Abstract
♦ Objective: To compare dietary intake of micronutrients by peritoneal dialysis (PD) patients according to their nutrition and inflammatory statuses.
♦ Design: This cross-sectional study evaluated 73 patients using subjective global assessment, 24-hour dietary recall, and markers of inflammation [C-reactive protein (CRP), tumor necrosis factor α, and interleukin 6].
♦ Results: Half the patients had an inadequate micronutrient intake. Compared with dietary reference intakes, malnourished patients had lower intakes of iron (11 mg) and of vitamins C (45 mg) and B6 (0.8 mg). Malnourished and well-nourished patients both had lower intakes of sodium (366 mg, 524 mg respectively), potassium (1555 mg, 1963 mg), zinc (5 mg, 7 mg), calcium (645 mg, 710 mg), magnesium (161 mg, 172 mg), niacin (8 mg, 9 mg), folic acid (0.14 mg, 0.19 mg), and vitamin A (365 μg, 404 μg). Markers of inflammation were higher in malnourished than in well-nourished subjects. Compared with patients in lower quartiles, patients in the highest CRP quartile had lower intakes (p < 0.05) of sodium (241 mg vs 404 mg), calcium (453 mg vs 702 mg), vitamin B2 (0.88 mg vs 1.20 mg), and particularly vitamin A (207 μg vs 522 μg).
♦ Conclusions: Among PD patients, half had inadequate dietary intakes of iron, zinc, calcium and vitamins A, B6, C, niacin, and folic acid. Lower micronutrient intakes were associated with malnutrition and inflammation. Patients with inflammation had lower intakes of sodium, calcium, and vitamins A and B2. Micronutrient intake must be investigated in various populations so as to tailor adequate supplementation.
Keywords: Micronutrients, nutrition, inflammation, CAPD
Protein–energy malnutrition is very common in peritoneal dialysis (PD) patients worldwide (1,2) and is associated with morbidity and mortality (3). There is a possibility that PD patients might develop micronutrient (vitamin and mineral) deficiencies, which may aggravate clinical conditions such as anemia, anorexia, decreased taste, cardiovascular disease, or sexual dysfunction (4,5).
Although micronutrients do not provide energy, they are essential to several metabolic reactions, energy production, maturation and growth of organs and tissues, and protection against some chronic diseases (5,6). Micronutrient deficiencies in PD patients may be caused by poor food intake, intestinal dysfunction, dialysate and urine losses, and abnormal metabolism (4,5). Additionally, inflammation—which is highly prevalent in PD patients (7)—may both regulate and be regulated by some micronutrients (8,9). Micronutrient status also depends on content in foods, bioavailability, and the presence of absorption inhibitors or promoters—all of which may vary by demographic region and traditional nutrition practices (10).
In PD patients, dietary intake of micronutrients and the association of intakes with nutrition status have rarely been evaluated, and in particular, no data about the association of intakes with inflammation are available. In the present study, we therefore aimed to compare dietary intakes of micronutrients (vitamins A, B1, B2, B6, B12, C, niacin, folic acid, and the minerals calcium, phosphorus, magnesium, sodium, potassium, iron, and zinc) by PD patients according to their nutrition status. Additionally, we investigated the relationship between micronutrient intakes and inflammatory status in the patients.
METHODS
This cross-sectional study at the Medical Research Unit for Renal Diseases (Hospital de Especialidades, CMNO, IMSS, Guadalajara, Mexico) enrolled 73 randomly selected patients on continuous ambulatory PD (CAPD). Patients had to have been on dialysis for at least 1 month and to be free of any infectious disease during the preceding 6 weeks. Patients were excluded if they had decompensated heart failure, liver disease, intestinal malabsorption, cancer, or acquired immunodefi ciency syndrome.
All patients underwent clinical, biochemical, dialysis adequacy, and nutrition measurements. For the nutrition evaluation, 4 tools were used: subjective global assessment (SGA), 24-hour dietary recall, and anthropometric and biochemical measurements. The SGA evaluates weight loss, anorexia, vomiting, muscle tissue and body fat loss, and presence of edema (11). Using the SGA, nutrition status is classified as 1 (normal), 2 (mild malnutrition), 3 (moderate malnutrition), or 4 (severe malnutrition). Macro- and micronutrient intakes were evaluated using a 24-hour dietary questionnaire, which was processed against a computerized database of nutritional values for the main foods consumed in Mexico [NutriPac 1.5: NutriPac, Mexico City, Mexico (http://nutripac.com.mx/software/soft.shtml)]. Plastic food models were used to more accurately estimate portions. The 24-hour dietary assessment did not consider calories from dialysate because the intention of the study was to measure only calories from diet (that is, those associated with micronutrient intake). Once the total intake was known, the percentage intakes of calories and protein were calculated and compared with the usual recommendations for individuals on PD. Micronutrient intakes were compared with the dietary reference intakes (DRIs) for a Mexican general population (10). Anthropometric variables were measured using standard techniques and calculated as traditionally recommended (12); lean body mass was estimated by creatinine kinetics (13).
Biochemical parameters—including creatinine, blood urea nitrogen, total cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, glucose, calcium, phosphorus, potassium, and hemoglobin—were measured at the Central Laboratory (personnel blinded to details about the patients) of the Hospital de Especialidades, CMNO, using standard techniques. Serum albumin was determined by the bromocresol green method. Protein nitrogen appearance (PNA) was calculated (14) and normalized (nPNA) to each patient’s actual weight. Additionally, serum samples were analyzed using high-sensitivity kits for the presence of C-reactive protein (CRP), tumor necrosis factor α (TNFα), and interleukin 6 (IL-6); CRP was determined by nephelometry, and TNFα and IL-6 by ELISA. At the Central Laboratory, inter- and intra-assay variations were, respectively, 3.4% and 3.4% for CRP, 3.9% and 4.6% for IL-6, and 2.8% and 6% for TNFα (15). Renal, dialysate, and total (dialysate + renal) clearances of creatinine and urea (Kt/Vurea) were calculated.
All patients used a double-bag system and standard glucose-based dialysis fluid (BenY: Laboratorios Pisa, Guadalajara, Mexico). The study was performed in accordance with the ethical standards of the local research committee on health investigation.
STATISTICAL ANALYSIS
Data are presented as mean ± standard deviation, median and 25% – 75% percentiles, or percentages, as appropriate. For comparison purposes, patients were classified into 2 groups by the SGA results: normal nutrition (score 1) and malnutrition of any grade (scores 2 – 4). For between-group comparisons, the Mann–Whitney U-test or the chi-square test was used, as appropriate. Correlation analysis was performed using the Spearman correlation coefficient. A value of p < 0.05 was considered significant.
