In children on peritoneal dialysis (PD), peritonitis is mainly a result of infectious causes (1). However non-infectious causes of peritonitis are recognized. Cloudy PD effluent can be attributed to either cellular or non-cellular constituents of peritoneal fluid. Polymorphonuclear leukocytes may be increased because of either intra- or juxta-peritoneal inflammation, or drug-induced chemical peritonitis. Increased eosinophils often represent a response to intraperitoneal (IP) air or an allergy to a component of the dialysis system (2).
Amino acid (AA)–containing PD solutions are used to improve protein nutrition status, particularly if low protein intake is a cause of the malnutrition in malnourished PD patients (3). In some adult studies, a significant improvement in nutrition was reported in patients on continuous ambulatory PD when 1 or 2 exchanges were replaced with 1.1% AA-containing solutions. In children, AA-containing PD solutions have also been shown to be safe and as effective as glucose-based solutions in achieving ultrafiltration and clearances (4). Similarly, the long-term combined administration of glucose- and AA-containing PD solutions in pediatric patients on automated PD (APD) has recently been reported to result in a marked positive nitrogen balance (3). Minor episodes of nausea, vomiting, or unspecified gastrointestinal symptoms have been reported with the use of AA-containing PD solutions, episodes that were dose-dependent. Use of the combined regimen was also associated with an increase in serum urea and a mild degree of metabolic acidosis (3). Solutions of 1.1% AAs were used in a single long daytime dwell in children on APD; the glucose-based solutions were used for night exchanges (5). Solution with AAs was also used as 25% – 40% of a mixed solution also containing glucose-based fluid in overnight exchanges for children on APD (6).
In this short communication, we report an unexpected finding of sterile peritonitis in children on APD who used a combined AA and glucose PD solution.
METHODS
We used a combined AA and glucose PD solution in 7 children (5 girls, 2 boys) as part of a study aimed at treating malnutrition in children on PD. Their mean age was 11.33 ± 3.7 years, and their mean duration on PD before the study was 15 ± 0.8 months. During the study period, children received hourly APD cycles for 10 hours using a mixture of a manufactured 1.1% AA-based solution and a glucose solution (1.36% or 2.27%) in a 1:1 ratio. White blood cells (WBCs) were checked regularly in the PD effluent as part of the monitoring during the study. Children were studied for 12 months.
RESULTS
