposed treatment goals (2). The emergence of new strategies based on the use of cinacalcet has improved outcomes in some fields (2,3). Some of the published clinical trials and observational studies of cinacalcet included a small percentage (6% – 12%) of peritoneal dialysis (PD) patients, but provided few data specific to PD (4,5).
The significant variation in the incidence and implications of CKD-MBD between patients on PD and those on hemodialysis (HD) may require different treatments producing different results (6). The aim of the present study was to evaluate the effectiveness and safety of cinacalcet therapy in moderate-to-severe secondary hyperparathyroidism (SHPT) in PD patients.
METHODS
This multicenter observational prospective cohort study enrolled, on a consecutive systematic basis, all patients who met the study criteria and who gave informed consent. The study criteria included presence of moderate-to-severe SHPT according to the guidelines from the Kidney Disease Quality Outcomes Initiative (K/DOQI) in force at the time of the study (7), based on 2 measurements of parathyroid hormone (PTH) above 400 pg/mL, without hypocalcemia, in the preceding 4 months; and treatment of the SHPT with diet and maximum tolerated doses of phosphate binders and vitamin D, or inability to treat with vitamin D because of hypercalcemia (>10.5 mg/dL) or hyperphosphatemia (>5.5 mg/dL).
The PD parameters in the patients were adjusted to maintain the weekly Kt/V and creatinine clearance (CCr) within the ranges recommended by the K/DOQI guidelines (7). Cinacalcet (Mimpara: Amgen, Thousand Oaks, CA, USA) was administered according to the package insert (initial daily dose of 30 mg); no restrictions were placed on the use of other medications. To prevent the risk of hypocalcemia associated with cinacalcet, the initial Ca concentration in the PD solution was 1.75 mEq/L. Depending on the patient’s response, Ca concentrations of 1.25 mEq/L were permitted according to the physician’s judgment.
Data were collected at initiation of cinacalcet and at 1, 3, 6, 9, and 12 months afterward. A luminescence immunoassay (Hoffmann–La Roche, Basel, Switzerland) was used by all centers to measure PTH. Measurements of Ca were corrected for serum albumin. The percentages of patients falling within ranges defined by K/DOQI (7) and Kidney Disease: Improving Global Outcomes (8) were calculated for each time point. Data were analyzed using the SPSS software application (version 11.0: SPSS, Chicago, IL, USA). The Wilcoxon test was used to compare laboratory values for each time point with baseline values. The median time to reach control was estimated using a Kaplan–Meier analysis.
RESULTS
From January 2007 to December 2008, the study enrolled 54 patients from 4 centers [61.4% men; mean age: 51.3 ± 15.5 years (standard deviation)]. At the start of cinacalcet therapy, the patients averaged 26.5 ± 22.4 months on PD. The patients were receiving automated PD (42.7%) or continuous ambulatory PD sufficient to reach adequate efficacy (mean Kt/V: 2.71 ± 0.76; mean weekly CCr: 95.4 ± 37 L), which was maintained within the target range during the follow-up.
At cinacalcet initiation, the percentage of patients using dialysis fluid with 1.75 mEq/L Ca was 100%; at 3 months, it was 74.5%; and at 1 year, it was 78.1%. The mean follow-up time for cinacalcet therapy was 10.8 months (range: 3 – 12 months). During the first year, 6 patients withdrew because of transplantation, 3 switched to HD, 3 discontinued cinacalcet because of digestive intolerance, and 2 were transferred to another center. One patient reduced the dose of cinacalcet because of digestive intolerance.
Median values for CKD-MBD were PTH, 271 pg/mL (final) versus 585 pg/mL (baseline); Ca, 9.45 mg/dL versus 9.80 mg/dL; and P, 4.99 mg/dL versus 5.80 mg/dL. In comparison with the baseline value, PTH had declined 42.8% at 6 months and 50.9% at 1 year (Table 1). Those patients who reached a PTH of less than 300 pg/mL maintained that range in 65.6% of subsequent determinations. At the end of the study, in the last lab value available for each patient, 14% had a PTH of less than 150 pg/mL, and 69.2% had a PTH of less than 350 pg/mL. Table 2 shows the progression in the percentage of patients reaching targets during the study.