RESULTS
By SGA results, only 14 patients (19%) had normal nutrition; 59 patients (81%) had some degree of malnutrition. Almost half the population in this sample showed mild malnutrition (Figure 1). Compared with the well-nourished patients, the malnourished patients had spent significantly more time on dialysis, had lower weights, and showed trends toward older age and lower systolic blood pressure (Table 1). Although the malnourished group contained higher proportions of women and of patients with diabetes, those differences were not statistically significant.
Figure 1.
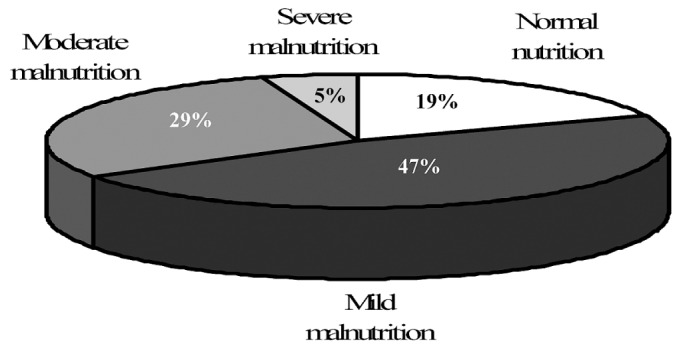
— Nutrition status of the study patients by subjective global assessment.
TABLE 1.
Demographics and Clinical Results by Nutrition Status of the Study Patients
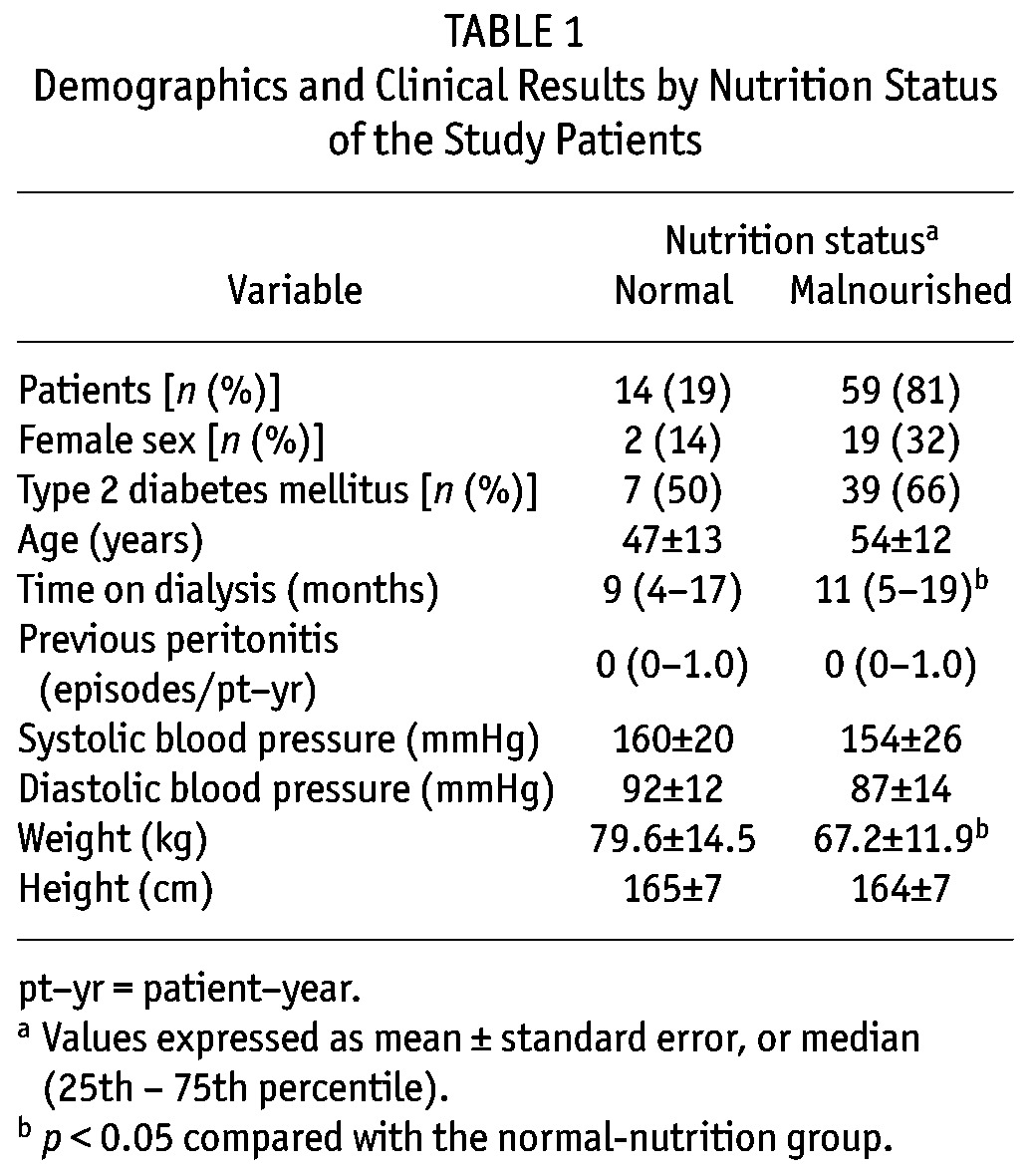
Table 2 shows the biochemical and inflammation results. Compared with patients having normal nutrition, those with malnutrition had significantly lower levels of hemoglobin, albumin, and potassium, and higher levels of LDL cholesterol. Inflammation markers were higher in malnourished than in well-nourished patients, but only TNFα reached statistical significance. On the other hand, the groups showed no significant differences in parameters of dialysis adequacy (peritoneal, renal, or total), in protein losses, or in volumes of drained dialysate and urine (Table 3).
TABLE 2.