We observed a rise in WBCs to more than 100/mL in the PD effluent in 5 children (71%) after a mean duration of 7 ± 1.4 months (range: 6 – 9 months) from the time the AA mixture was started (Table 1). The rise in WBCs was observed during laboratory tests in asymptomatic patients. Cloudy PD effluent was not reported by any of the patients. The differential showed mainly monocytes, and the increased WBCs were not associated with a clinical picture of peritonitis or an elevation in acute-phase reactants such as C-reactive protein or peripheral WBCs. All cultures were negative for bacteria and fungi. All the children were treated with IP antibiotics, with no improvement in the effluent cell counts.
TABLE 1.
Changes in Effluent White Blood Cell (WBC) Levels in the Study Patients
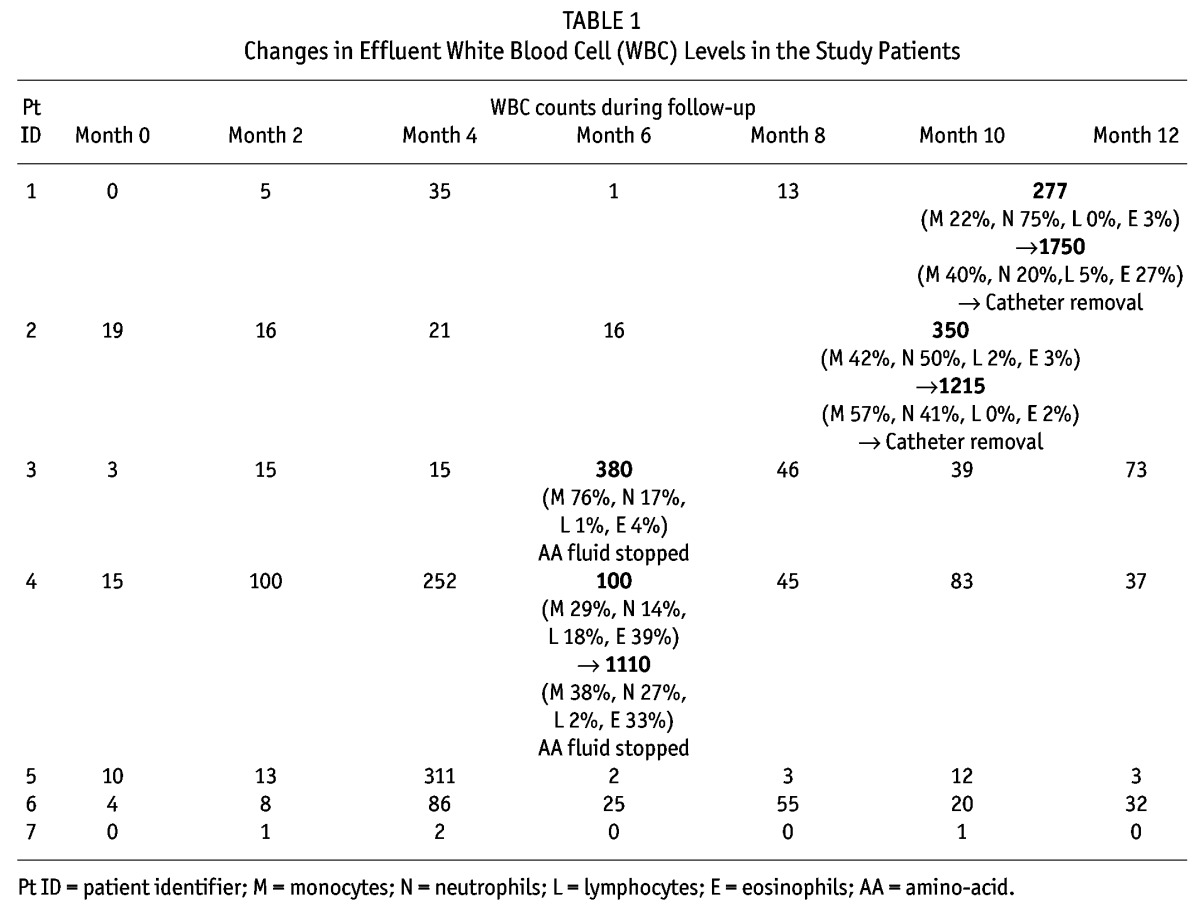
In view of the lack of improvement, the catheter was removed in 2 children, who were temporarily switched to hemodialysis. In the first patient, the effluent cell count rose to 277/mL, and after 3 weeks of IP antibiotics, the effluent cell count reached 1750/mL. The catheter was therefore removed. In the second child, the effluent cell count rose to 350/mL, and after 3 weeks of IP antibiotics, it increased to 1215/mL. The catheter was therefore removed (Table 1).
We stopped the AA dialysate in 2 children, and their effluent cell count rapidly improved to less than 100/mL. In one child, the effluent cell count increased to 380/mL from 15/mL. No response was obtained with IP antibiotics for 3 weeks. The AA-containing solution was stopped, and after 10 days, the WBCs dropped to 62/mL and remained low. Similarly, the PD effluent cell count increased in the other child to 1110/mL from 100/mL. There was no response to 3 weeks of IP antibiotics. The AA-containing solution was stopped, and the WBCs dropped to 46/mL after 1 week and remained low.
Only 3 children completed the study. One had a high effluent cell count of 311/mL with 75% monocytes, but spontaneously improved after few weeks. Another experienced WBC increases to 86/mL (70% monocytes, 11% neutrophils) and 55/mL (78% monocytes, 15% neutrophils), but completed the study. The final child showed an effluent cell count of 0/mL – 2/mL throughout the study. We nevertheless assessed the PD effluent by sending samples for WBC counts and cultures, all of which proved to be clear. However, we lack the facilities to measure endotoxins.
We observed no difference in the results of peritoneal equilibration tests conducted before the study started and after the study ended. Similarly ultrafiltration did not change before, during, or after the study. Mean Kt/V before the study was 2.04 ± 0.33; after the study, it was 1.7 ± 0.78 (p = 0.32).
DISCUSSION
We observed asymptomatic rises in peritoneal WBCs in 5 of our patients, which unfortunately led to catheter removal in 2. Cell counts declined to normal in 2 other children after AA-containing solution was stopped, and counts declined spontaneously in the last child. To the best of our knowledge, no similar observations have previously been reported in PD patients.