TABLE 1.
Evolution of Parameters of Mineral Bone Disease and of Medications During Follow-Up
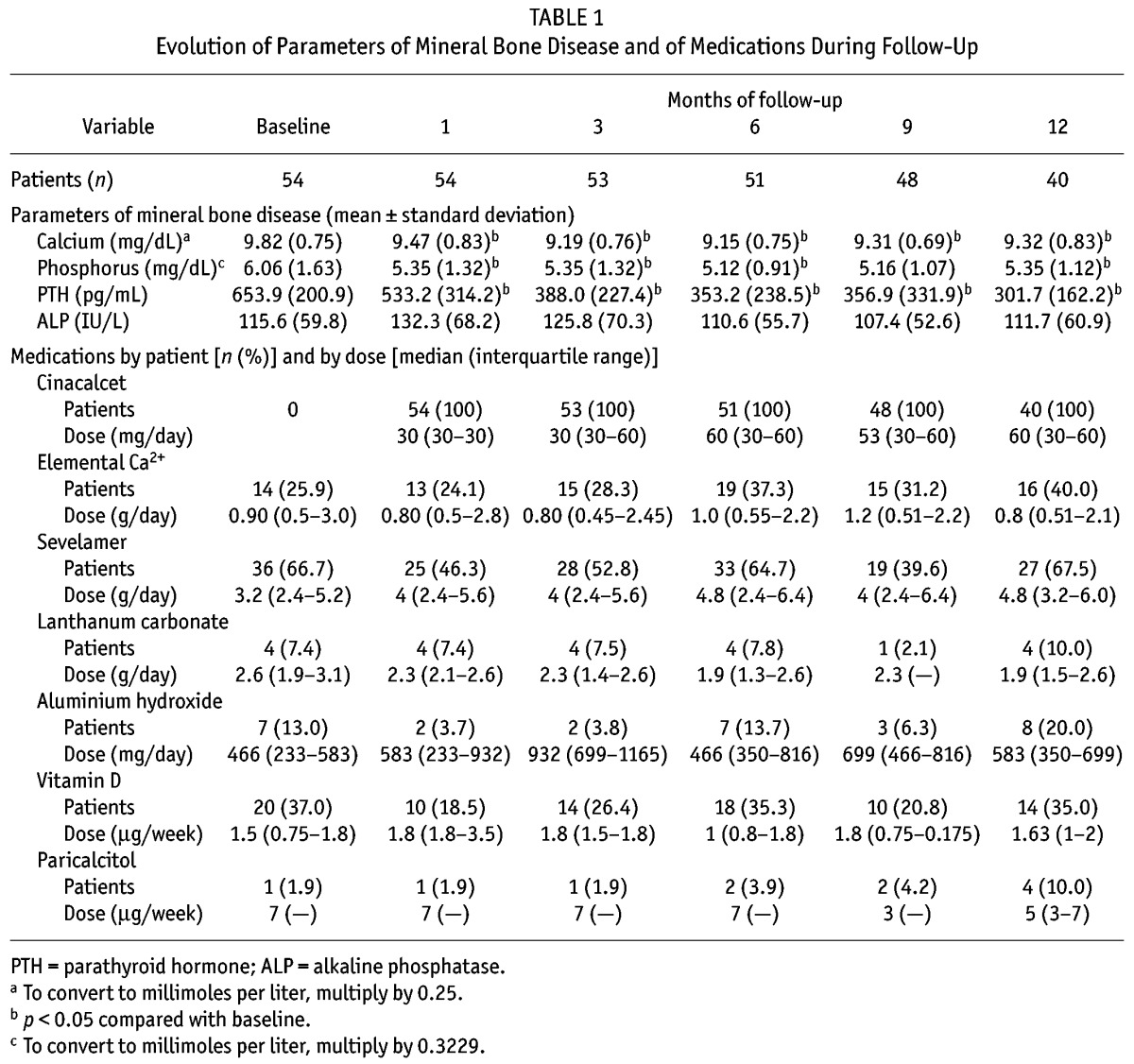
TABLE 2.