Biochemical and Inflammation Results by Nutritional Status of the Study Patients
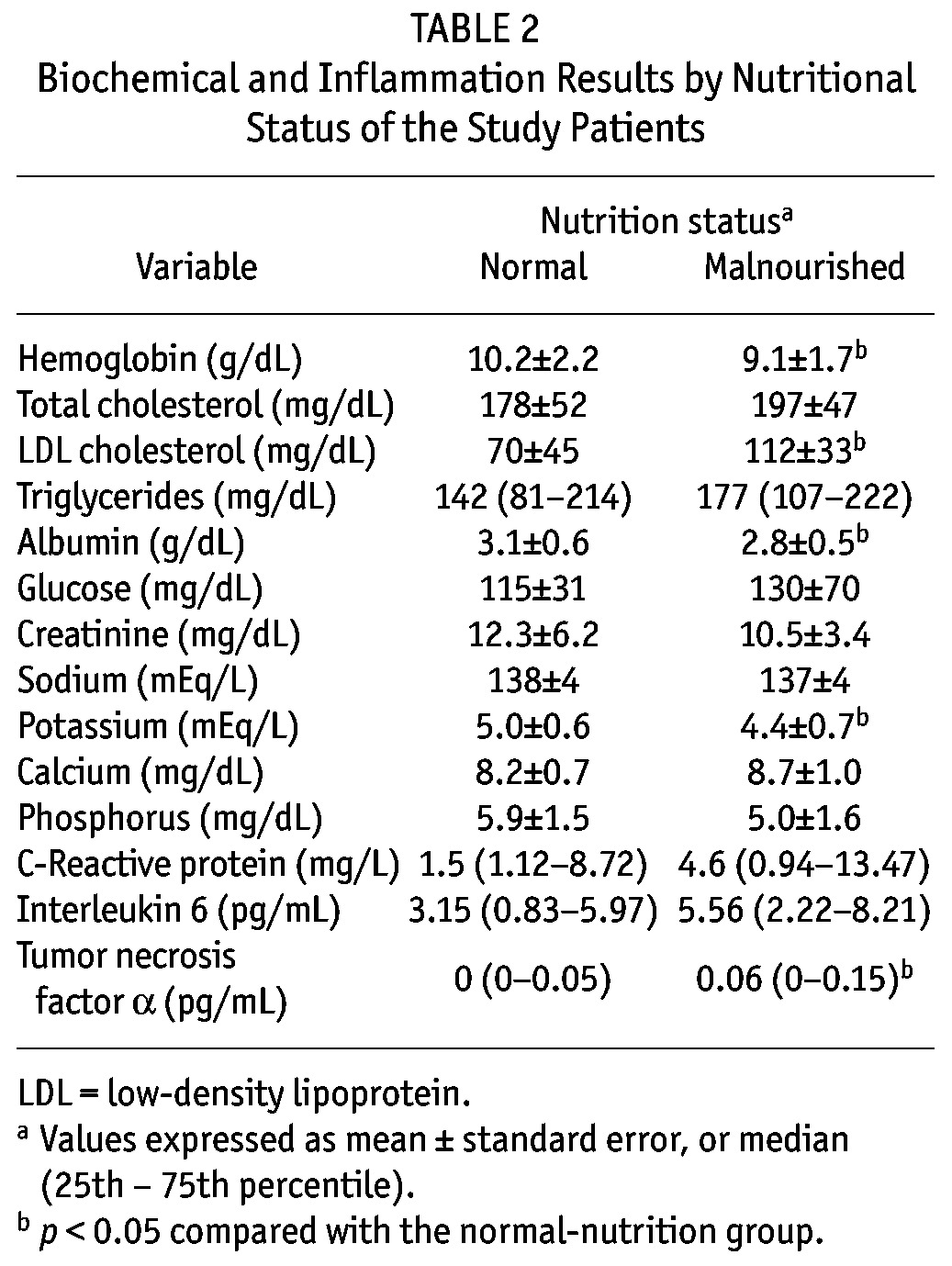
TABLE 3.
Dialysis Adequacy Results by Nutrition Status of the Study Patients
RESULTS OF THE NUTRITION EVALUATIONS
Table 4 shows macronutrient intake results. Compared with the well-nourished patients, the malnourished patients had lower intakes of cholesterol and total calories; however, after adjusting for weight, the difference in calorie intake was no longer significant. No other differences in macronutrient intake were found between the groups. However, disregarding nutrition status, the intakes of calories, protein, monounsaturated and polyunsaturated fats, and fiber were lower than the usually recommended intakes in both groups. Intakes of saturated fat, on the other hand, were higher than recommended.
TABLE 4.
Macronutrient Intake by Nutrition Status of the Study Patients, with the Levels Usually Recommended for Patients on Peritoneal Dialysis
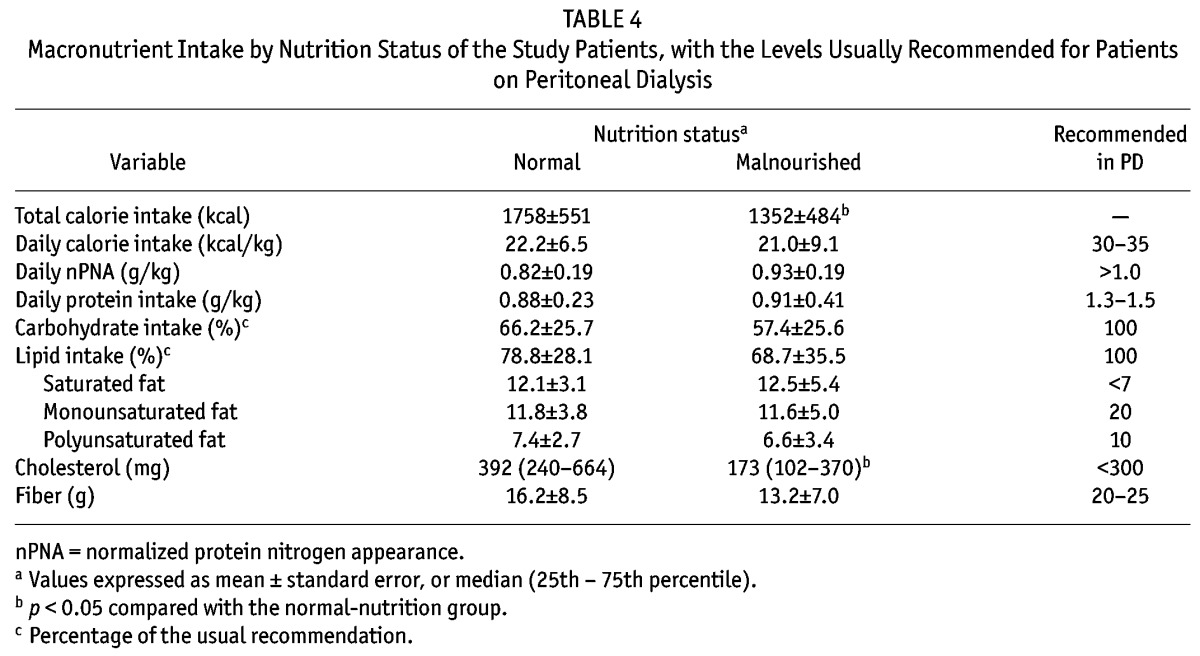
Figure 2 shows, for the overall patient sample, the adequacy of micronutrient intakes as compared with DRIs. Intakes were considered adequate if 100% of the recommended micronutrient intake was fulfilled. Remarkably, only half the patients (or fewer) had adequate intakes of iron, zinc, calcium, vitamin C, vitamin B6, niacin, folic acid, and vitamin A. In particular, folic acid and zinc were ingested in adequate amounts by fewer than 15% of the patients.