Cloudy PD effluent because of noninfectious rises in cell counts of neutrophils, eosinophils, or monocytes has been reported because of visceral IP inflammation, endotoxin-contaminated PD fluid, allergic reaction, pharmaceutical administration, or IP irritation from blood or air introduced during placement of new catheter (7). Cloudy effluent has also been reported with varicella infection (8). The explanation for the rise in effluent WBCs in our patients might be chemical peritonitis, because the condition improved spontaneously after discontinuation of the AA-containing solution in patients 3 and 4. The doses of AA-containing solution in our study were also relatively larger (50% of APD fluid for 10 hours nightly) than those used in most previous trials (4,5), where the AA solution was administered as a single daytime dwell or in a lower concentration of 25% – 40% of total APD fluid for 8 – 10 hours nightly (8,9). Allergy is unlikely, given that no eosinophilia was present and that the increased WBCs developed in most patients after 6 – 10 months of exposure. Intrinsic contamination of the PD solution with endotoxin is a possibility that cannot be excluded, because the endotoxin level in the PD solution was not measured. Contamination of PD solution with endotoxin has been reported as the cause of an outbreak of sterile peritonitis (10).
The reason that the increase in WBCs was observed in some children and not in others is unclear. The spontaneous improvement in one child is interesting, but the rise in WBCs was transient.
CONCLUSIONS
We report an asymptomatic rise in peritoneal WBCs in children dialyzed using relatively high doses of AA-containing solution in APD. The AA dialysate provided effective small-solute clearance and ultrafiltration. We speculate that lower doses of AA-containing PD solution may be necessary, at least in children.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
Acknowledgments
We thank the Deanship of Scientific Research, King Abdulaziz University; and Baxter Healthcare for sponsoring our study.
REFERENCES
- 1. Akman S, Bakkaloglu SA, Ekim M, Sever L, Noyan A, Aksu N. Peritonitis rates and common microorganisms in continuous ambulatory peritoneal dialysis and automated peritoneal dialysis. Pediatr Int 2009; 51:246–9 [DOI] [PubMed] [Google Scholar]
- 2. Teitelbaum I. Cloudy peritoneal dialysate: it’s not always infection. Contrib Nephrol 2006; 150:187–94 [DOI] [PubMed] [Google Scholar]
- 3. Shockley TR, Martis L, Tranæus AP. New solutions for peritoneal dialysis in adult and pediatric patients. Perit Dial Int 1999; 19(Suppl 2):S429–34 [PubMed] [Google Scholar]
- 4. Canepa A, Verrina E, Perfumo F. Use of new peritoneal dialysis solutions in children. Kidney Int Suppl 2008; (108):S137–44 [DOI] [PubMed] [Google Scholar]
- 5. Qamar IU, Secker D, Levin L, Balfe JA, Zlotkin S, Balfe JW. Effects of amino acid dialysis compared to dextrose dialysis in children on continuous cycling peritoneal dialysis. Perit Dial Int 1999; 19:237–47 [PubMed] [Google Scholar]
- 6. Brem AS, Maaz D, Shemin DG, Wolfson M. Use of amino acid peritoneal dialysate for one year in a child on CCPD. Perit Dial Int 1996; 16:634–6 [PubMed] [Google Scholar]
- 7. Rocklin MA, Teitelbaum I. Noninfectious causes of cloudy peritoneal dialysate. Semin Dial 2001; 14:37–40 [DOI] [PubMed] [Google Scholar]
- 8. Braun A, Querfeld V, Mehls O. “Sterile peritonitis” caused by varicella in a child on maintenance peritoneal dialysis treatment. Pediatr Nephrol 1991; 5:95–6 [DOI] [PubMed] [Google Scholar]
- 9. Canepa A, Verrina E, Perfumo F, Carrea A, Menoni S, Delucchi P, et al. Value of intraperitoneal amino acids in children treated with chronic peritoneal dialysis. Perit Dial Int 1999; 19(Suppl 2):S435–40 [PubMed] [Google Scholar]
- 10. Mangram AJ, Archibald LK, Hupert M, Tokars JI, Silver LC, Brennan P, et al. Outbreak of sterile peritonitis among continuous cycling peritoneal dialysis patients. Kidney Int 1998; 54:1367–71 [DOI] [PubMed] [Google Scholar]