Percentage of Patients Who, Simultaneously or Separately, Reached Biochemical Targets Recommended by the Kidney Disease Outcomes Quality Initiative (K/DOQI) and Kidney Disease: Improving Global Outcomes (KDIGO)
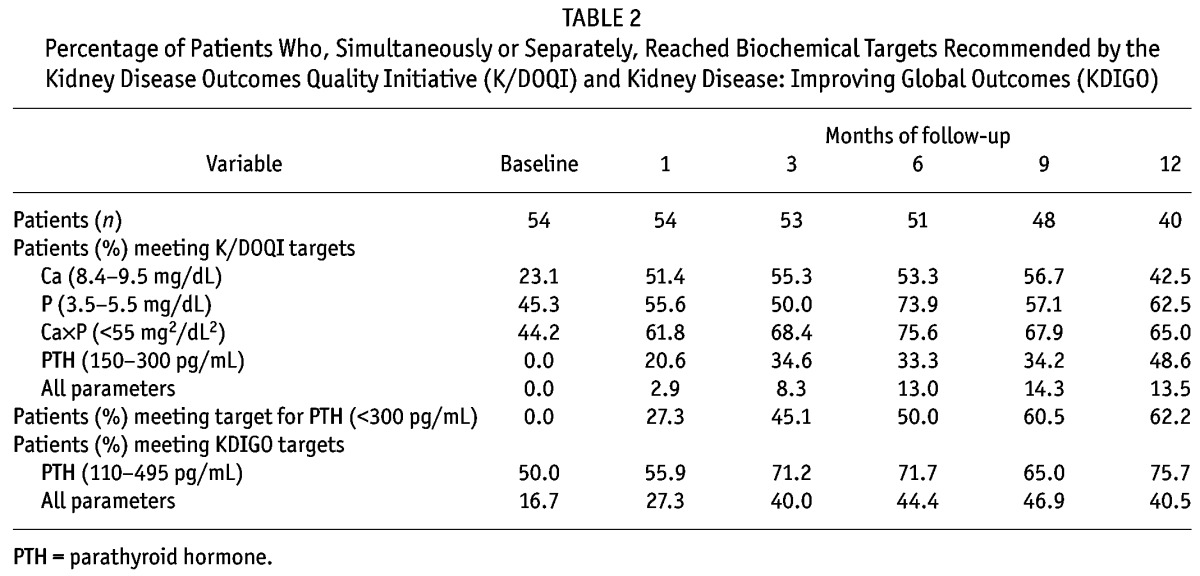
The initial dose of cinacalcet was 30 mg in all cases, with a mean dose of 60 mg at both 6 and 12 months. There was suspicion of low compliance in 3 patients. The median time required to reach a PTH value within the K/DOQI range was 4.4 months (95% confidence interval: 2.1 to 6.7 months), and the median time to reach all the targets was 7.0 months (95% confidence interval: 5.7 to 8.2 months).
Of 301 Ca determinations, only 3 showed a Ca level less than 7.5 mg/dL, and in only 1 patient were associated symptoms of hypocalcemia noted. No parathyroidectomies were performed, but in 2 patients (including 1 noncompliant subject) whose PTH values remained above 1000 pg/mL, a subtotal parathyroidectomy was performed at 3 and at 6 months from the end of follow-up.
DISCUSSION
This is the first 1-year multicenter observational study to have been conducted specifically in PD patients with SHPT treated with cinacalcet. We achieved a 50.9% reduction in PTH levels, with tolerability at least comparable to that reported in HD patients by other authors (3–5). The response in our PD patients seems to be better than that reported in studies conducted exclusively or primarily in HD patients. In a larger published observational study (5) with a mean baseline PTH of 721 pg/mL, a greater-than-50% reduction in PTH was achieved; 28% reached PTH targets; and 18% reached the targets for all biochemical values combined. Another 3-year observational study in HD patients (4) found that, with a mean dose of more than 90 mg, 33% of patients reached the combined PTH and CaχP target (4).