Figure 2.
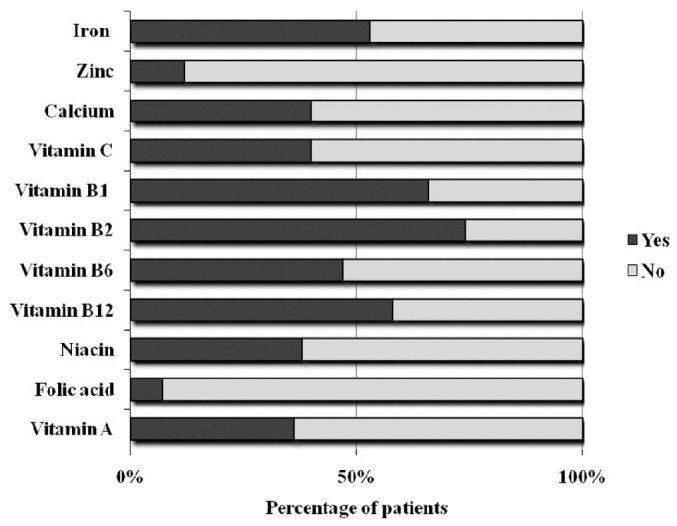
— Percentage of patients with adequate micronutrient intake (100% of the recommended intake) according to the dietary reference intake for the Mexican population.
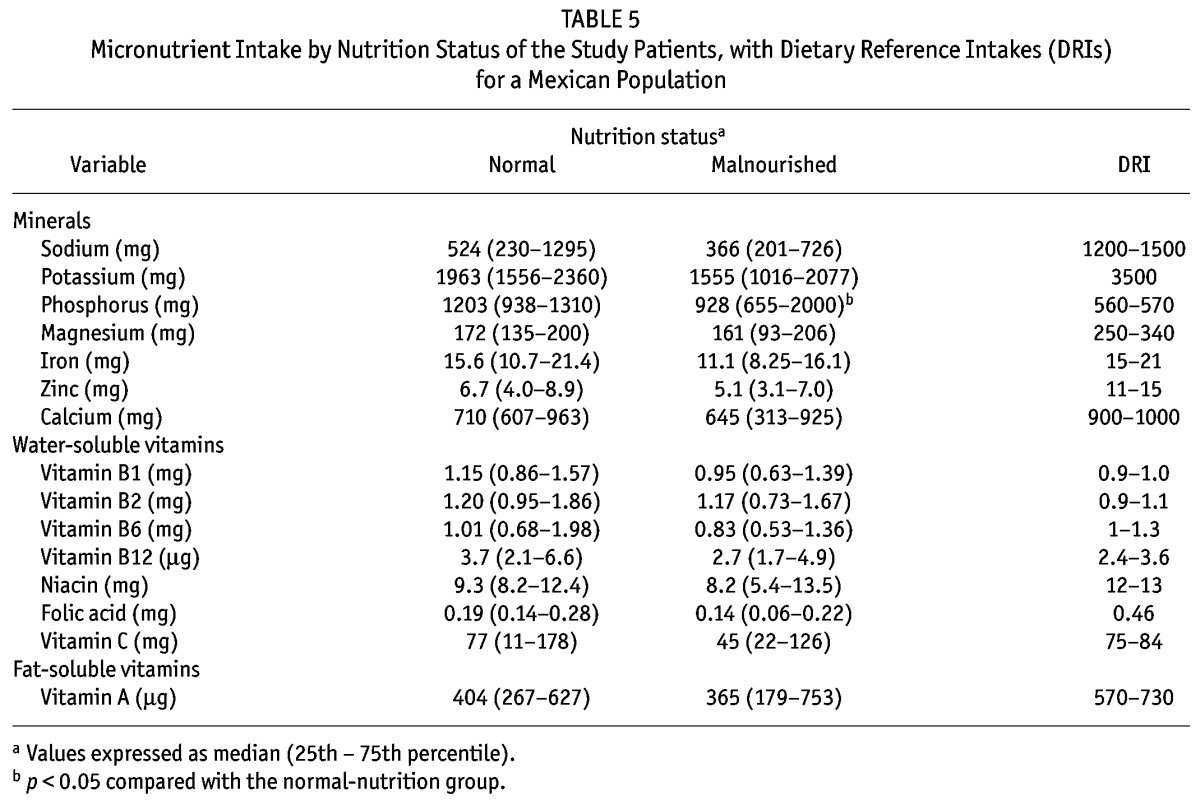
Table 5 compares micronutrient intakes by the nutrition status of the patients. Compared with the well-nourished patients, malnourished patients had a significantly lower intake of phosphorus and a non-significant trend (p = 0.08) to lower iron and folic acid intakes. But those findings were not a result of different dietary patterns in the two groups, because the median nutritional densities for the groups (well-nourished compared with malnourished) were not significantly different: phosphorus, 723 mg/Mcal (628 – 813 mg/Mcal) and 712 mg/Mcal (559 – 878 mg/Mcal); iron, 8.7 mg/Mcal (7.5 – 10.7 mg/Mcal) and 8.1 mg/Mcal (7.0 – 11.4 mg/Mcal); folic acid, 99 mg/Mcal (46 – 153 mg/Mcal) and 127 mg/Mcal (70 – 159 mg/Mcal) respectively. Although no other significant difference in micronutrient intake was found, the dietary intakes of iron and of vitamins C and B6 were noteworthy in being lower than the DRIs only in patients with malnutrition. On the other hand, intakes of sodium, potassium, zinc, calcium, magnesium, niacin, folic acid, and vitamin A were lower than the DRIs in patients of both groups. According to the DRIs, median intakes were adequate in both the malnourished and the well-nourished patients only in the cases of vitamins B1, B2, and B12.
TABLE 5.
Micronutrient Intake by Nutrition Status of the Study Patients, with Dietary Reference Intakes (DRIs) for a Mexican Population
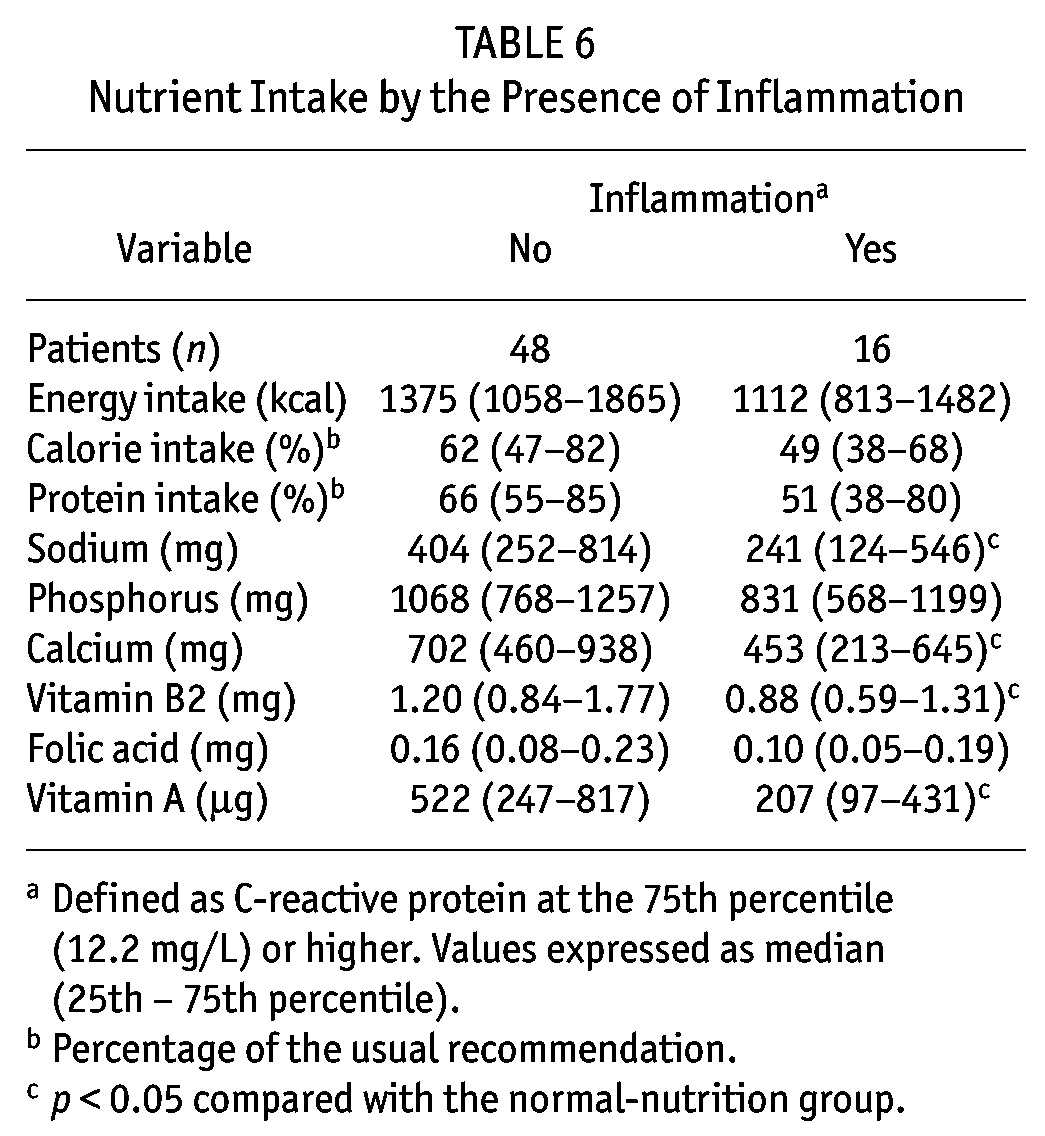
With regard to inflammation (Table 6), patients in the highest quartile of serum CRP had significantly lower intakes of sodium, calcium, vitamin B2, and vitamin A. Additionally, a nonsignificant trend to lower percentage intakes of calories and protein (from the recommended levels), p = 0.07 and p = 0.09 respectively, was found. With regard to dietary patterns, the only difference was a significantly lower (p = 0.02) intake of vitamin A–rich foods in patients with inflammation than in patients without inflammation [nutritional density: 170 mg/Mcal (83 – 318 mg/Mcal) vs 324 mg/Mcal (196 – 584 mg/Mcal) respectively]. Additionally, the following correlations were found: IL-6 with lean body mass (r = –0.66, p < 0.0001), CRP with albumin (r = –0.32, p = 0.01), and TNFα with lean body mass (r = –0.34, p = 0.05), with body mass index (r = –0.30, p = 0.02), and with mid-arm muscle area (r = –0.30, p = 0.02).
TABLE 6.
Nutrient Intake by the Presence of Inflammation
As expected, most of the anthropometric variables were lower in malnourished than in well-nourished patients, respectively: body mass index, 25.1 ± 4.0 kg/m2 and 29.0 ± 3.8 kg/m2, p = 0.001; tricipital skinfold, 19.7 ± 8.1 mm and 23.1 ± 8.3 mm, p = 0.13; subscapular skinfold, 19.6 ± 8.2 mm and 26.8 ± 8.2 mm, p = 0.07; mid-arm muscle area, 29.0 ± 8.5 cm2 and 41.1 ± 9.8 cm2, p < 0.0001; mid-arm fat area, 25.0 ± 11.3 cm2 and 33.3 ± 13.1 cm2, p = 0.02; and lean body mass, 40.2 ± 10.3 kg and 52.9 ± 19.1 kg, p = 0.07.
DISCUSSION
In CAPD, assessments of nutrient intake are essential for identifying subjects at increased risk of malnutrition and for developing strategies to improve nutrition status. However, research has focused primarily on energy–protein intake, with little attention being paid to micronutrient data. In the present study, this latter issue was addressed, and although intakes of micronutrients were strikingly inadequate in malnourished patients, low intakes of several minerals and vitamins were also observed in many of the patients considered to be well-nourished.
Particularly remarkable were the low intakes of folic acid and zinc in both groups, with fewer than 15% of patients having adequate intakes. In the Mexican general population, the median daily intakes of folic acid and zinc have been described as lower than recommended at 0.23 mg and 7.3 mg respectively (16), but intakes were worse in our CAPD patients. Folic acid deficiency is associated with hyperhomocysteinemia, increased risk for cardiovascular disease, and anemia (17,18). Patients on dialysis have levels of homocysteine that are 2 – 3 times those in healthy individuals; these levels can be lowered with folic acid supplementation. Although prospective studies have shown contradictory results for the predictive value of hyperhomocysteinemia with regard to cardiovascular disease in uremia (19), folic acid supplementation is still proposed because this vitamin has no apparent side effects and is inexpensive. On the other hand, zinc deficiency is related to complications such as anorexia and alterations of taste and smell (20,21), which may also lower a patient’s nutrient intake in uremia. Low plasma zinc concentrations have been reported in pre-dialysis and dialysis patients, with improvement noted after zinc supplementation (22).