Reaching CKD-MBD targets depends on the combined action of all treatments used, including aluminum hydroxide, whose use was permitted for periods of less than 2 months throughout the present study to avoid toxicity. Doses of cinacalcet used in our study were relatively low, probably because of the mild SHPT found in the study patients, and because of the prudence endorsed by the current Kidney Disease: Improving Global Outcomes guidelines—that is, their suggestion to avoid changing the prescription for an isolated PTH value (8).
The improved response in PD may involve a risk of excessive suppression of PTH. In our study, 14% reached a PTH of less than 150 pg/mL, which, according to some authors, is within an at-risk range for adynamia (9). Hypocalcemia develops in 3.7% of HD patients on cinacalcet therapy (10). However, the parallel percentage seems to be less in PD patients because of the constant presence of an exchange fluid in which Ca levels can be regulated. We were expecting that digestive tolerability would be worse because of the fullness and increased abdominal pressure associated with PD, but the appearance of side effects was even lower than that reported for HD. The main study limitations were the small size of the study cohort and the lack of a control group.
CONCLUSIONS
Cinacalcet is well tolerated and may improve biochemical control of SHPT in PD patients.
DISCLOSURES
Joint funding of Grupo Centro de Diálisis Peritoneal is provided by Baxter Healthcare, Fresenius Medical Care, and Gambro Lundia through an agreement with the Madrid Nephrology Foundation (SOMANE). The authors have no other financial conflicts of interest to declare.
Acknowledgments
The authors thank Dr. Neus Valveny from Trial Form Support SL (Barcelona, Spain) for assistance in the preparation of the manuscript. This writing assistance was supported by Amgen SA, but that company did not participate in the study design, data collection, or analyses.
REFERENCES
- 1. Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT. The Kidney Disease Outcomes Quality Initiative (K/DOQI) Guideline for Bone Metabolism and Disease in CKD: association with mortality in dialysis patients. Am J Kidney Dis 2005; 46:925–32 [DOI] [PubMed] [Google Scholar]
- 2. Strippoli GF, Palmer S, Tong A, Elder G, Messa P, Craig JC. Meta-analysis of biochemical and patient-level effects of calcimimetic therapy. Am J Kidney Dis 2006; 47:715–26 [DOI] [PubMed] [Google Scholar]
- 3. Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 2004; 350:1516–25 [DOI] [PubMed] [Google Scholar]
- 4. Sprague SM, Evenepoel P, Curzi MP, González MT, Husserl FE, Kopyt N, et al. Simultaneous control of PTH and Ca×P is sustained over three years of treatment with cinacalcet HCl. Clin J Am Soc Nephrol 2009; 4:1465–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ureña P, Jacobson SH, Zitt E, Vervloet M, Malberti F, Ashman N, et al. Cinacalcet and achievement of the NKF/K-DOQI recommended target values for bone and mineral metabolism in real-world clinical practice—the ECHO observational study. Nephrol Dial Transplant 2009; 24:2852–9 [DOI] [PubMed] [Google Scholar]
- 6. Torres A, Lorenzo V, Hernández D, Rodríguez JC, Concepción MT, Rodríguez AP, et al. Bone disease in predialysis, hemodialysis, and CAPD patients: evidence of a better bone response to PTH. Kidney Int 1995; 47:1434–42 [DOI] [PubMed] [Google Scholar]
- 7. National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42(Suppl 3):S1–202 [PubMed] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009; (113):S1–130 [DOI] [PubMed] [Google Scholar]
- 9. Barreto FC, Barreto DV, Moysés RM, Neves KR, Canziani ME, Draibe SA, et al. K/DOQI-recommended intact-PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int 2008; 73:771–7 [DOI] [PubMed] [Google Scholar]
- 10. Messa P, Macário F, Yaqoob M, Bouman K, Braun J, von Albertini B, et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Sc Nephrol 2008; 3:36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]