Vitamin A is important for normal vision, immunologic response, gene expression, cellular growth and differentiation, and protection against oxidant-mediated damage. Previous studies have shown adequate or excessive ingestion and serum concentrations of vitamin A (23–25) in dialysis patients. In the present study, however, vitamin A intake was lower than recommended in both groups. That finding may be related to the fact that the main sources of vitamin A in the Mexican diet are red-orange and green vegetables (26), which are also usually rich in potassium and therefore typically restricted in these patients. Studies measuring serum concentrations of vitamin A are required, especially when supplementation of this vitamin could be considered (as in populations such as ours).
Intakes of niacin, which participates in many biologic reduction and oxidation reactions, were also deficient in both patient groups. In chronic kidney disease patients, niacin has the potential for lipid-lowering and anti-inflammatory effects, and it may be a promising phosphate binder (27,28). Niacin deficiency has been not much studied in renal failure and CAPD; however, it may, like other water-soluble vitamins, be lost through dialysate (29). Thus, niacin supplementation according to the DRI seems to be a prudent recommendation.
Calcium intake was also low in both patient groups, probably because of the common restrictions on dairy products (a typical strategy to reduce dietary phosphorus). Calcium intake in the form of phosphorus binder was not considered in the present study; in our setting, it is usual to prescribe about 1.0 – 1.2 g of elemental calcium, which may broadly compensate for low intake.
Although dietary intakes of iron, vitamin C, and vitamin B6 were low in this sample, only malnourished patients had intakes strictly below the DRIs. These nutrients are involved in erythropoiesis and anemia prevention (17), and low levels may contribute to the low hemoglobin concentrations in these patients. When iron intake is compared with that in other international studies (23,24), our population seems to have similar or even higher intakes; however, because of local characteristics (high tannin intake and low iron bioavailability), the iron requirement is higher in a Mexican population (10). It is recommended that 75% of total iron intake come from heme sources; however, in our country, the main iron sources are cereals and vegetables (non-heme iron). Meats (heme iron) provide only 14% of the total intake (26). Dietary iron is considered safe, but when chronically supplemented above tolerable upper intake levels (especially in dialysis patients), attention must be paid to avoiding adverse effects (30). Vitamin C is a potent antioxidant; however, this nutrient is easily lost during dialysis, and because the main dietary sources (citrus fruits, leafy vegetables) are frequently restricted in renal patients, deficiency may be very common in this population (31). Low intake of vitamin C has previously been reported in dialysis patients (23,24), but it was more remarkable in the present study. Large doses of vitamin C may result in significant increase of calcium oxalate crystallization and urolithiasis (32); thus, research is necessary to guide supplementation with this vitamin. With regard to pyridoxine, this vitamin also participates in erythropoiesis and acts as a cofactor in enzymatic reactions, importantly in homocysteine metabolism (33). Pyridoxine replacement is recommended to prevent deficiencies and to maintain adequate stores (34).
Remarkable findings in the present study were the presence of a higher systemic inflammatory status in malnourished patients, and the association between inflammation and lower dietary intakes of calories, protein, and some micronutrients (sodium, calcium, and vitamins B2 and A). It has been shown that inflammation increases protein catabolism (35) and anorexia (36), which, in conjunction with an already low nutrient intake and dialysis losses, may worsen the overall nutrition status of PD patients. Vitamin B2 has potential anti-inflammatory effects through the release of nitric oxide, the opening of ATP-sensitive K+ channels, and possibly inhibition of the synthesis of inflammatory cytokines (37). Whether the relationship between riboflavin and inflammation is causal or not in PD patients deserves to be investigated; meanwhile, dietary supplementation seems to be reasonable in these patients. The association between inflammation and low intake of vitamin A is particularly intriguing. Low serum concentrations of retinol have been shown to be an independent predictor of overall and cardiovascular mortality in hemodialysis patients (38), and in the presence of inflammation and a diet rich in saturated fat and low in fiber, as in our patients, a pre-existing cardiovascular disease could worsen (39). In the case of patients with inflammation, reduced vitamin A intake seems to be a result of lower consumption of foods rich in this vitamin (as judged by lower nutritional density). Thus, in PD patients with inflammation, advice to increase their consumption of vitamin A–rich meals may be necessary, and (if our finding is further confirmed) oral supplementation could be applied in populations such as ours. Dietary sodium restriction, commonly prescribed in PD to help avoid water retention and high blood pressure, may have negative effects in certain circumstances. Very low intake of sodium has been associated with an increased risk of general and cardiovascular mortality in PD patients (40); such a low intake may be associated with lower global intakes of calories and nutrients, increased insulin resistance, activation of the renin–angiotensin system, and increased activity of the sympathetic system (40). The association between low sodium intake and inflammation in PD (and a possibly worse cardiovascular outcome) deserves further investigation. On the other hand, the relationship between inflammation and low calcium intake found in the present study is difficult to evaluate, especially given that the intake of calcium-based phosphate binders was not specifically measured. However, it is interesting to note that diets high in calcium have been shown to lower oxidative and inflammatory stress in mice and humans (41).
Loss of residual renal function (associated with longer dialysis vintage) and lower solute clearance have been shown to be risk factors for malnutrition and low nutrient intake (42); however, in the present study, we observed no differences in renal, dialysis, and total creatinine clearance or in Kt/Vurea between the well-nourished and the malnourished patients. Subjects in the present study seemed to have a slightly higher residual renal function than has been seen in other studies (24,42), which could attenuate the more negative nutritional effects of anuria.
Calories from dialysate do not contribute to micronutrient intake, and therefore they were not considered in the present study. If calories from dialysate had been considered, the micronutrient density calculation might be altered. Patients with malnutrition had higher concentrations of total and LDL cholesterol, a result that is not completely clear, but that may be related to a lower fiber intake than that in well-nourished patients (43).
Limitations of the present study include its cross-sectional nature and the lack of measured serum micronutrient concentrations. Other causes of malnutrition in PD—such as loss of micronutrients in dialysate, metabolic acidosis, endocrine disorders, and the peritoneal transport rate—were also not evaluated. In a 24-hour dietary recall, real food consumption can be underestimated by 10% – 20% because only the previous day is evaluated; however, longer dietary questionnaires may not completely solve the problem and may entail more difficulty in acquiring information (44). Additional longitudinal investigations with larger numbers of patients, especially with respect to the foregoing limitations, are necessary to confirm our results. Micronutrient deficiencies such as those shown in the present study could play a role in the PD survival reported in our setting (45,46).
CONCLUSIONS
Half our PD patients had inadequate dietary intakes of iron, zinc, calcium, and vitamins A, B6, C, niacin, and folic acid. Low nutrient intake was associated with malnutrition and inflammation, but not with renal or dialysis clearance. Patients with inflammation had lower intakes of sodium, calcium, vitamin B2, and especially vitamin A. Micronutrient intake and supplementation must be investigated in various populations so that adequate supplementation can be tailored and deficiencies avoided according to need. In populations such as ours, multivitamin and mineral supplements (including at least zinc, folic acid, niacin, and vitamins A, B6, and C) could be advised; alternatively, zinc and folic acid supplementation might be prioritized to improve anorexia and appetite, in the hope of subsequently increasing dietary intake of other micronutrients.
DISCLOSURES
This study was partially supported by grants 2005/1/I/108 and 2006/1A/I/007 from the Fondo de Fomento a la Investigación, IMSS.
Acknowledgments
Special acknowledgment goes to chemists Adolfo Cota and Eva Barajas.
REFERENCES
- 1. Young GA, Kopple JD, Lindholm B, Vonesh EF, De Vecchi A, Scalamogna A, et al. Nutritional assessment of continuous ambulatory peritoneal dialysis patients: an international study. Am J Kidney Dis 1991; 17:462–71 [DOI] [PubMed] [Google Scholar]
- 2. Martín-Del-Campo F, González–Espinoza L, Rojas–Campos E, Ruiz N, González J, Pazarín L, et al. Conventional nutritional counselling maintains nutritional status of patients on continuous ambulatory peritoneal dialysis in spite of systemic inflammation and decrease of residual renal function. Nephrology (Carlton) 2009; 14:493–8 [DOI] [PubMed] [Google Scholar]
- 3. de Mutsert R, Grootendorst DC, Axelsson J, Boeschoten EW, Krediet RT, Dekker FW. on behalf of the NECOSAD Study Group. Excess mortality due to interaction between protein–energy wasting, inflammation and cardiovascular disease in chronic dialysis patients. Nephrol Dial Transplant 2008; 23:2957–64 [DOI] [PubMed] [Google Scholar]
- 4. Kalantar–Zadeh K, Kopple JD. Trace elements and vitamins in maintenance dialysis patients. Adv Ren Replace Ther 2003; 10:170–82 [DOI] [PubMed] [Google Scholar]
- 5. Makoff R, Gonick H. Clinical dilemmas: renal failure and the concomitant derangement of micronutrient metabolism. Nutr Clin Pract 1999; 14:238–46 [Google Scholar]
- 6. Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults: scientific review. JAMA 2002; 287:3116–26 [Erratum in: JAMA 2002; 288:1720] [DOI] [PubMed] [Google Scholar]
- 7. Cueto–Manzano AM, González–Espinoza L, Martin del Campo F, Fortes PC, Pecoits–Filho R. Inflammation in peritoneal dialysis: a Latin American perspective. Perit Dial Int 2007; 27:347–52 [PubMed] [Google Scholar]
- 8. Tomkins A. Assessing micronutrient status in the presence of inflammation. J Nutr 2003; 133(Suppl 2):1649S–55S [DOI] [PubMed] [Google Scholar]
- 9. Saldeen TG, Mehta JL. Dietary modulations in the prevention of coronary artery disease: a special emphasis on vitamins and fish oils. Curr Opin Cardiol 2002; 17:559–67 [DOI] [PubMed] [Google Scholar]
- 10. Bourges R, Casanueva E, Rose JL. Nutrient Intake Recommendations for the Mexican Population [Spanish] México DF, México: Editorial Médica Panamericana; 2005. [Google Scholar]
- 11. Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is a subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr 1987; 11:8–13 [DOI] [PubMed] [Google Scholar]
- 12. Frisancho AR. New standards of weight and body composition by frame size and height for assessment of nutritional status of adults and the elderly. Am J Clin Nutr 1984; 40:808–19 [DOI] [PubMed] [Google Scholar]
- 13. Keshaviah PR, Nolph KD, Moore HL, Prowant B, Emerson PF, Meyer M, et al. Lean body mass estimation by creatinine kinetics. J Am Soc Nephrol 1994; 4:1475–85 [DOI] [PubMed] [Google Scholar]
- 14. Bergström J, Heimbürger O, Lindholm B. Calculation of the protein equivalent of total nitrogen appearance from urea appearance. Which formulas should be used? Perit Dial Int 1998; 18:467–73 [PubMed] [Google Scholar]
- 15. Cueto–Manzano AM, Rojas–Campos E, Martínez–Ramírez HR, Valera–González I, Medina M, Monteón F, et al. Can the inflammation markers of patients with high peritoneal permeability on continuous ambulatory peritoneal dialysis be reduced on nocturnal intermittent peritoneal dialysis? Perit Dial Int 2006; 26:341–8 [PubMed] [Google Scholar]
- 16. Barquera S, Hernández–Barrera L, Campos–Nonato I, Espinosa J, Flores M, Ab J, et al. Energy and nutrient consumption in adults: analysis of the Mexican National Health and Nutrition Survey 2006. Salud Publica Mex 2009; 51(Suppl 4):S562–73 [DOI] [PubMed] [Google Scholar]
- 17. Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr 2004; 24:105–31 [DOI] [PubMed] [Google Scholar]
- 18. Brattström L, Wilcken DE. Homocysteine and cardiovascular disease: cause or effect? Am J Clin Nutr 2000; 72:315–23 [DOI] [PubMed] [Google Scholar]
- 19. Suliman ME, Bárány P, Kalantar–Zadeh K, Lindholm B, Stenvinkel P. Homocysteine in uraemia—a puzzling and conflicting story. Nephrol Dial Transplant 2005; 20:16–21 [DOI] [PubMed] [Google Scholar]
- 20. Grzegorzewska AE, Mariak I. Zinc as a marker of nutrition in continuous ambulatory peritoneal dialysis. Adv Perit Dial 2001; 17:223–9 [PubMed] [Google Scholar]
- 21. Mahajan SK, Prasad AS, Lambujon J, Abbasi AA, Briggs WA, McDonald FD. Improvement of uremic hypogeusia by zinc: a double-blind study. Am J Clin Nutr 1980; 33:1517–21 [DOI] [PubMed] [Google Scholar]
- 22. Roozbeh J, Hedayati P, Sagheb MM, Sharifian M, Hamidian Jahromi A, Shaabani S, et al. Effect of zinc supplementation on triglyceride, cholesterol, LDL, and HDL levels in zinc-deficient hemodialysis patients. Ren Fail 2009; 31:798–801 [DOI] [PubMed] [Google Scholar]
- 23. Kalantar–Zadeh K, Kopple JD, Deepak S, Block D, Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr 2002; 12:17–31 [DOI] [PubMed] [Google Scholar]
- 24. Wang AY, Sea MM, Ip R, Law MC, Chow KM, Lui SF, et al. Independent effects of residual renal function and dialysis adequacy on dietary micronutrient intakes in patients receiving continuous ambulatory peritoneal dialysis. Am J Clin Nutr 2002; 76:569–76 [DOI] [PubMed] [Google Scholar]
- 25. Henderson IS, Leung AC, Shenkin A. Vitamin status in continuous ambulatory peritoneal dialysis. Perit Dial Bull 1984; 4:143–5 [Google Scholar]
- 26. Avila CA, Shamah LT, Chavez VA, Galindo GC. Survey of Urban Food and Nutrition at the Metropolitan Zone of Mexico City 2002 (Spanish). México DF, México: Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Instituto Nacional de Salud Pública; 2003. [Google Scholar]
- 27. Ahmed MH. Niacin as potential treatment for dyslipidemia and hyperphosphatemia associated with chronic renal failure: the need for clinical trials. Ren Fail 2010; 32:642–6 [DOI] [PubMed] [Google Scholar]
- 28. Cho KH, Kim HJ, Kamanna VS, Vaziri ND. Niacin improves renal lipid metabolism and slows progression in chronic kidney disease. Biochim Biophys acta 2010; 1800:6–15 [DOI] [PubMed] [Google Scholar]
- 29. Wolk R. Micronutrition in dialysis. Nutr Clin Pract 1993; 8:267–76 [DOI] [PubMed] [Google Scholar]
- 30. Kovesdy CP, Kalantar–Zadeh K. Iron therapy in chronic kidney disease: current controversies. J Ren Care 2009; 35(Suppl 2):14–24 [DOI] [PubMed] [Google Scholar]
- 31. Lim SL, Lee EJ, Myint CC, Ong KT, Tay ME, Yusuf N, et al. Oral intake and serum levels of ascorbic acid in continuous ambulatory peritoneal dialysis patients. Adv Perit Dial 2001; 17:215–18 [PubMed] [Google Scholar]
- 32. Baxmann AC, De O G Mendonça C, Heilberg IP. Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Kidney Int 2003; 63:1066–71 [DOI] [PubMed] [Google Scholar]
- 33. Alemán G, Tovar AR, Torres N. Metabolism of homocysteine and cardiovascular disease risk: importance of the nutritional status of folic acid, vitamins B6 and B12 (Spanish). Rev Invest Clin 2001; 53:141–51 [PubMed] [Google Scholar]
- 34. Kopple JD, Mercurio K, Blumenkrantz MJ, Jones MR, Tallos J, Roberts C, et al. Daily requirement for pyridoxine supplements in chronic renal failure. Kidney Int 1981; 19:694–704 [DOI] [PubMed] [Google Scholar]
- 35. Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 2010; 91:1128S–32S [DOI] [PubMed] [Google Scholar]
- 36. Dukkipati R, Kopple JD. Causes and prevention of protein–energy wasting in chronic kidney failure. Semin Nephrol 2009; 29:39–49 [DOI] [PubMed] [Google Scholar]
- 37. Granados–Soto V, Terán–Rosales F, Rocha–González HI, Reyes–García G, Medina–Santillán R, Rodríguez–Silverio J, et al. Riboflavin reduces hyperalgesia and inflammation but not tactile allodynia in the rat. Eur J Pharmacol 2004; 492:35–40 [DOI] [PubMed] [Google Scholar]
- 38. Kalousová M, Kubena AA, Kostírová M, Vinglerová M, Ing OM, Dusilová–Sulková S, et al. Lower retinol levels as an independent predictor of mortality in long-term hemodialysis patients: a prospective observational cohort study. Am J Kidney Dis 2010; 56:513–21 [DOI] [PubMed] [Google Scholar]
- 39. Lairon D, Arnault N, Bertrais S, Planells R, Clero E, Hercberg S, et al. Dietary fiber intake and risk factors for cardiovascular disease in French adults. Am J Clin Nutr 2005; 82:1185–94 [DOI] [PubMed] [Google Scholar]
- 40. Dong J, Li Y, Yang Z, Luo J. Low dietary sodium intake increases the death risk in peritoneal dialysis. Clin J am Soc Nephrol 2010; 5:240–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zemel MB, Sun X. Dietary calcium and dairy products modulate oxidative and inflammatory stress in mice and humans. J Nutr 2008; 138:1047–52 [DOI] [PubMed] [Google Scholar]
- 42. Wang AY, Sea MM, Ip R, Law MC, Chow KM, Lui SF, et al. Independent effects of residual renal function and dialysis adequacy on actual dietary protein, calorie and other nutrient intake in patients on continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 2001; 12:2450–7 [DOI] [PubMed] [Google Scholar]
- 43. Solà R, Bruckert E, Valls RM, Narejos S, Luque X, Castro–Cabezas M, et al. Soluble fibre (Plantago ovata husk) reduces plasma low-density lipoprotein (LDL) cholesterol, triglycerides, insulin, oxidised LDL and systolic blood pressure in hypercholesterolaemic patients: a randomised trial. Atherosclerosis 2010; 211:630–7 [DOI] [PubMed] [Google Scholar]
- 44. Griffiths A, Russell L, Breslin M, Russell G, Davies S. A comparison of two methods of dietary assessment in peritoneal dialysis patients. J Ren Nutr 1999; 9:6–31 [DOI] [PubMed] [Google Scholar]
- 45. Cueto–Manzano AM, Quintana–Piña E, Correa–Rotter R. Survival on CAPD: 12-year experience of a single Mexican center. Perit Dial Int 2001; 21:148–53 [PubMed] [Google Scholar]
- 46. Paniagua R, Amato D, Vonesh E, Correa–Rotter R, Ramos A, Moran J, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 2002; 13:1307–20 [DOI] [PubMed] [Google Scholar]


